Abstract
Coenzyme Q (CoQ) has three well-characterized functions in mitochondria, namely (i) transfer of reducing equivalents in the electron transport chain, (ii) generation of superoxide anion radical (O2˙̄), and (iii) quenching of free radicals. The main purpose of this review is to discuss the effects of CoQ10 intake for relatively prolonged periods on mitochondrial respiratory capacity, indicators of oxidative stress, and life span of animals, in context of the broader issue of whether or not the overall progression of the aging process can be modified by CoQ10 administration. Comparative studies on different mammalian species have indicated that the rate of mitochondrial superoxide anion radical generation is directly correlated with mitochondrial CoQ9 content and inversely related to amounts of CoQ10, particularly the CoQ10 bound to mitochondrial membrane proteins. Contrary to the historical view, dietary supplementation of mice and rats with CoQ10 has been demonstrated to augment the endogenous CoQ content (CoQ9 + CoQ10) in mitochondria and homogenates of various tissues, albeit to varying extent. Ingestion of CoQ10 results in the elevation of endogenous CoQ9, the predominant homologue in mice and rats. In our studies, there was no indication of a discernable effect of CoQ10 intake reflecting enhancement of mitochondrial respiratory activity, antioxidant capacity and pro-oxidant potentiation or prolongation of life span. The possibility that CoQ10 intake affects certain other biological functions by as yet unelucidated mechanisms cannot be ruled out as CoQ has been shown to broadly alter gene expression in mice.
Keywords: Coenzyme Q, aging, mitochondria, oxidative stress, vitamin E, antioxidants, free radicals
1. Introduction
Coenzyme Q (CoQ) or ubiquinone (2,3-dimethoxy-5-methyl-6-multiprenyl-1,4-benzoquinone) molecules are located in the hydrophobic domain of the phospholipid bilayer of cellular membranes (Battino et al., 1990; Lenaz et al., 1999). CoQ is composed of a tyrosine-derived quinone ring, linked to a polyisoprenoid side chain, consisting of 9 or 10 subunits in higher invertebrates and mammals (Ernster and Dallner, 1995). The benzoquinone ring can assume three alternate redox states: the fully oxidized or ubiquinone (Q); the univalently reduced (1e− + 1H+) ubisemiquinone (•QH), a free radical; and the fully reduced (2e− + 2H+) ubiquinol. The polyisoprenyl chain apparently facilitates the stability of the molecule within the hydrophobic lipid bilayer. In addition, the length of the CoQ isoprenoid chain seems to affect the mobility, intermolecular interaction with membrane proteins, and autoxidizability (Matsura et al., 1992; James et al., 2004). The physiological roles of CoQ in biological systems are most well characterized in the inner mitochondrial membrane, where three of its main functions are: (i) carrier of electrons from respiratory complexes I and II to complex III, (ii) generation of superoxide anion radical by autoxidation of ubisemiquinone and (iii) anti-oxidant quenching of free radicals (Crane and Navas, 1997; James et al., 2004; Turunen et al., 2004). The apparently paradoxical property of mitochondrial CoQ to potentially act both as a pro-oxidant and an antioxidant would seem to suggest that it may also be a modulator of the cellular redox state under physiological and/or pathological conditions, and particularly it may play a role in the aging process.
It is now widely recognized that during aging there is a pro-oxidizing shift in the cellular redox state, accompanied by an accrual of the amounts of oxidatively damaged molecules, which in combination may play a causal role in senescence (Sohal and Weindruch, 1996; Sohal et al., 2002). The main premise of this hypothesis, often referred to as the oxidative stress hypothesis, is that the imbalance between pro-oxidant generation and anti-oxidant defenses, or the level of oxidant load or stress, increases during aging, and mitochondria play a critical role in this homeostatic perturbation. The reason why mitochondria are thought to be implicated is that the rates of mitochondrial O2˙̄/H2O2 production and oxidative damage to mitochondrial DNA, proteins and lipids increase during aging (Sohal and Sohal, 1991; Sohal et al., 1994 a, b; Sohal and Dubey, 1994). An important consequence of this apparently self-initiated damage is the acceleration of the rate of reactive oxygen species (ROS) generation, a phenomenon first reported in (Sohal and Sohal, 1991; Sohal and Dubey, 1994).
Another major age-related mitochondrial alteration is that ADP-stimulated (state 3) and the maximal (uncoupled) rates of mitochondrial oxygen consumption decline during aging (Ferguson et al., 2005). These twin changes, i.e., elevation of oxidative stress/damage due to enhanced O2˙̄/H2O2 production, and the decline in mitochondrial ability to synthesize ATP, are thought to progressively attenuate the functional capacity of various physiological systems. CoQ is suspected to be involved in both of these age-related alterations because as an electron carrier, CoQ is a component of the oxidative phosphorylation system, and because CoQ is also an ROS generator and a quencher. The main question though is whether there is a cause-and-effect relationship between age-related changes in mitochondria and the CoQ-related parameters. In this context, the main purpose of this review is to examine whether variations in CoQ content or the relative abundance of its homologues affect mitochondrial function and have an impact on the aging process.
2. Effect of age on CoQ content in different tissues
CoQ content as well as the ratios of Q9 and Q10 vary in different organelles, tissues and species. For instance, lysosomes and Golgi membranes generally contain relatively higher concentrations of CoQ than mitochondrial membranes or microsomes (Dallner and Sindelar, 2000). In mice, the variation in CoQ content among homogenates of different tissues is about 100-fold, with rank order: kidney > heart > skeleton > muscle > brain > liver (Lass et al., 1999a; Lass and Sohal, 2000). Compared to the homogenates, CoQ (CoQ9 + CoQ10) content of mitochondria was greater by six-times in liver, three-times in kidney, four-times in heart and 23-times in the skeletal muscle. CoQ concentrations in mitochondria also varied in different tissues, with heart containing 3.6-, 3.3-, 2.7- and 1.5-times higher amounts than those in the kidney, liver, brain, and skeletal muscle, respectively.
Results of the various studies in the literature on the age-related changes in CoQ levels do not support the existence of a common trend. For instance, Kalen et al. (1989) reported an age-related loss of CoQ content in homogenates of human tissues. Beyer et al. (1985) found no age-related changes in CoQ level in homogenates of rat brain and lungs, an increase in the liver, and a decrease in the heart, kidney and skeletal muscles. Battino et al. (1995) reported a decline in CoQ content in nonsynaptic mitochondria from mouse brain between 2 and 18 months of age, followed by an increase at 24 months of age.
Studies in this laboratory suggest that age-associated changes in CoQ content are most evident in mitochondria, which also preferentially sequester CoQ, rather than in the homogenate. For instance, in the rat, there were no significant differences in the amounts of CoQ9 or CoQ10 in the plasma or the tissue homogenates of liver, heart and kidney at 4-, 19-, or 24 months of age (Kamzalov and Sohal, 2004). In contrast, mitochondrial content of CoQ9, which is the predominant homologue, declined with age in all three tissues. A previous study showed a similar loss in mitochondria of rat skeletal muscle (Lass et al., 1999b). Homogenates of mouse liver, heart, kidney, skeletal muscle and brain also showed no age-related loss in CoQ9 or CoQ10 content (Sohal et al., 2006). Rather, during the period of 7 to 19 months of age, CoQ9 content increased in brain and skeletal muscle, respectively, by 20% and 12%, while CoQ10 content increased 33% and 52%. The aforementioned discrepancies between the findings from different laboratories may be due to the specific ages of the sampled animals or the procedure used for extraction and quantification of CoQ, or differences in species/strains/diets.
To summarize, it seems that during aging, decreases in CoQ content may occur in mitochondria of some tissues in certain species, but such losses are selective rather than ubiquitous. It has also been experimentally shown that physiological concentrations of CoQ in mitochondria do not exceed those required for kinetic saturation of NADH-Q-oxidoreductase, suggesting that CoQ is rate limiting in the electron transport chain (Estornell, et al., 1992). Therefore, decreases of CoQ below the physiological levels can potentially affect mitochondrial respiratory function, which may indeed occur under specific pathological conditions.
3. Inter-species variations in mitochondrial CoQ content and superoxide anion radical generation
The variations observed in the amounts of CoQ and ratios of the CoQ homologues among different tissues raise the question whether they carry any physiological significance, particularly whether differential amounts of CoQ9 or CoQ10 affect mitochondrial functions. This issue was addressed by us by first comparing the concentrations of CoQ homologues in cardiac mitochondria from nine different mammalian species, namely mouse, rat, guinea pig, rabbit, goat, sheep, pig, cow and horse (Lass et al., 1997; Sohal et al., 1999). The total amount of CoQ (CoQ9 + CoQ10) present in different species varied about 2-fold with the rank order: horse = mouse = cow = sheep = goat > rat > pig = rabbit > guinea pig; however, the ratios of CoQ10:CoQ9 in different species varied 60-fold. The total CoQ content was not found to be correlated with maximum life span (MLS) of the species. In contrast, CoQ9 content was inversely and CoQ10 directly correlated with the MLS of the species (Fig. 1). For instance, CoQ9 concentration was 40 to 60-times greater in mitochondria of shorter-lived species such as mouse or rat compared to the longer-lived horse or cow.
Fig. 1.

Relationship between CoQ9 (A) and CoQ10 (B) content of heart mitochondria from different species and maximum lifespan potential (MLSP) of the species, expressed as log10 units. (Adapted from data in Lass et al., 1997).
In separate studies involving comparisons of different mammalian species (Sohal et al., 1989; Ku et al., 1993; Sohal et al., 1999), we found that species-specific maximum life span was inversely correlated with mitochondrial rates of O2˙̄ and H2O2 generation (Fig. 2). It was thus of interest to further examine whether CoQ9 or CoQ10 content or the ratio of the two were also correlated with the rate of mitochondrial O2˙̄ generation (Lass et al., 1997; Sohal et al., 1999). CoQ9 content of cardiac mitochondria was found to correlate directly and CoQ10 inversely with the rate of O2˙̄ generation (Fig. 3).
Fig. 2.
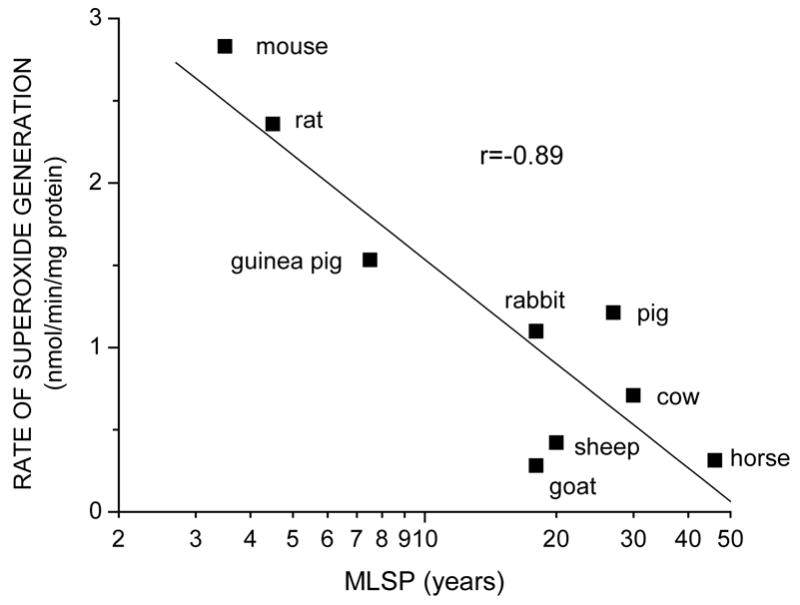
Relationship between rate of superoxide anion radical generation by cardiac submitochondrial particles and maximum life span potential (MLSP) of different species, expressed as log10 units. (Adapted from data in Lass et al., 1997).
Fig. 3.
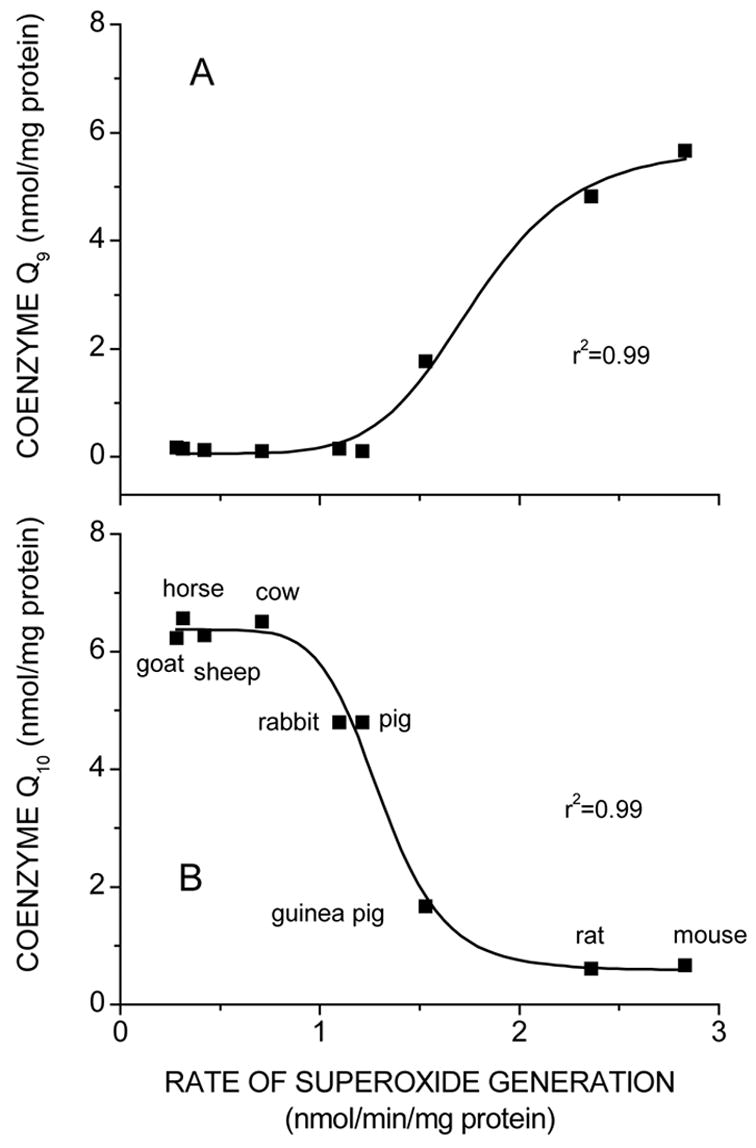
Relationship between rates of superoxide anion radical generation by cardiac submitochondrial particles from different species and amounts of CoQ9 (A) and CoQ10 (B) extracted from heart mitochondria. (From Lass et al., 1997).
Such correlations led to the hypothesis that CoQ9 content of mitochondria might be a determinant of the rate of O2˙̄ generation. This possibility was addressed in CoQ depletion/repletion studies on submitochondrial particles (SMPs) of rat and cow, which differ 16-fold in total CoQ content (Fig. 4). Depletion of CoQ, followed by repletion with different amounts of CoQ9 or CoQ10, indicated that at relatively lower concentrations, there were no significant differences in O2˙̄ generation between repletions with equal amounts of CoQ9 or CoQ10 (Lass et al., 1997). However, at repletions with relatively higher concentrations of CoQ9 or CoQ10, the rates of O2˙̄ generations were greater in CoQ9- than CoQ10-augmented SMPs. This can be interpreted to suggest that increased CoQ9 content may lead to relatively higher rates of O2˙̄ generation in vivo. Interestingly, an increase in the rate of O2˙̄ generation due to CoQ9 augmentation of SMPs of rat was an order of magnitude higher than that in cow SMPs, suggesting that structural organization of the inner mitochondrial membrane also plays a determining role in the rate of O2˙̄ production.
Fig. 4.

Rates of superoxide anion radical generation by CoQ-depleted/repleted rat (A) and bovine (B) heart submitochondrial particles (SMPs). Freeze-dried SMPs were depleted of native CoQ by six repeated extractions with pentane and reconstituted with specific amounts of CoQ homologues, followed by measurements of rates of O2˙̄ generation. (From Lass et al., 1997).
To further examine the possibility that binding of CoQ and mitochondrial membrane proteins (presumably the oxidoreductases of the electron transport chain), modulates the rate of O2˙̄ generation, proteins in mitochondrial membranes were isolated as micelles from five different mammalian species that varied in their relative amounts of CoQ9 and CoQ10 (Lass and Sohal, 1999). It was found that up to 32% of the total mitochondrial CoQ is bound to proteins, the rest presumably belonged to the freely diffusible pool. Notably, the amount of protein-bound CoQ in micelles of mitochondria from heart of different species was found to be inversely related to the rate of mitochondrial O2˙̄ generation (Figure 5). For instance, micelles from cow and pig contained two-fold higher amounts of CoQ than those from rat and mouse. It is worth pointing out that those micelles with relatively high CoQ:protein ratio were derived from species that are longer-lived and have CoQ10-rich mitochondria with relatively low rates of O2˙̄ generation. On the basis of such studies, it can be hypothesized that variations in longevity among different species co-evolved with the increases in the amounts of CoQ10 bound to the mitochondrial proteins.
Fig. 5.
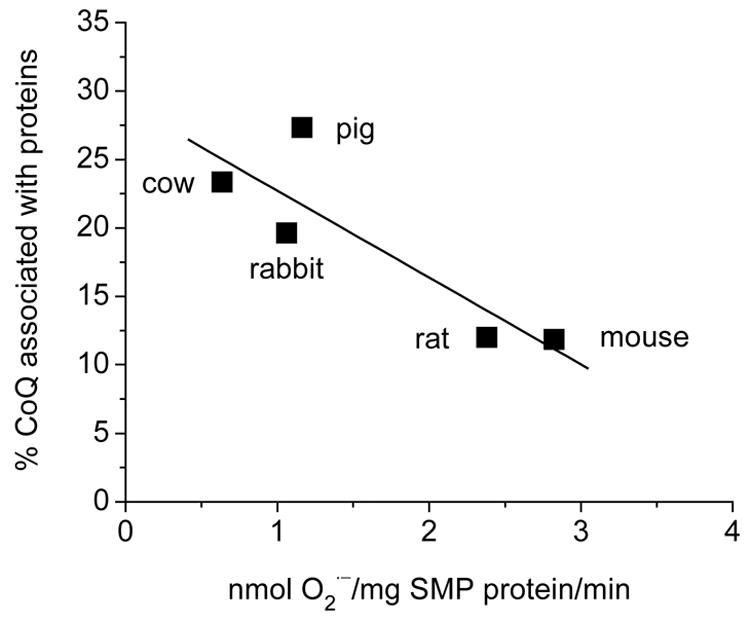
Relationship between amounts of CoQ associated with membrane proteins and rate of superoxide anion radical generation by cardiac mitochondria of different mammalian species. Mitochondrial membrane proteins were isolated as deoxycholic acid (DOC) pelletable micelles, and the CoQ9 and CoQ10 content was determined by HPLC. The CoQ content (CoQ9 + CoQ10), retained in the mitochondrial DOC micelles in different species, is expressed as percent of the total amount of CoQ present in whole mitochondria and is plotted against the rate of O2˙̄ generation, reported previously by Lass et al., 1997.
Altogether, results of the various correlational studies discussed above suggest that variations in the relative amounts of CoQ homologues may be associated with rate of mitochondrial O2˙̄ generation as well as longevity of different species; notwithstanding, such associations do not establish a cause-and-effect relationship. Experimental tests of this idea would require manipulations of the relative amounts of CoQ homologues.
4. Effect of CoQ administration on endogenous CoQ content
CoQ is synthesized endogenously by the mevalonate pathway (Ernster and Dallner, 1995). The historical view about the contribution of the dietary sources to endogenous levels of CoQ (Reahal and Wigglesworth, 1992; Zhang et al., 1995; 1996), reiterated recently (Bentinger et al., 2003), is that although ~6% of the orally administered CoQ permeates the gastrointestinal tract into the blood and is transferred to liver and spleen, CoQ uptake by other tissues such as heart, kidney, brain and skeletal muscle is low or completely absent, unless the endogenous levels have fallen below a critical threshold. More recent studies, however, have reported that this historical perspective was based on administration of relatively low dosages of CoQ for a comparatively short period. It was first shown by Matthews et al. (1998) that CoQ10 intake by 12- or 24-month-old rats increased CoQ content in brain mitochondria and had a neuroprotective effect against 3-nitropropionic acid. Subsequently, a series of studies conducted by us (Lass et al., 1999a, b; Lass and Sohal, 2000; Kwong et al., 2002; Kamzalov et al., 2003; Rebrin et al., 2004) demonstrated that CoQ10 administration via food to young adult mice or rats caused an increase in amounts of both CoQ9 and CoQ10 homologues in plasma, and in homogenates and mitochondria of liver, heart and skeletal muscle (Fig. 6). In the brain, the increase was of a lesser magnitude and occurred primarily in mitochondria and not the homogenate. In all the tissues, the amount of CoQ augmentation was greater in mitochondria than in the homogenate, suggesting its preferential sequestration in mitochondria. These studies also indicated that CoQ10 administration enhanced endogenous CoQ9, by a mechanism that remains to be elucidated. In general, greater augmentations of CoQ (CoQ9 + Co10) could be achieved with increases in the duration of the CoQ10 administration. These augmentations were equivalent to or greater than those achieved by short-term increases in CoQ dosage. Furthermore, when compared with the relatively hydrophobic CoQ powder, a water-miscible CoQ10 formulation (Q-Gel from Tishcon) resulted in more consistent augmentation of endogenous CoQ, particularly in brain (see Kwong et al., 2002; Kamzalov et al., 2003 versus Lass et al., 1999a; Sohal et al., 2006). Notwithstanding, it should be noted that the uptake of CoQ and other lipoidal substances is a complex process dependent upon a number of different factors.
Fig. 6.
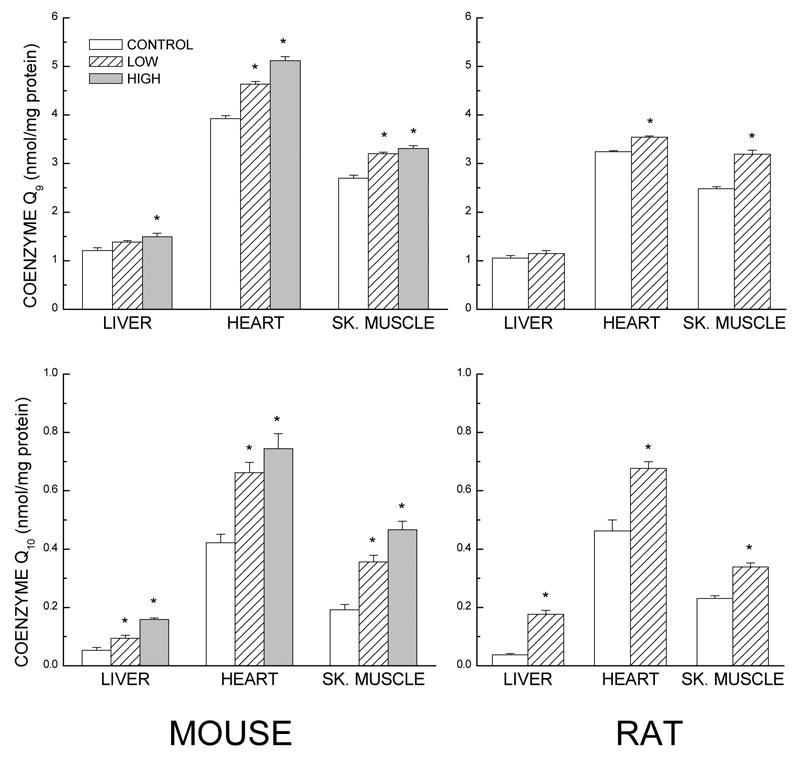
Effect of CoQ10 intake on CoQ9 (top panels) and CoQ10 (bottom) content in mitochondria from different tissues of young adult mice (left) or rats (right). In studies on mice (Kamzalov et al., 2003), CoQ10 was added to the food yielding daily CoQ intakes of 148 or 654 mg/kg body weight for 11 weeks. Rats were fed an amount equivalent to the low dose (150 mg/kg/d) for 13 weeks prior to tissue collection (Kwong et al., 2002). Values are means ± SE for 5–8 mice or 6–7 rats; * p<0.05 when compared with the control group (planned individual comparison within one-way ANOVA). The mouse data are from Kamzalov et al., 2003.
5. Antioxidant roles of CoQ and α-tocopherol in inner mitochondrial membrane
The inner mitochondrial membrane contains CoQ as well as α-tocopherol, both of which have antioxidant properties, thereby raising the issue about their respective roles in quenching the free radicals generated in inner mitochondrial membrane. In solutions, CoQ has been shown to inhibit lipid peroxidation in mitochondrial membranes that have been depleted of α-tocopherol (Mellors and Tappel, 1966; Takayanagi et al., 1980). α-Tocopherol has also been unambiguously demonstrated to be capable of scavenging lipid peroxyl radicals, thereby preventing the propagation of chain reactions during lipid peroxidation (McCay, 1985). Notwithstanding, the reactivity of α-tocopherol with peroxyl radical, which generates α-tocopheroxyl radical, far exceeds that of peroxyl radicals with ubiquinol, thereby suggesting that ubiquinol is unlikely to be a direct radical scavenger in vivo. Current evidence suggests that ubiquinol and α-tocopherol act in concert to scavenge radicals during autoxidation of mitochondrial membranes (Kagan et al., 1990; Stoyanovsky et al., 1995; Lass and Sohal, 1998; 2000; Sohal, 2004). α-Tocopherol seems to act as a direct scavenger forming tocopheroxyl radical, whereas ubiquinol reacts with tocopheroxyl radical to regenerate α-tocopherol. Several studies seem to confirm the sparing/regenerative effect of CoQ on α-tocopherol in vivo (reviewed in Sohal, 2004). In young adult mice, CoQ10 intake effectively augmented the α-tocopherol concentration in tissue homogenates and mitochondria from liver, heart, and skeletal muscle (Kamzalov et al., 2003). A similar effect was observed in homogenates and mitochondria of rats (Fig. 7).
Fig. 7.

Effect of coenzyme Q10 intake on α-tocopherol concentration in mitochondria from different tissues of young adult mice or rats. In studies on mice (Kamzalov et al., 2003), CoQ10 was added to the food in different amounts yielding daily CoQ intakes of 148 (low) or 654 (high) mg/kg body weight for 11 weeks. Rats consumed an amount equivalent to the low dose (150 mg/kg/d) for 13 weeks prior to tissue collection (Kwong et al., 2002). Values are means ± SE for 5–8 mice or 6–7 rats; * p<0.05 when compared with the control group (planned individual comparison within one-way ANOVA). Data for mouse are from Kamzalov et al., 2003; α-tocopherol concentrations in rat mitochondria are from previously unpublished data from the study described by Kwong et al., 2002.
6. CoQ intake, mitochondrial function and life span in mice
Because CoQ can act as a generator or a quencher of ROS and because it is widely consumed by humans as a dietary supplement motivated us to conduct studies on the effects of long-term intake of CoQ10 on the aging process. Specifically, we examined whether CoQ10 intake affects the level of oxidative stress, mitochondrial respiratory functions, or the survival of the animals (Sohal et al., 2006). Mice were fed diets providing daily supplements of 0, 93, or 371 mg CoQ10/kg body weight, starting at 3.5 months of age. Amounts of CoQ9 and CoQ10, determined at 7 and 21 months of age, were found to increase in relation to dosage and duration of CoQ10 administration. Augmentations of CoQ were detected in homogenates and mitochondria of liver, heart, kidney, and skeletal muscle, and were evidently maintained throughout life. Different tissues tended to vary in their capacity for CoQ accretion, with liver and skeletal muscle exhibiting the highest elevations, and the brain showing the least. Thus, these studies confirmed our previous observations in young adult mice and rats, firmly refuting the long-held notion that CoQ content of tissues other than plasma, liver or spleen cannot be significantly augmented by dietary administration of CoQ10. Indeed these demonstrations have provided the necessary rationale for testing the effects of CoQ10 intake on a broad range of parameters associated with mitochondrial functions, oxidative stress, and life span.
Long-term CoQ10 intake, lasting from 3.5 up to 25 months had no effect on activities of the major antioxidant enzymes, such as catalase, glutathione peroxidase and superoxide dismutase in liver, kidney, skeletal muscle or brain. Similarly, long-term CoQ10 intake had no effect on mitochondrial respiratory chain, measured as activities of oxidoreductases such as NADH ferricytochrome c reductase (complex I/III) and ferrocytochrome c oxidase (complex IV). The rates of oxygen consumption by liver mitochondria, measured as glutamate/malate and succinate supported state-3 respiration, were also not affected by CoQ10 intake. To further determine whether CoQ10 intake affected the level of oxidative stress, rates of O2˙̄ generation were measured in SMPs from heart, kidney and skeletal muscle of 25-month old experimental and control mice. No significant differences were detected between the groups. Levels of protein carbonyls and GSH:GSSG ratios also showed no notable effect of CoQ10 intake. Thus, prolonged CoQ10 intake seemingly failed to modulate mitochondrial respiratory capacity or levels of oxidative stress.
CoQ10 administration, starting at 3.5 months of age, also had no significant effect on the long-term survival of the mice (Fig. 8). Two previous studies on mice and rats in other laboratories, in which CoQ10 was administered at dosages lower than the highest dosage used by us, also reported no effect on life span (Lonnrot et al., 1998; Lee et al., 2004). Results of studies in other species are, however, quite contradictory and species-specific. For instance, different groups have reported that life span of the worm, C. elegans, is prolonged by CoQ10 supplementation (Asencio et al., 2003), or, paradoxically, by a deficiency of CoQ in the diet (Larsen and Clarke, 2002) or even by the inability of a mutant strain to synthesize the normal CoQ homologue (Branicky et al., 2002). One explanation of these contradictory findings may be the unusual life history and the mode of adaptation of C. elegans to stress. A deficiency of CoQ induces a hypometabolic or a dauer-like state, which in nature facilitates survival under adverse conditions. For instance, the life span of the worm is extended by the intake of antimycin A, an inhibitor of the electron transport chain, which is extremely toxic to most other aerobic species (Dillin et al., 2002). Notwithstanding, the results of studies on the effects of CoQ10 intake on the life span of mammals are in agreement that CoQ10 supplementation has no effect on longevity.
Fig. 8.

Survival plots for groups of 50 mice fed a control diet or low or high amounts of CoQ10. The low (0.72 mg/g of food) and high (2.81 mg/g) CoQ diets were introduced when the mice were 3.5 months of age and yielded daily CoQ intakes of approximately 93 or 371 mg/kg body weight throughout life. Survivorship, expressed as Kaplan-Meier probability, was not significantly different from the control for the mice with low or high intakes of CoQ (p>0.065, Tarone-Ware). Adapted from Sohal et al., 2006.
7. CoQ intake and brain function in mice
Concurrently with our studies of long-term intake of CoQ10 on the aging process in mice, we also examined the effect of supplementation of CoQ 10 alone, α-tocopherol alone or CoQ 10 + α-tocopherol on cognitive and motor functions of 24 month old mice, which had been treated for 13 weeks (McDonald et al., 2005). CoQ10 intake alone increased α-tocopherol concentrations in plasma, liver, heart, and skeletal muscle of these 24-month-old mice, however, in the brain tissue of old mice, the mitochondrial α-tocopherol concentration was augmented only when CoQ10 and α-tocopherol were administered together. This treatment (CoQ10+ α-tocopherol) also partially ameliorated impaired learning of an avoidance problem by these old mice, whereas treatments with CoQ10 alone or α-tocopherol alone were relatively less effective. None of the treatments improved age-related impairment of the mice in tests of motor performance. In a follow-up experiment, much higher doses of CoQ10 administration also failed to improve avoidance performance of the old mice. These studies suggested that intake of α-tocopherol and CoQ10 together has a synergistic effect.
To conclude, although CoQ may act as a pro-oxidant or an antioxidant in vitro, it has no notable in vivo effects on mitochondrial respiratory functions or levels of oxidative stress. There is also no clear indication of its ability to influence the life span or brain function of mammals, however, it is conceivable that beneficial effects of CoQ10 intake may occur under certain pathological conditions. It is also worth noting that CoQ10 intake has been shown to broadly affect the pattern of gene expression (Lee et al., 2004; Groneberg et al., 2005), indicated by the abundance of mRNAs, however, the nature of the physiological impact remains to be demonstrated.
Acknowledgments
Our studies on CoQ10 were supported by the grant RO1 AG17525 from the National Institute on Aging-National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asencio C, Rodrigues-Aguilera JC, Ruiz-Ferrer M, Navas P. Silencing of ubiquinone biosynthesis genes extends life span in Caenorhabditis elegans. FASEB J. 2003;17:1135–1137. doi: 10.1096/fj.02-1022fje. [DOI] [PubMed] [Google Scholar]
- Battino M, Ferri E, Gorini A, Federico Villa R, Rodriguez Huertas JF, Fiorella P, Genova ML, Lenaz G, Marchetti M. Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem. 1990;9:179–190. doi: 10.3109/09687689009025839. [DOI] [PubMed] [Google Scholar]
- Battino M, Gorini A, Villa RF, Genova ML, Bovina C, Sassi S, Littarru GP, Lenaz G. Coenzyme Q content in synaptic and non-synaptic mitochondria from different brain regions in the ageing rat. Mech Ageing Dev. 1995;78:173. doi: 10.1016/0047-6374(94)01535-t. [DOI] [PubMed] [Google Scholar]
- Bentinger M, Dallner G, Choinacki T, Sweiezewsk E. Distribution and breakdown of labelled coenzyme Q10 in rat. Free Radic Biol Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- Beyer RE, Burnett B, Cartwright KJ, Edington DW, Falzon MJ, Kneitman KR, Kuhn TW, Ramp BJ, Rhee SYS, Rossenwasser MJ, Stein M, An LCI. Tissue coenzyme Q (ubiquinone) and protein concentrations over the life span of the laboratory rat. Mech Ageing Dev. 1985;32:267–281. doi: 10.1016/0047-6374(85)90085-5. [DOI] [PubMed] [Google Scholar]
- Branicky R, Benard C, Hekimi S. clk-1, mitochondria and physiological rates. Bioessays. 2000;22:48–56. doi: 10.1002/(SICI)1521-1878(200001)22:1<48::AID-BIES9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Crane FL, Navas P. The diversity of coenzyme Q function. Molec Aspects Med. 1997;18:81–86. doi: 10.1016/s0098-2997(97)00016-2. [DOI] [PubMed] [Google Scholar]
- Dallner G, Sindelar P. Regulation of ubiquinone metabolism. Free Radic Biol Med. 2000;29:285–294. doi: 10.1016/s0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Estornell E, Fato R, Castelluccio C, Cavazzoni M, Castelli GP, Lenaz G. Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett. 1992;311:107–109. doi: 10.1016/0014-5793(92)81378-y. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg DA, Kindermann B, Althammer M, Klapper M, Vormann J, Littarru GP. Coenzyme Q10 affects expression of genes involved in cell signaling, metabolism and transport of genes involved in cell signaling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol. 2005;37:1208–1218. doi: 10.1016/j.biocel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- James AM, Smith RA, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Kagan V, Serbinova E, Packer L. Antioxidant effects of ubioquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophy Res Commun. 1990;169:851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- Kalen A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- Kamzalov S, Sumien N, Forster MJ, Sohal RS. Coenzyme Q intake elevates the mitochondrial and tissue levels of coenzyme Q and α-tocopherol in young mice. J Nutr. 2003;133:3175–3180. doi: 10.1093/jn/133.10.3175. [DOI] [PubMed] [Google Scholar]
- Kamzalov S, Sohal RS. Effect of age and caloric restriction on coenzyme Q and [alpha]-tocopherol levels in the rat. Exp Gerontol. 2004;39:1199. doi: 10.1016/j.exger.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- Kwong L, Kamzalov S, Rebrin I, Bayne ACV, Jana CK, Morris P, Forster MJ, Sohal RS. Effects of coenzyme Q10 administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic Biol Med. 2002;33:627–638. doi: 10.1016/s0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Larsen PL, Clarke CF. Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science. 2002;295:120–123. doi: 10.1126/science.1064653. [DOI] [PubMed] [Google Scholar]
- Lass A, Agarwal S, Sohal RS. Mitochondrial ubiquinone homologues, superoxide radical generation and longevity in different mammalian species. J Biol Chem. 1997;272:19199–19204. doi: 10.1074/jbc.272.31.19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of α-tocopherol inhibits autooxidation of mitochondrial membranes. Arch Biochem Biophys. 1998;352:229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- Lass A, Sohal RS. Comparisons of coenzyme Q bound to mitochondrial membrane proteins among different mammalian species. Free Radic Biol Med. 1999;27:220–226. doi: 10.1016/s0891-5849(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Lass A, Forster MJ, Sohal RS. Effects of coenzyme Q10 and α-tocopherol administration on their tissue levels in the mouse: Elevation of mitochondrial α-tocopherol by coenzyme Q10. Free Radic Biol Med. 1999a;26:1375–1382. doi: 10.1016/s0891-5849(98)00330-x. [DOI] [PubMed] [Google Scholar]
- Lass A, Kwong L, Sohal RS. Mitochondrial coenzyme Q content and aging. Biofactors. 1999b;9:199–205. doi: 10.1002/biof.5520090215. [DOI] [PubMed] [Google Scholar]
- Lass A, Sohal RS. Effect of coenzyme Q10 and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. FASEB J. 2000;14:87–94. doi: 10.1096/fasebj.14.1.87. [DOI] [PubMed] [Google Scholar]
- Lee CK, Pugh TD, Klopp RG, Edwards J, Allison DB, Weindruch R, Prolla TA. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic Biol Med. 2004;36:1043–1057. doi: 10.1016/j.freeradbiomed.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Fato R, Di Bernardo S, Jarreta D, Costa A, Genova ML, Parenti Castelli G. Localization and mobility of coenzyme Q in lipid bilayers and membranes. Biofactors. 1999;9:87–93. doi: 10.1002/biof.5520090202. [DOI] [PubMed] [Google Scholar]
- Lonnrot K, Holm P, Lagerstedt A, Huhtala H, Alho H. The effects of lifelong ubiquinone Q10 supplementation on the Q9 and Q10 tissue concentrations and life span of male rats and mice. Biochem Mol Biol Int. 1998;44:727–737. doi: 10.1080/15216549800201772. [DOI] [PubMed] [Google Scholar]
- Matsura T, Yamada K, Kawasaki T. Difference in antioxidant activity between reduced coenzyme Q9 and reduced coenzyme Q10 in the cell: studies with isolated rat and guinea pig hepatocytes treated with a water-soluble radical initiator. Biochim Biophys Acta. 1992;1123:309–315. doi: 10.1016/0005-2760(92)90012-k. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci USA. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay PB. Vitamin E: interactions with free radicals and ascorbate. Annu Rev Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- McDonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Mellors A, Tappell AL. The inhibition of mitochondrial peroxidation by ubiquinone and ubquinol. J Biol Chem. 1966;241:4353–4356. [PubMed] [Google Scholar]
- Reahal S, Wigglesworth J. Tissue concentrations of coenzyme Q10 in the rat following its oral and intraperitoneal administration. Drug Metab Dispos. 1992;20:423–427. [PubMed] [Google Scholar]
- Rebrin I, Sohal RS. Distribution and measurement of coenzyme Q. Methods Enzymol. 2004;378:146–151. [Google Scholar]
- Sohal RS, Svensson I, Sohal BH, Brunk UT. Superoxide anion radical production in different animal species. Mech Ageing Dev. 1989;49:129–135. doi: 10.1016/0047-6374(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release and aging. Free Radic Biol Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994a;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 Mice. Mech Ageing Dev. 1994b;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Lass A, Yan LJ, Kwong LK. Mitochondrial generation of reactive oxygen species and oxidative damage during aging: roles of coenzyme Q and tocopherol. In: Cadenas E, Packer L, editors. Understanding the Process of Aging. Marcel Dekker; New York: 1999. pp. 119–142. [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS. Coenzyme Q and vitamin E interactions. Methods Enzymol. 2004;378:146–151. doi: 10.1016/S0076-6879(04)78010-6. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Kamzalov S, Sumien N, Ferguson M, Rebrin I, Heinrich KR, Forster MJ. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med. 2006;40:480–487. doi: 10.1016/j.freeradbiomed.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanovsky DA, Osipov AN, Quinn PJ, Kagan VE. Ubiquinone-dependent recycling of vitamin E radicals by superoxide. Arch Biochem Biophys. 1995;323:343–351. doi: 10.1006/abbi.1995.9955. [DOI] [PubMed] [Google Scholar]
- Takayanagi R, Takeshige K, Minakami P. NADH- abd NADPH-dependent lipid peroxidation in bovine heart submitochondrial particles. Dependence on the rate of electron flow in the respiratory chain and an antioxidant role of ubiquinol. Biochem J. 1980;192:853–860. doi: 10.1042/bj1920853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Turunen M, Appelkvist EL. Restricted uptake of dietary coenzyme Q is in contrast to the unrestricted uptake of α-tocopherol into rat organs and cells. J Nutr. 1996;126:2089–2097. doi: 10.1093/jn/126.9.2089. [DOI] [PubMed] [Google Scholar]


