Abstract
Objective:
To evaluate the relationship between CSF hypocretin-1 levels and clinical profiles in narcolepsy and CNS hypersomnia in Norwegian patients.
Method:
CSF hypocretin-1 was measured by a sensitive radioimmunoassay in 47 patients with narcolepsy with cataplexy, 7 with narcolepsy without cataplexy, 10 with idiopathic CNS hypersomnia, and a control group.
Results:
Low hypocretin-1 values were found in 72% of the HLA DQB1*0602 positive patients with narcolepsy and cataplexy. Patients with low CSF hypocretin-1 levels reported more extensive muscular involvement during cataplectic attacks than patients with normal levels. Hypnagogic hallucinations and sleep paralysis occurred more frequently in patients with cataplexy than in the other patient groups, but with no correlation to hypocretin-1 levels.
Conclusion:
About three quarters of the HLA DQB1*0602 positive patients with narcolepsy and cataplexy had low CSF hypocretin-1 values, and appear to form a distinct clinical entity. Narcolepsy without cataplexy could not be distinguished from idiopathic CNS hypersomnia by clinical symptoms or biochemical findings.
Citation:
Heier MS; Evsiukova T; Vilming S; Gjerstad MD; Schrader H; Gautvik K. CSF hypocretin-1 levels and clinical profiles in narcolepsy and idiopathic CNS hypersomnia in norway. SLEEP 2007;30(8):969-973.
Keywords: Hypocretin, narcolepsy, cataplexy, CNS hypersomnia
INTRODUCTION
Narcolepsy is a sleep disorder with a prevalence of 0.2‰ −0.52‰1–4 It is characterized by daytime hypersomnia, sleep attacks, and cataplexy (sudden, bilateral loss of postural muscle tone, triggered by sudden emotions). Associated symptoms like hypnagogic hallucinations, sleep paralysis, automatic behavior, nightmares, parasomnias, and fragmented nocturnal sleep are frequently reported. The diagnosis is based on anamnestic data and supported by the presence of REM in ≥2 sleep onset periods (SOREM) in a multiple sleep latency test (MSLT). It is associated with HLA-type DQB1*0602 in all ethnic groups, with a frequency of 95% in patients with severe cataplexy and 40%–60% in patients with milder forms. However, as about 25% of the general European population also has this HLA-type,5,6 it cannot be used as a diagnostic test alone.
In 1996 a new mRNA in the rat brain restricted to the lateral hypothalamus and parafornical area was isolated.7 The corresponding peptide was called hypocretin (orexin), and was shown to exist as two variants: hypocretin-1, and hypocretin-2, with distinct receptors.8 The hypocretins are neuroexitatory, and hypocretin-producing neurons were shown to have projections innervating systems with regulatory functions on sleep and wake mechanisms.8–14 Narcolepsy with cataplexy in humans has been shown to be associated with low levels of CSF hypocretin-1 in many, but not all patients, and measurement of CSF hypocretin-1 levels is now being evaluated as a diagnostic tool.15–21
The objective of the present study was to determine which clinical criteria were associated with reduced CSF hypocretin-1 levels and HLA DQB1*0602 type in a Norwegian population of patients with narcolepsy. For comparison we also included patients with idiopathic CNS hypersomnia.
PATIENTS AND METHODS
Sixty-four previously diagnosed patients were recruited, the majority from the neurological outpatient department of Ullevål University Hospital in Oslo. Others were recruited from other main university hospitals and from the patient organisation of sleep disorders. All were Caucasians of Norwegian parentage. Only patients who fulfilled internationally accepted criteria for narcolepsy or idiopathic CNS hypersomnia were included. The patients answered a questionnaire on sleep habits, daytime sleepiness, accessory symptoms, duration, and treatment. The patients with cataplexy also had a structured interview by a senior neurologist, with special emphasis on a detailed description of their cataplectic attacks, triggering factors, and frequency.
Inclusion criteria for narcolepsy with cataplexy were excessive daytime sleepiness for more than 3 months and typical cataplexy. Results of previous MSLT were available for 32 of the 47 patients with cataplexy, showing mean sleep latencies ≤5 min and ≥2 SOREM. Cataplexy was defined, based on internationally accepted criteria, as sudden bilateral loss of postural muscle tone of short duration (<5 minutes), triggered by sudden, intense emotion. Patients who did not meet these criteria were either excluded from the study or reclassified as narcolepsy without cataplexy or CNS hypersomnia, depending on clinical symptoms and results of MSLT. Inclusion criteria for narcolepsy without cataplexy were excessive daytime sleepiness for more than 3 months, absence of other disorders that could explain the symptoms, and mean sleep latency ≤5 min and ≥2 SOREM in an MSLT. Patients with idiopathic CNS hypersomnia with and without long sleep periods,22 were included if they had documented prolonged sleep period of >10 hours and/or excessive daytime sleepiness for more >6 months, MSLT with mean sleep latency ≤8 minutes, no SOREMs, and no other causes of daytime sleepiness. Results from previous MSLTs were reviewed, and new sleep recordings performed when necessary.
Fifty controls were recruited consecutively from patients without hypersomnia, admitted to the neurological department at Ullevål university hospital for lumbar puncture as part of diagnostic procedures for other neurological disorders, such as mononeuropathies, polyneuropathies, and unspecified paresthesias. Lumbar puncture was performed between 10:00–12:00 in patients and controls, and spinal fluid analyzed for hypocretin-1. A blood sample was analyzed for HLADQB1*0602-type.
The study was sanctioned by the regional committee for medical ethics and both patients and controls gave their informed verbal and written consent according to the Helsinki declaration. All regulations in the Norwegian law of biotechnology and biobanking were met.
Biochemical Analysis
Hypocretin-1 was measured by radioimmunoassay (RIA), using a polyclonal antibody as described by the producer (Phoenix Pharmaceutical, St. Joseph, MO, USA). Two ml CSF was centrifuged and stored immediately at −70° until assay. The samples were never thawed more than once. CSF portions of 1 ml were chromatographed on Sephadex (Sep) columns after pre-treatment with 1.0 ml of 0.1% trifluoroacetic acid (TFA), mixing, and applied to the columns after 20 minutes in room temperature. Each column was washed twice with 3 ml 0.1% TFA and eluted with 3 ml 60% acetonitril in 0.1% TFA. The eluate was collected as 3 ml fractions which were freeze dried. The precipitate was resuspended in 0.5 ml RIA buffer and mixed well; 100 μl in duplicates were analysed in RIA. The detection limit of the RIA was 5.0 ± 0.7 pg/ml. The intra assay coefficient of variation was 4.8%, determined by 7 replicate analysis of the same sample. The interassay coefficient of variation was 15.5%, determined from measuring the same sample in 7 different assays. The standard curve range was from 1–128 pg/ml. In all assays, 3 pooled internal standards were included, representing low (ca 100 pg/ml and ca 180 pg/ml) and normal (ca 475 pg/1) values. The recovery of peptide added directly to CSF after purification, concentration, and resuspension, was 71% ± 4.5% (mean ± SD). Dilution curves of CSF samples were parallel to the standard curve.
Statistics
The significance of different muscular involvement in eataplexy was calculated by 2-sided Fisher's exact test. Differences in frequency of sleep paralysis and hypnagogic hallucinations, and in number of relatives with sleep disorder were calculated by uncorrected chi-square test.
RESULTS
Patient Categories
Sixty-four patients were included (Table 1). Forty-seven patients had narcolepsy with cataplexy, with 43 of them having HLA type DQB1*0602. Seven had narcolepsy without cataplexy, with 2 of them being HLA DQB1*0602 positive; and 10 had a confirmed diagnosis of idiopathic CNS hypersomnia with 4 HLA DQB1*0602 positive patients.
Table 1.
Demographic and Clinical Data
| N+ Cataplexy | N+ Cataplexy | N− Cataplexy | Idiopathlc CNS | Controls | |
|---|---|---|---|---|---|
| Low hcrt | Normal hcrt | Hypersomnia | |||
| n = 31 | n = 16 | n = 7 | n = 10 | n = 50 | |
| Female/male | 20/11 | 12/4 | 3/4 | 8/2 | 27/23 |
| Median age | 59 (16–76) | 46 (23–72) | 35 (13–57) | 39 (29–60) | 40 (16–80) |
| Age at symptom onset | 15 (4–50) | 16 (5–50) | 23 (4–55) | 20 (4–20) | |
| Duration of disorder | 26 (1–60) | 22.5 (9–50) | 10 (2–30) | 15 (5–25) | |
| Hypnagogic hallucinations | 20 (71%)* | 15 (94%) | 5 (71%) | 7 (70%) | |
| Sleep paralysis | 19 (68%)* | 15 (94%) | 3 (42%) | 3 (30%) | |
| Patients with relatives with sleep disorder | 11 (36%)* (18 relatives) |
7 (43%) (14 relatives) |
1 (14%) (1 relative) |
2 (20%) (3 relatives) |
Table 1 shows the number and median age of patients in each group, and median age at symptom debut and median duration of the disorder in each patient group. It also shows the number and percentage of patients in each group who reported hypnagogic hallucinations, sleep paralysis and relatives with similar sleep disorder. (*Number of patients reduced to 28 due to incomplete answers to the questionnaire). The total number of reported relatives in each group is given in brackets. N = Narcolepsy, hcrt = hypocretin-1
CSF Hypocretin Levels and Association with HLA DQB1*0602
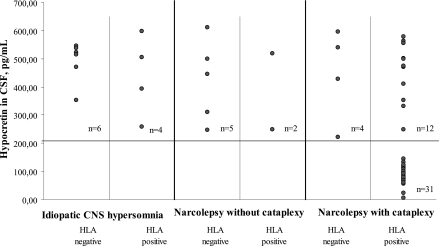
The mean CSF hypocretin-1-level in the controls was 399.8 pg/ml (SD = 95.5) with no sex related difference. The lower limit for normal hypocretin concentration was set at 2 SD below the mean level for the controls (209 pg/ml). In the 43 patients with narcolepsy with cataplexy and positive HLA type there was a clustering of 31 patients with markedly reduced values (mean 85.9 pg/ml, range 5–144 pg/ml), including one below 5 pg/ml. In the remaining 12 the values were within the normal range (mean 460.0 pg/ml), not significantly different from the narcolepsy patients without cataplexy (mean 411.5 pg/ml) and those with idiopathic CNS hypersomnia (mean 487.2 pg/ml) (Figure 1).
Figure 1.
Distribution of HLADQB1*0602 types in each diagnostic group. CSF hypocretin-1 levels in HLA DQB1*0602 positive and negative patients with narcolepsy with and without cataplexy, and patients with CNS hypersomnia. The horizontal line is 2 SD below the mean hypocretin level of the controls.
Correlation Between CSF Hypocretin and Duration of Disorder
The median age at symptom debut for the patients with narcolepsy with cataplexy was 15 years for the patients with low hypocretin values and 16 for those with normal values. Duration of the disorder was measured as the number of years between the first symptoms and the time of the study. There was no correlation between the duration of the disorder and the CSF levels of hypocretin-1. The values for the patients with low hypocretin-1 levels seemed to distribute as a separate population, independent of disease duration.
Relationship Between CSF Hypocretin -1 levels and Clinical Description of Cataplexy
Except for one patient who could not be reached, the remaining 46 patients with cataplexy had a thorough interview to get a detailed description of their cataplectic attacks (Table 2). Involvement of jaw and face muscles was reported by 90% of the patients with low hypocretin values and by 56% of the patients with normal values (P = 0.020). Similarly, involvement of neck muscles was reported by 86% with low values, and by 50% with normal values (P = 0.013). In contrast, involvement of the leg muscles was not significantly different in the 2 groups. Laughter was the main triggering factor in both patient groups.
Table 2.
Muscle Groups Involved in Cataplexy and Triggering Factors
| Muscle groups involved in cataplexy |
Triggering factors |
||||
|---|---|---|---|---|---|
| Hcrt<209 | Hcrt>209 | Hcrt<209 | Hcrt>209 | ||
| N=30 | N=16 | N=30 | N=16 | ||
| Jaw/face | 27 (90%) | 9 (56%) | Laughter | 30 (100%) | 13 (81%) |
| Neck | 26 (86%) | 8 (50%) | Surprise | 21 (69%) | 8 (50%) |
| Arms/hands | 24 (79%) | 11 (69%) | Sudden fright | 14 (45%) | 7 (44%) |
| Legs | 27 (90%) | 16 (100%) | Anger | 15 (52%) | 5 (31%) |
Muscle groups involved in cataplexy and the most frequent emotional stimuli causing cataplectic attacks in patients with low (hcrt-1<209pg/ml) and normal CSF hypocretin-1 levels.
Hypnagogic Hallucinations and Sleep Paralysis
These symptoms were reported in all diagnostic groups (Table 1). There was no significant difference in frequency of hypnagogic hallucinations and sleep paralysis between the patients with cataplexy with low and normal CSF hypocretin values. The patients with CNS hypersomnia or narcolepsy without cataplexy reported a slightly lower frequency of sleep paralysis than the patients with cataplexy with low or normal levels of hypocretin (P = 0.03), whereas there was no significant difference in the frequency of hypnagogic hallucinations.
Relatives with Sleep Disorder
The number of relatives with sleep disorder reported by patients with cataplexy with normal or low hypocretin-1 values was significantly higher than the number reported by the patients without cataplexy or with CNS hypersomnia (P = 0.001) (Table 1). Eleven patients with narcolepsy, cataplexy, and low hypocretin values (36%), reported a total of 10 first-degree relatives and 8 second-degree relatives with similar symptoms. Seven of the patients with cataplexy and normal hypocretin values (43%), reported 10 first-degree relatives and 4 second-degree relatives with similar symptoms. Among the patients with narcolepsy without cataplexy, only one patient (14%) reported one symptomatic first-degree relative, whereas 2 of the patients with idiopathic CNS hypersomnia (15%) reported 3 affected first-degree relatives. The affected relatives had either a confirmed diagnoses of narcolepsy or undiagnosed hypersomnia with or without associated symptoms.
DISCUSSION
The mean hypocretin value in the control group was 399 pg/ml with the lower limit for normal values at 209 pg/ml (2 SD below the mean). This is comparable to the levels previously found by others.15,17,19,20 The detection limit in our study was 5 pg/ml whereas detection limits in the previous studies varied between 10 and 100 pg/ml.15,17,19,20 The differences may partly be due to the use of different batches of polyclonal antibodies and partly to differences in method, as some studies have used a direct assay and others assay from extracted CSF, like the present study.
A previous study15 using a direct hypocretin assay set the cut off for narcolepsy with cataplexy to 110 pg/ml, representing onethird of the mean of the controls, based on sensitivity/specificity ratio. This has been proposed as the diagnostic hypocretin-1 level in The International Classification of Sleep Disorders, Second Edition (ICSD-2).22
In our study a cut off value at one-third of the mean value for the controls is 134 pg/ml and defines a cluster of 30 patients. In addition, one patient with a slightly higher value (144 pg/ml) seems to cluster with the others and is therefore included in this group, indicating that a somewhat higher cut-off value might be feasible. The limit of 134 pg/ml is not necessarily valid for measurements using assay of extracted CSF, which is recommended by the producer of the test kit. Furthermore, details in methodology and antibody batch variability may influence the cut-off value.
The previous studies have reported low hypocretin-1 values (below 110 pg/ml) in approximately 90% of narcolepsy patients with cataplexy and HLA type DQB1* 0602.15,17,19 In the present study 72% of such patients had low values (≤134 pg/ml). In diagnosing cataplexy there is a danger of including patients with atypical cataplexy or cataplexy-like symptoms of other origin. Our recruitment procedures were especially designed to avoid this. We therefore conclude that contamination of the cataplexy group with patients without typical cataplexy is unlikely and does not explain the higher percentage of patients with normal hypocretin-1 levels. As narcolepsy with cataplexy with normal and low hypocretin-1 values seem to represent separate pathogenetic entities, the relative percentage of each category may vary in different ethnic populations. So far, little has been done to clarify this, and more extensive studies of different patient populations are needed.
Previous studies based on single patient observations, have suggested a lack of correlation between hypocretin-1 levels and the duration of the disorder.15 The hypocretin-1 level was measurable in all our patients with narcolepsy with cataplexy, and it was therefore possible to correlate the CSF levels to the duration of the disorder. The results show no such correlation, and support the assumption that patients with low and normal hypocretin-1 values represent separate clinical subgroups with different pathogenetic mechanisms. It also indicates that the patients with low CSF hypocretin already had low values in the early phase of the disorder, without evidence of further decline, which might have been expected in a progressive degenerative or immunological process. The mechanism causing similar symptoms in patients with normal hypocretin values must be different. The existence of nonfunctional hypocretin receptors has been postulated, similar to the situation in inherited narcolepsy in dogs.10
The present results confirm the strong association between cataplexy, HLA DQB1*0602, and low hypocretin-1 levels. The patients with low hypocretin values described cataplectic attacks with significantly more extensive muscular involvement of face, jaw, and neck than the patients with normal levels. This has also been indicated by previous studies.15
Although both hypnagogic hallucinations and sleep paralysis were most frequently reported by patients with cataplexy, there was no correlation to low hypocretin-1 values. Hypnagogic hallucinations and sleep paralysis are usually thought to be isolated components of REM sleep in the waking state. Similarly, cataplexy has been considered as an isolated manifestation of REM sleep atonia, triggered by emotional stimuli. However, the close correlation of low hypocretin values with cataplexy, and not with sleep paralysis or hypnagogic hallucinations, and the occurrence of hypnagogic hallucinations and sleep paralysis in disorders without cataplexy, indicate that cataplexy may involve other neuronal pathways than hypnagogic hallucinations and sleep paralysis. This is in accordance with observations by others.23 Narcolepsy with cataplexy seems to contain a subgroup in which reduction or lack of hypocretin-1 aggravates the muscle paralysis in cataplexy, pointing to the importance of hypocretin neurons in balancing muscle tone.
The 7 patients with narcolepsy without cataplexy all had normal hypocretin values similar to the 10 patients with idiopathic CNS hypersomnia. The median duration of the disorders was 10 and 15 years, respectively (Table 1), which supports the concept of the disorders as separate entities from narcolepsy with cataplexy, and not early stages of this disorder. Although daytime sleep episodes in narcolepsy sometimes differ from idiopathic CNS hypersomnia by being more refreshing, the clinical manifestations of daytime sleepiness and sleep attacks are often similar. In our study hypnagogic hallucinations and sleep paralysis occurred with the same prevalence in both conditions. With normal hypocretin values and no HLA association, narcolepsy without cataplexy is only objectively distinguishable from idiopathic CNS hypersomnia by having ≥2 SOREMs in MSLT, which may occasionally be found in other conditions with marked daytime sleepiness.24,25
An issue which is not settled is how familial occurrence of narcolepsy is transmitted as an increased risk to relatives.18,26–28 As our data were collected from the questionnaire, without possibility of examining the relatives, we cannot be sure of their diagnosis. However this lack of precision is the same for all the patient groups, and there still is a marked difference, with a higher number of reported family members with narcolepsy or similar sleep disorders among the patients with narcolepsy and cataplexy both with low and normal hypocretin values, suggesting that hereditary factors may play a role in both these groups, compared to the other diagnostic groups. Genetic studies, however, have found no significant association between narcolepsy susceptibility in humans and gene polymorphism to hypocretin or its receptors.29,30 Mutations in genes regulating the expression of hypocretin or its receptors have been suggested as possible alternatives.
CONCLUSION
Measurement of CSF hypocretin-1 level in a Norwegian patient population identified 72% of the patients with narcolepsy, cataplexy, and HLA DQB1*0602 type as a well-defined subgroup with low hypocretin-1 levels. In clinical diagnostics of narcolepsy both PSG, MSLT, HLA-typing and hypocretin measurements are needed, beginning with PSG and MSLT in most cases. CSF hypocretin-1 measurement will confirm the diagnosis in most, but not all HLA DQB1*0602 positive patients with cataplexy, and is particularly valuable in patients where MSLT is inconclusive or cannot be performed. Patients with low hypocretin values reported cataplectic attacks with more extensive muscular involvement. Hypnagogic hallucinations and sleep paralysis were not statistically correlated to low hypocretin-1 levels. There was no correlation between duration of the disorder and CSF hypocretin-1 level.
Patients with narcolepsy without cataplexy and idiopathic CNS hypersomnia all had normal hypocretin-1 levels. No significant clinical or biochemical differences were found between these patient groups.
As the number of reported studies of CSF hypocretin-1 measurements so far is low, with variations in methods, sensitivity and ethnic diversity, the recommended cut off value at 110 pg/ml, based on only one previous study ought to be open for revalidation.
ACKNOWLEDGMENTS
The authors thank Professor Emmanuel Mignot for support, interest and advice and MS Tine Slettedal for excellent technical support and assistance in handling the database.
The project was supported financially by VIRUUS and The Department of Clinical Chemistry, Ullevål University Hospital
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Heier, Evsiukova, Vilming, Gjerstad, Schrader, and Gautvik have indicated no financial conflicts of interest.
REFERENCES
- 1.Ohayon MM, Pries RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58:1826–33. doi: 10.1212/wnl.58.12.1826. [DOI] [PubMed] [Google Scholar]
- 2.Hublin C, Partinen M, Kaprio J, Koskenvuo M, Guilleminault C. Epidemiology of narcolepsy. Sleep. 1994;17:S7–S12. doi: 10.1093/sleep/17.suppl_8.s7. [DOI] [PubMed] [Google Scholar]
- 3.Hublin C, Kaprio J, Partinen M, et al. The prevalence of narcolepsy: an epidemiologic study of the Finnish twin cohort. Ann Neurol. 1994;35:709–16. doi: 10.1002/ana.410350612. [DOI] [PubMed] [Google Scholar]
- 4.Dement W, Zarcone W, Varner V. The prevalence of narcolepsy. Sleep Res. 1972;1:148. [Google Scholar]
- 5.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–20. [PubMed] [Google Scholar]
- 6.Okun ML, Lin L, Pelin Z, Hong S, Mignot E. Clinical aspects of narcolepsy-cataplexy across ethnic groups. Sleep. 2002;25:27–35. doi: 10.1093/sleep/25.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Gautvik KM, de Lecea L, Gautvik VT, et al. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional PCR subtraction. Proc Natl Acad Sci USA. 1996;93:8733–8. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 10.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 11.de Lecea L, Sutcliffe JG. The hypocretins and sleep. FEBS Journal. 2005;272:5675–88. doi: 10.1111/j.1742-4658.2005.04981.x. [DOI] [PubMed] [Google Scholar]
- 12.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (Orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broberger C, de Lecea L, Sutcliffe JG, Hökfeldt T. Hypocretin/orexin and melanin-concentrating hormone expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to neuropeptide Y innervation. J Comp Neurol. 1998;402:460–74. [PubMed] [Google Scholar]
- 14.Ebrahim O, Howard RS, Kopelman MD, Sharief MK, Williams AJ. The hypocretin/orexin system. J R Soc Med. 2002;95:227–30. doi: 10.1258/jrsm.95.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurements in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 16.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. CSF hypocretin/orexin deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 17.Ripley B, Overeem S, Fujiki N, et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–58. doi: 10.1212/wnl.57.12.2253. [DOI] [PubMed] [Google Scholar]
- 18.Krahn LE, Pancratz VS, Oliver L, Boeve BF, Silber MH. Hypocretin (orexin) levels in cerebrospinal fluid of patients with narcolepsy: relationship to cataplexy and HLADQB1*0602 status. Sleep. 2002;25:733–6. doi: 10.1093/sleep/25.7.733. [DOI] [PubMed] [Google Scholar]
- 19.Dauvilliers Y, Baumann CR, Carlander B, et al. CSF hypocretin-1 levels in Narcolepsy, Kleine-levin syndrome, and other hypersomnias and neurological conditions. J Neurol Neurosurg Psychiaty. 2003;74:1667–73. doi: 10.1136/jnnp.74.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassetti C, Gugger M, Bischof M, et al. The narcoleptic borderland. A multimodal diagnostic approach including cerebrospinal fluid levels of hypocretin-1 (orexin A). Sleep Med. 2003;4:7–12. doi: 10.1016/s1389-9457(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 21.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:467–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep Disorders, [Google Scholar]
- 23.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J. Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 24.Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep. 1997;20:620–9. [PubMed] [Google Scholar]
- 25.Aldrich MS. Diagnostic aspects of narcolepsy. Neurology. 1998;50(suppl. 1):S1–S7. doi: 10.1212/wnl.50.2_suppl_1.s2. [DOI] [PubMed] [Google Scholar]
- 26.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. Frequency of narcolepsy symptoms and other sleep disorders in narcoleptic patients and their first degree relatives. J Sleep Res. 2005;14:437–5. doi: 10.1111/j.1365-2869.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- 27.Hartwig G, Harsh J, Ripley B, Nishino S, Mignot E. Low cerebrospinal fluid hypocretin levels found in familial narcolepsy. Sleep Med. 2001;2:451–3. doi: 10.1016/s1389-9457(01)00077-6. [DOI] [PubMed] [Google Scholar]
- 28.Nevsimalova S, Mignot E, Sonka K, Arrigoni JL. Familial aspects of narcolepsy-cataplexy in the Czech republic. Sleep. 1997;20:1021–6. doi: 10.1093/sleep/20.11.1021. [DOI] [PubMed] [Google Scholar]
- 29.Olafsdottir BR, Rye DB, Scammel TE, Matheson JK, Stefansson K, Gulcher JR. Polymorphism in hypocretin/orexin pathway genes and narcolepsy. Neurology. 2001;57:1896–9. doi: 10.1212/wnl.57.10.1896. [DOI] [PubMed] [Google Scholar]
- 30.Hungs M, Lin L, Okun M, Mignot E. polymorphism in the vicinity of the hypocretin/orexin are not associated with human narcolepsy. Neurology. 2001;57:1893–5. doi: 10.1212/wnl.57.10.1893. [DOI] [PubMed] [Google Scholar]