Abstract
Study Objective:
To determine sleep and EEG spectra in rats during surgical recovery.
Design:
Sleep, activity, and EEG spectral power were examined in rats via telemetry on days 1, 2, 3, 7, 14, and 15 after implantation surgery.
Results:
NREM sleep and total sleep were increased on days 1 and 2 compared to later days. REM sleep was decreased on days 2 and 3 compared to days 14 and 15, and activity was decreased on days 1 and 2 compared to later days. EEG power (0.5–5 Hz for NREM and wakefulness, and 5.5–10 Hz for REM and wakefulness) was increased on days 1–3 compared to days 7, 14, and 15.
Conclusion:
The results are discussed in terms of their implications for post-surgery stabilization of sleep and potential relevance for sleep after injury.
Citation:
Tang X; Yang L; Sanford LD. Sleep and EEG spectra in rats recorded via telemetry during surgical recovery. SLEEP 2007;30(8):1057-1061.
Keywords: Sleep, radio telemetry, surgery, EEG Power, activity
INTRODUCTION
RESEARCH INVOLVING INVASIVE SURVIVAL SURGICAL PROCEDURES IN ANIMALS TYPICALLY ALLOWS A PERIOD OF TIME FOR RECOVERY PRIOR TO BEGINNING experiments. However, the actual impact of surgical stress on sleep, how sleep varies with recovery, and the approximate time required for sleep to stabilize in the post-surgery period remains unclear.
Chronic surgical implantation of transmitters into the bodies of animals is often used for telemetrically recording a variety of physiological parameters including electroencephalogram (EEG), electromyogram (EMG), electrocardiograph, activity, temperature, and blood pressure.1,2 These implantation procedures and the possibility for immediate and continuous monitoring via telemetry thus provide an opportunity to examine the influence of surgical trauma and recovery from surgery on sleep in rodents. Therefore, we examined post-surgery sleep architecture and EEG spectra after implantation of a telemetry transmitter in rats. This work may also provide information regarding the influence of general acute injury on sleep patterns in rats.
METHODS
Subjects
Eight 90-day-old male Wistar rats (Harlan, Indianapolis, IN) served as subjects. They were singly housed (cage size 45 cm [length] × 24 cm [width] × 20 cm [height]) and given ad libitum access to food and water upon arrival and throughout the experiment. The same room was used for housing the rats and recording sleep. The room was kept on a 12:12 h light:dark cycle with lights on from 07:00 to 19:00, and ambient temperature was maintained at 24.5±0.5° C. Light intensity was 90–110 lux in the room, about 60 lux in the cage during light period, and less than 1 lux during dark period.
Surgery
Each rat was implanted with a transmitter (DataSciences ETA10F20) for recording EEG and activity via telemetry as described previously.1 The body of the transmitter was implanted subcutaneously off midline and posterior to the scapula, and it was attached to the skin with 3 sutures for stabilization. Leads from the transmitter were led subcutaneously to the skull and the bare ends placed in contact with the dura through holes in the skull (A: 2.0 [Bregma], L: 1.5; P: 7.0 [Bregma], L: 1.5 contralateral). The electrodes were anchored to the skull with screws and dental cement. All surgical procedures were performed stereotaxically under aseptic conditions. Surgical anesthesia was achieved with isoflurane (5% induction; 2% maintenance). Three days before and after surgery, water containing ibuprofen (0.1 mg/ml) was provided to alleviate potential postoperative pain. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 04-006).
Data Collection
Telemetric recording of EEG and activity was conducted using procedures similar to those we have used in mice2 and rats.1 For the EEG signal, the gain of transmitters was set at −0.5/+0.5 volts per/units × 2 and raw signals generated from the transmitter were in the range of 0.5–200 Hz. The signals were processed by a DataSciences analog converter and routed to an AD converter (Eagle PC30) housed in a PC class computer. The AD converter digitized the EEG and activity signals at 128 Hz. The digitized data were transferred to the computer and displayed graphically by the program on the computer monitor. An on-line fast Fourier transformation (FFT) was performed on EEG data in every 2 sec of data (256 samples) after a Harming window treatment. The FFT analyses generated the power density values from 0.0 to 63.5 Hzat a resolution of 0.5 Hz. The FFT data were further averaged in the range of 0 to 40 Hz for every 10 sec. The sleep data and FFT results were saved to the hard disk every 10 seconds for additional off-line analyses. Movement of the animal in relation to the telemetry receiver generated transistor-transistor logic (TTL) pulses that were collected and counted as a measure of activity.
Implant surgeries were conducted between 10:00 to 16:00 over 2 days (4 rats for each day). Recording began at the onset of the dark period of the day that the animal received surgery and 24-h EEG and activity were recorded on days 1, 2, 3, 7, 14, and 15 after surgery in all 8 rats.
Determination of Behavioral States and Analysis in EEG Power
Trained observers visually scored the EEG and activity records in 10-s epochs to determine wakefulness, NREM sleep and REM sleep. Wakefulness was scored based on the presence of low-voltage, fast EEG and irregularly spaced and clustered TTL pulses on the activity channel. NREM was scored based on the presence of spindles interspersed with slow waves in the EEG. During REM, the EEG was characterized by a regular, higher-amplitude theta rhythm. NREM and REM were characterized by the absence of TTL pulses on the activity channel, though infrequent sporadic pulses occurred. Compared to quiet wakefulness, EEG power during REM is significantly reduced in lower frequencies (0.5–4 Hz) and increased in the range of theta activity (6.5–9 Hz, peak at 7.5 Hz). A concurrent display of EEG power spectra for each epoch thus was used to aid in discriminating REM from wakefulness. The EEG power density data were sorted according to behavioral states. In each state, absolute EEG power density was calculated.
Data Analysis
The time spent (min) in NREM, REM, and total sleep time (NREM+REM), as well as activity counts, were processed to obtain total 24-h, and 12-h light and 12-h dark period totals for each day. The numbers of NREM and REM episodes and the average duration of NREM and REM episodes were also analyzed for the total 24-h and the 12-h light and dark periods. The absolute EEG power during wakefulness, NREM, and REM were calculated in 0.5 Hz bins from 0.5 to 30 Hz for the entire 24-h records of each recording day. Afterwards, the EEG power in selected frequency bands was analyzed.
All statistical analyses were conducted using SigmaStat software (SPSS, Inc.). One-way repeated measures of analysis of variance (ANOVA) procedures were used in the data analyses across days. After significant ANOVAs, post hoc comparisons of means were conducted with Tukey tests.
RESULTS
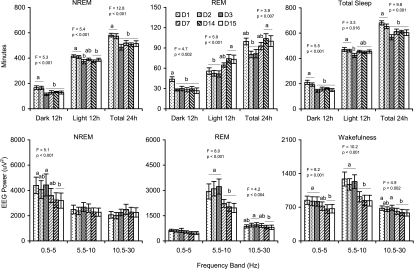
As shown in the upper panels of Figure 1, the rats exhibited increased 24-h NREM and total sleep time for the first 2 post-surgery days compared to later days. Increases in NREM during day 1 and day 2 were similar for both light and dark periods, with significant increases compared to later days, the exception being a slightly elevated amount of NREM during the light period of day 7 that did not significantly differ from any of the other recording days. Total sleep was elevated during the dark period of day 1 and day 2 compared to all other days, whereas during the light period day 1 and day 2 were elevated only compared to day 3.
Figure 1.
Time spent (min) in NREM, REM, and total sleep during 12-h dark and light periods and during total 24-h period (Upper Panels), and absolute EEG power during NREM, REM, and wakefulness in 3 selected frequency bands (Lower Panels) on days 1–3, 7, 14, and 15 after surgery. Values are means ± SEM. Different letters above the bars indicate significant differences across days (Tukey test, P <0.05). F and P values for the overall ANOVA are presented above each plot.
Twenty-four-hour REM amounts were significantly decreased on days 2–3 compared to days 14–15, but no other comparisons reached significance. Dark period REM was significantly increased during day 1 but did not otherwise differ across days. By comparison, light period REM was decreased on days 1–3. REM on day 7 was intermediate to that on days 2–3 and days 14–15 and did not significantly differ from earlier or later days.
Table 1 contains the number and average length of NREM and REM episodes for the 12-h light and dark periods and total 24-h period for each recording day. In general, the time course for post-surgery stabilization of these parameters paralleled those for amounts of both states. For both numbers of episodes and average lengths, there were no significant differences for NREM episodes among days 3, 7, 14, and 15, and no significant differences obtained for REM episodes among days 7, 14, and 15.
Table 1.
Number and average length of NREM and REM episodes during 12-h dark and light periods and during total 24-h period on days 1–3, 7, 14, and 15 after surgery. Values are means (SEM). Different letters within the columns indicate significant differences across days (Tukey test, P < 0.05).
| NREM 12-h Dark |
12-h Light | 24-h Total | REM 12-h Dark |
12-h Light | 24-h Total | |
|---|---|---|---|---|---|---|
| Number of Episode (Number) | ||||||
| D1 | 38.1 (4.1) | 79.6 (3.9) b | 117.8 (7.3) b | 23.1 (2.0) | 30.4 (2.6) | 53.5 (2.6) |
| D2 | 47.3 (5.3) | 93.5 (6.0) ab | 140.8 (7.7) ab | 17.9 (0.9) | 28.6 (1.4) | 46.5 (1.2) |
| D3 | 34.4 (3.5) | 99.4 (6.4) a | 133.8 (8.2) ab | 17.9 (1.7) | 31.3 (2.5) | 49.1 (3.0) |
| D7 | 50.3 (6.8) | 100.9 (5.1) a | 151.1 (11.) a | 18.6 (2.2) | 33.4(1.6) | 52.0 (3.6) |
| D14 | 49.5 (3.1) | 90.0 (5.0) ab | 139.5 (6.6) ab | 17.6(1.4) | 34.5 (2.3) | 52.1 (2.0) |
| D15 | 49.1 (3.6) | 93.6 (5.3) ab | 142.8 (6.1) a | 16.5 (1.8) | 36.3 (2.3) | 52.8 (2.6) |
| F (5, 35) = | 2.8 | 4.6 | 3.8 | |||
| P < | 0.028 | 0.003 | 0.008 | NS | NS | NS |
| Average Length of Episode (Min) | ||||||
| D1 | 4.4 (0.19) a | 5.3 (0.32) a | 5.0 (0.24) a | 1.9 (0.10) a | 1.8 (0.08) ab | 1.9 (0.08) ab |
| D2 | 3.6 (0.27) ab | 4.5 (0.25) ab | 4.1 (0.21) ab | 1.6 (0.09) b | 1.8 (0.10) ab | 1.7 (0.08) ab |
| D3 | 3.4 (0.20) bc | 3.8 (0.27) c | 3.7 (0.20) c | 1.7 (0.10) b | 1.6 (0.07) b | 1.7 (0.08) b |
| D7 | 2.7 (0.24) c | 3.9 (0.20) bc | 3.5 (0.21) bc | 1.5 (0.05) b | 2.0 (0.11) ab | 1.8 (0.09) ab |
| D14 | 2.8 (0.19) c | 4.2 (0.24) bc | 3.7 (0.19) bc | 1.7 (0.09) b | 2.2 (0.15) a | 2.0 (0.11) a |
| D15 | 2.6 (0.18) c | 4.2 (0.29) bc | 3.6 (0.19) bc | 1.6 (0.10) b | 2.0 (0.17) ab | 1.9 (0.13) ab |
| F (5, 35) = | 11.6 | 6.9 | 12.0 | 3.7 | 3.7 | 2.8 |
| P < | 0.001 | 0.001 | 0.001 | 0.008 | 0.008 | 0.03 |
The lower panels of Figure 1 present absolute EEG power in 3 selected frequency bands across days for wakefulness, NREM, and REM. Overall, during wakefulness and REM, the rats appeared to have greater EEG power in all 3 selected frequency bands during days 1 through 3 compared to the other recording days. Significant differences were found across days in the 5.5–10 Hz frequency band (days 1–3>7=14=15) during both wakefulness and REM. During wakefulness, significant differences were also observed in the 0.5–5 Hz frequency band (days 1=2=3>14=15) and differences in the 10.5–30 Hz frequency band (days 1=3>7=14=15). During REM, significant differences across days were seen in the 10.5–30 Hz frequency band (days 2=3>14=15). During NREM, significant differences across days were in the 0.5–5 Hz frequency band (days 1–3>14=15).
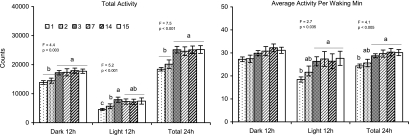
Figure 2 presents total activity count and average activity count per waking min across days. Significant differences were found for total activity across days for the dark and total 24-h periods (day 1=2<7=14=15) and for the light period (day 1<2<3 and day 1<7=14=15). Significant differences in average activity per waking min were found for the light and total 24-h periods (day 1<3=7=14=15). No significant differences across days were found for average activity per waking min in the dark period.
Figure 2.
Total activity and average activity counts per waking min during 12-h dark and light periods and during total 24-h period on days 1–3, 7, 14, and 15 after surgery. Values are means ± SEM. Different letters above the bars indicate significant differences across days (Tukey test, P <0.05). F and P values for the overall ANOVA are presented above each plot.
DISCUSSION
The results revealed that, compared to days 14 and 15, the rats exhibited increased NREM and total sleep time on days 1 and 2 after surgery. In contrast, similar comparisons showed that the rats displayed decreased REM time during days 1–3, except for an increase during the dark period of day 1 (surgical day). REM time was still somewhat decreased during day 7 compared to days 14 and 15, though the difference was not significant. These results suggest that stabilization of REM time after this type of surgery required a longer recovery period (>7 days) than required for stabilization of NREM time (<3 days).
The increase in REM during the dark period on the surgical day may have been associated with a number of contributing factors. In addition to surgical trauma, the rats experienced a period of sleep interruption and novel handling procedures as well as isoflurane anesthesia during the surgery itself. Thus, it was not possible to determine if the alterations in sleep were due to isoflurane anesthesia in this study. However, it is worth mentioning that rats anesthetized via intravenously administered propofol for 12 h during their normal sleep period did not sleep more in the subsequent 8 h than did control rats that were allowed to sleep spontaneously.3 Indeed, after propofol, post-anesthesia NREM and REM were significantly reduced compared to controls during the first 4 h, but only slightly reduced during the second 4 h, of the recording period.3 This suggests the possibility that post-anesthesia sleep may vary with anesthetic agent and/or time under anesthesia.
Decreases in spontaneous behaviors, e.g. locomotor activity, feeding, and grooming have been observed in rats and mice after surgery.4,5 Consistent with those observations, we found decreases in activity for 2 days after surgery compared to later days. Similarly, NREM and total sleep were significantly increased during the first 2 days after surgery. In addition to the influences from possible endogenous inflammatory factors induced by injury, the potential for pain may affect spontaneous activity. However, stabilized levels of total sleep, total NREM, and spontaneous activity (compared to 14 and 15 days post surgery) were reached within similar periods after surgery (<3 days), suggesting an alleviation of the possible effects of pain over that period.
Although compared to later days, NREM and total sleep amounts were significantly elevated only on post-surgery days 1 and 2, increases in EEG power in different frequency bands (0.5–5 Hz for NREM and wakefulness and 5.5–10 Hz for REM and wakefulness) were also found on post-surgery day 3. This suggests that other surgery-induced changes in sleep architecture may persist even after amounts have stabilized. It should be noted that long-term trends in sleep including decreased REM were reported in mice recorded over 60 days post surgery.13 Thus, while we used sleep at 14–15 days as our index of stability, it is possible sleep may continue to change over time with longer recordings than we performed.
Compared to implantation of electrodes for cable recording, the surgical implantation of telemetry transmitters may result in greater surgical trauma.1,2 For example, an implant for cable recording may require only one incision site over the skull, whereas implantation of the transmitter requires a surgical site to affix electrodes into the skull and a separate site for the transmitter itself. This site can be intraperitoneal2 or subcutaneous, e.g., off midline and posterior to the scapula as in the current study. Thus, there is the possibility that the extent of surgery-related changes in sleep may vary with type and extent of surgery and amount of discomfort of the implant site itself.
Reports in the literature are consistent that various painful stimuli decrease both NREM and REM, and thus increase wakefulness.6,7 In our study, ibuprofen was provided in the drinking water 3 days before and after surgery. This regimen could have been a factor as ibuprofen appears to be an effective analgesic as indicated by increased post-surgery locomotor activity in rodents.4,5 In addition, the fact that the rats did not display typical pain-induced decreases in NREM and REM after surgery suggests that sleep may be strongly influenced by the inflammatory reactions resulting from surgical trauma. Indeed, the increases in NREM and decreases in REM (after the increase during the first dark period) we observed after surgical trauma appear similar to those associated with host defense responses to viral and bacterial inoculations.8–10 Host defense systems produce increases in NREM and decreases in REM in response to various viral and bacterial infections.8–10 Host defense responses similar to those seen after infection also may be evoked by somatic injury from trauma or surgery. For example, mediators released by injured tissues, neural and nociceptive input originating from the site of injury, and baroreceptor stimulation from intravascular volume depletion activate stress reactions in endocrine, metabolic, and immunologic systems.11 Increases in many cytokines, e.g., tumor necrosis factor-α and interleukin-1, which play critical roles in promoting NREM after infection, are also seen in host responses to injury.8,12 The mode and time course of changes in hormone and immune systems in host responses to infection may differ compared to those after injury, however, the same cytokines may be activated by inflammatory reactions in both infection and injury.11
In conclusion, the results demonstrate that sleep, activity, and EEG spectra stabilize at different rates after implantation surgery, with REM taking the longest period to reach stability. The exact time course until sleep stabilizes could vary with type of surgery, strain, or species, and with other factors that influence recovery of individual animals.
ACKNOWLEDGMENTS
This work was by supported by NIH research grant MH64827 and MH61716. The authors would like to thank Dr. Jidong Fang (Perm State University) for the comments on the manuscript.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Sanford has received industry support from Sepracor. Dr. Tang and Ms. Yang have indicated no financial conflicts of interest.
REFERENCES
- 1.Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–8. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002;25:691–9. [PubMed] [Google Scholar]
- 3.Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg. 2001;92:1232–6. doi: 10.1097/00000539-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Goecke JC, Awad H, Lawson JC, Boivin GP. Evaluating postoperative analgesics in mice using telemetry. Comp Med. 2005;55:37–44. [PubMed] [Google Scholar]
- 5.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci. 2000;39:18–23. [PubMed] [Google Scholar]
- 6.Lavigne GJ, McMillan D, Zucconi M. Pain and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1246–1255. [Google Scholar]
- 7.Schutz TC, Andersen ML, Tufik S. Sleep alterations in an experimental orofacial pain model in rats. Brain Res. 2003;993:164–71. doi: 10.1016/j.brainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Krueger JM, Majde JA. Host defense. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 256–265. [Google Scholar]
- 9.Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM. Influenza viral infections enhance sleep in mice. Proc Soc Exp Biol Med. 1995;210:242–52. doi: 10.3181/00379727-210-43945. [DOI] [PubMed] [Google Scholar]
- 10.Krueger JM, Majde JA. Microbial products and cytokines in sleep and fever regulation. Crit Rev Immunol. 1994;14:355–79. doi: 10.1615/critrevimmunol.v14.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 11.Lin E, Lowry SF, Calvno SE. The sysemic response to injury. In: Schwarts SI, editor. Principles of Surgery. 7th Edition. Vol. 2. New York: McGraw-Hill; 1999. pp. 3–51. [Google Scholar]
- 12.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:520–50. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 13.Sinton CM, Jouvet M. Paradoxical sleep and coping with environmental change. Behav Brain Res. 1983;9:151–63. doi: 10.1016/0166-4328(83)90125-0. [DOI] [PubMed] [Google Scholar]