Abstract
The hypothalamus, the seat of neuroendocrine control, is exquisitely sensitive to gonadal steroids. For decades it has been known that androgens, estrogens and progestins, acting through nuclear hormone receptors, elicit both organizational and activational effects in the hypothalamus and basal forebrain that are essential for reproductive function. While changes in gene expression mediated by these classical hormone pathways are paramount in governing both sexual differentiation and the neural control of reproduction, it is also clear that steroids impart critical control of neuroendocrine functions through nongenomic mechanisms. Specifically, endogenous neurosteroid derivatives of deoxycorticosterone, progesterone and testosterone, as well and synthetic anabolic androgenic steroids that are self-administered as drugs of abuse, elicit acute effects via allosteric modulation of γ-aminobutyric acid type A receptors. GABAergic transmission within the hypothalamus and basal forebrain is a key regulator of pubertal onset, the expression of sexual behaviors, pregnancy and parturition. Summarized here are the known actions of steroid modulators on GABAergic transmission within the hypothalamus/basal forebrain, with a focus on the medial preoptic area and the supraoptic/paraventricular nuclei that are known to be central players in the control of reproduction.
Keywords: neurosteroids, GABAA receptors, anabolic androgenic steroids, reproduction, supraoptic nucleus, medial preoptic area, GnRH, oxytocin
1. Introduction
Compounds classified as neurosteroids or neuroactive steroids encompass both natural and synthetic derivatives of the gonadal steroids, progesterone and testosterone, and the adrenal steroid, deoxycorticosterone. Naturally occurring neurosteroids are synthesized from cholesterol in both peripheral organs and in the central nervous system (CNS), primarily by glial cells, and can be classified as positive (enhancing) or negative (blocking) allosteric modulators of the GABAA receptor (for review, Baulieu, 1998; Compagnone and Mellon, 2000; Belelli and Lambert, 2005). Synthetic steroids, including anesthetics such as alphaxalone and ganaxolone (for review, Belelli and Lambert, 2005) and the anabolic androgenic steroids (AAS) (for review, Clark et al., 2004; 2006) also act as allosteric modulators of the GABAA receptor. Reproductive biologists have demonstrated an indisputable role for nuclear hormone receptor-mediated steroid signaling in governing reproductive processes (for review, Course and Korach, 1998) and neuroendocrinologists have firmly established that allosteric modulation γ-aminobutyric acid type A (GABAA) receptors has a critical role in mediating aggression, stress and anxiety; behaviors that are known to vary with hormonal state (for review, Finn et al., 2004; Henderson and Jorge, 2004; Smith, 2004). Less is known about the intersection of allosteric modulation of GABAA receptors and reproductive control, and the purpose of this review is to highlight the literature to date indicating that this mechanism of steroid action is important in neural regulation of reproduction.
2. Mechanisms of Steroid Modulation of GABAA Receptors
Neurosteroids can be metabolized in the CNS from plasma-derived steroids or directly synthesized in the brain (for review, Baulieu 1998; Compagnone and Mellon, 2000). A-ring reduced neurosteroids, such as allopregnanolone (3α-hydroxy-5α-pregnan-20-one), THDOC (5α-pregnane-3α,21-diol-20-one) and 3α-diol (5α-androstane-3α,17β-diol) are positive allosteric modulators of the GABAA receptor that augment channel burst durations by increasing the opening frequency (and therefore the relative proportion of long duration single channel events) without concomitant changes in the open duration time constants themselves (for review, Henderson and Jorge, 2004). These positive modulators also slow the rate of recovery from desensitization, thus prolonging deactivation since receptors can transit from high-affinity, bound desensitized states to open states at times after GABA has been cleared from the synaptic cleft (for review, Jones and Westbrook, 1996; Vicini, 2004). At concentrations higher than 100nM, positive neurosteroids can directly gate the GABAA receptor (for review, Belelli et al., 2006), although such actions are unlikely to have physiological effects except, perhaps, during pregnancy when neurosteroids are reported to reach such levels (see Section 4). A-ring reduction at the 3 position, as well as a D-ring C20 or C17 keto group, are believed to be critical for positive neurosteroid binding (for review, Lambert et al., 1995) (Figure 1).
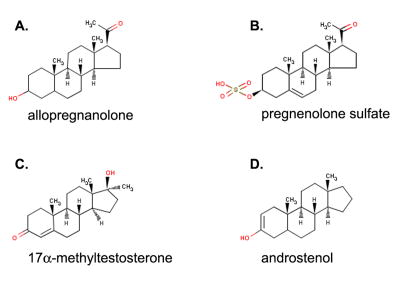
Figure 1.
Representative steroid modulators of the GABAA receptor for A) the positive neurosteroids: allopregnanolone, b) the negative neurosteroids: pregnenolone sulfate, C) the AAS: 17α-methyltestosterone and D) pheromones: androstenol. Structures are from the ChemIDplus website: http://chem.sis.nlm.nih.gov/chemidplus/.
The best-characterized negative allosteric modulators include the sulfate-derivatives of pregnenolone (5–pregnen-3β-ol-20-one; pregnenolone sulfate) and dehydroepiandrosterone (3α-ol-5-androstene-17-one; DHEAS) and the 3β-hydroxypregnane steroids (Wang et al., 2002; for review, Henderson and Jorge, 2004) (Figure 1). These compounds are believed to act as non-competitive antagonists at site(s) separate from those that bind the positive neurosteroid modulators. These steroid modulators promote a reversible inhibition of peak GABA-elicited currents by enhancing the rate of slow desensitization and by decreasing channel opening frequency, while having negligible effects on open or burst durations (Mienville and Vicini, 1989; Shen et al., 2000; Akk et al., 2001; Wang et al., 2003; for review, Henderson and Jorge, 2004).
The anabolic androgenic steroids (AAS) are modified, synthetic derivatives of testosterone originally designed for therapeutic purposes, but whose ranks now include greater than 60 compounds that are used broadly and illicitly as ergogenic agents (for review, Shahidi, 2001; Clark and Henderson, 2003). Few of the AAS have been assessed with respect to the mechanism of allosteric modulation, but the commonly abused AAS, 17α-methyltesosterone (17α-MeT), can both potentiate and antagonize GABAA receptor-mediated currents depending on the subunit composition of the receptor (for review Clark et al., 2004; Section 3.3). Unlike the positive neurosteroids, high concentrations of 17α-MeT (1-10 μM) cannot directly gate the GABAA receptor (Jorge-Rivera et al., 2000; Yang et al., 2002). Moreover, none of the AAS has a hydroxyl group in the α configuration at C3 and at the C20 or C17 keto group; signature substituents of the positive neurosteroids (for review, Lambert et al., 1995) (Figure 1). Finally, modeling studies indicate that the AAS have actions on state transitions of the GABAA receptor that are distinct from those associated with neurosteroid binding (Yang et al., 2002; 2005).
3. Subunit-specificity of steroid allosteric modulation
3.1 Subunit expression in the hypothalamus/basal forebrain
The native GABAA receptor is a pentameric ionotropic transmembrane protein for which sixteen different receptor subunit genes (α1-α6, β1-β3, γ1-γ3, δ, ε, π, and θ) have been identified in mammals (for review, Henderson and Jorge, 2004). Subunit composition is regulated with age and development, in a region-specific manner and in response to exposure to both exogenous and endogenous substances. Subunit composition, in turn, determines not only basic biophysical properties of the receptor, but also the sensitivity of this protein to a host of psychoactive compounds whose predominant mechanism of actions is allosteric modulation of channel function (for review, Sieghart, 1995). As a general rule, the hypothalamus/basal forebrain does not undergo the characteristic postnatal shift during which there is a significant up-regulation of the α1 subunit and concomitant down-regulation of the α2/3 subunits (Fritschy et al., 1994). In rodents, the medial preoptic areas (mPOA), supraoptic nucleus (SON) and paraventricular nucleus (PVN) are characterized by high levels of α2 subunit expression through adolescence and into adulthood, resulting in a relative abundance of α2 subunit greater than or equal to that of α1 subunit, and with lower, but appreciable, levels of α3 and α5 subunits (Wisden et al., 1992; Fénelon et al., 1995; Fritschy and Mohler, 1995; Davis et al., 2000; McIntyre et al., 2002; Penatti et al 2005). In the mPOA and the SON, β2/3 subunits are expressed at notably higher levels than is the β1 subunit, (Wisden et al., 1992; Fritschy and Mohler, 1995; Fénelon et al., 1995; Herbison and Fénelon, 1995). Delta subunit mRNA subunit expression is undetectable in the hypothalamus (Wisden et al., 2002), but while Fritschy and Mohler (1995) report no detectable level of δ subunit immunoreactivity throughout the mPOA/hypothalamus, Pirker et al. (2000) describe expression of α5, β1 and δ subunits on SON perikarya and α1, α2, and β1-3 subunit immunoreactivities on dendritic networks in this region. As is characteristic of most of the brain, expression of the γ2 subunit predominates in the SON/PVN (Fénelon et al., 1995; Fénelon and Herbison, 1996b). In contrast, high levels of the γ1 subunit mRNA are evident in a limited subset of interconnected forebrain regions, including the mPOA and lateral septum (LS), which are known to be important in the regulation of reproductive behaviors (Ymer et al., 1990; Araki et al., 1992, 1993; Wisden et al., 1992; Herbison and Fénelon, 1995; Clark et al., 1998). Like the γ1 subunit, ε expression is limited to a small number of CNS regions and is enriched in hypothalamic/forebrain regions, including the mPOA and the ventromedial nucleus of the hypothalamus (VMN), that are involved in the generation of reproductive behaviors (for review, Clark and Henderson, 2003; Clark et al., 2006). Of particular relevance, ε subunit is expressed at abundant levels in gonadotropin releasing hormone (GnRH) neurons, and virtually all GnRH neurons in the mPOA/anterior hypothalamus express this subunit (Moragues et al., 2003).
3.2 Neurosteroids
Mutational analysis of recombinant α1β2γ2 receptors indicates that four transmembrane residues in the α1 subunit are critical for both potentiation and direct activation of the receptor by the positive neurosteroids (Hosie et al., 2006). These residues are conserved among the α and β subunit family members (Hosie et al., 2006), suggesting substitution of different α or β family members within the 2α2βγ complex may not result in drastically different functional effects of the positive neurosteroids. This conclusion is supported by electrophysiological studies indicating that changes in α or β subunit composition have only a modest effect on positive neurosteroid potentiation. Specifically, results from recombinant GABAA receptors demonstrate that α subunit composition has a modest, albeit significant, effect on potency and no effect on efficacy for the positive A-ring reduced derivatives at recombinant αxβ1γ2L receptors. Substitution of different β subunit isoforms is without effect (for review, Belelli and Lambert, 2005; Belelli et al., 2006). With respect to α subunit composition, α6-containing recombinant receptors show the greatest sensitivity to modulation by positive neurosteroids, and those containing α1 or α3 subunits are slightly more sensitive than those containing α2 or α4 (for review Belelli et al., 2002). Published reports diverge with respect to the sensitivity of α5-containing receptors to positive neurosteroids (Belelli et al., 2002; Rahman et al., 2006). Neither the α4 nor the α6 subunit is expressed in hypothalamic/basal forebrain regions that regulate reproductive control, and studies addressing the relevance of α subunit composition with respect to positive neurosteroid modulation of reproductive behaviors have focused on α1 versus α2 expression, which changes with hormonal state (see Section 7).
In contrast to the minimal differences imparted by α and β subunit composition, substitution of a δ for the more commonly expressed γ subunit results in receptors with a markedly higher sensitivity to the positive neurosteroids; an action that is believed to reflect the ability of these modulators to shift δ-containing receptors from a low efficacy, non-desensitizing mode that normally characterizes them and promote a conversion of the effects of GABA from partial to full agonism (Wohlfarth et al., 2002; Bianchi and Macdonald, 2003; for review, Bianchi and Macdonald, 2004). Positive neurosteroid modulators elicit potentiation of both γ1- and γ2-containing GABAA receptors, although variable effects of γ subunit composition on efficacy and potency have been reported (Puia et al., 1993; for review, Belelli et al., 2002; Lambert et al., 2003). The ε subunit shares the greatest sequence homology with the γ subunits, and so it is generally thought to take the place of the single γ subunit in the pentameric receptor. However studies examining the sensitivity of recombinant ε-containing receptors to the positive neurosteroids have provided varying results in which the extent of enhancement by the positive neurosteroids of current through recombinant GABAA receptors appears to be inversely proportional to the level of ε subunit expression (Davies et al., 1997; 2001; Whiting et al., 1997; Thompson et al., 1998). A recent study by Thompson et al. (2002) suggest that the discordant findings with respect to steroid sensitivity of these ε-containing receptors can be reconciled if it is assumed that ε can substitute for not only for the γ, but also for non-γ subunits within the pentamer such that receptors with alternative stoichiometries are produced as ε subunit expression increases.
Fewer studies have directly assessed the subunit-specificity of negative neurosteroid modulators, although mutations in the α1, but not homologous mutations in the β2 or γ2S subunits, reduce the rate of block elicited by pregnenolone sulfate by 30-fold (Akk et al., 2001). A recent report by Rahman et al. (2006) indicates that substitution of an α5 for an α1 subunit in recombinant αxβ2γ2L receptors results in enhanced efficacy, but no effect on potency, for a panel of inhibitory modulators including pregnenolone sulfate, DHEAS, and a number of 3β-hydroxypregnane steroids. In contrast to the positive neurosteroids, substitution of a δ for the γ2 subunit in α4β3x recombinant receptors has no effect on the inhibitory modulation elicited by pregnenolone sulfate (Brown et al., 2002). Both pregnenolone sulfate and DHEAS inhibit spontaneous currents through α1β3ε recombinant receptors, but the sensitivity of these receptors to the negative modulators is low, with IC50 = 10.1 μM and 3.9 μM for DHEAS and pregnenolone sulfate, respectively (Maksay et al., 2003).
3.3 Anabolic androgenic steroids
In contrast to the positive neurosteroids, α subunit composition has a significant impact on AAS modulation. Specifically, the AAS, 17α-MeT, has no effect on rates of deactivation, desensitization or recovery from desensitization for α1β3γ2 recombinant receptors under conditions that mimic synaptic transmission (phasic application of 1-10 mm GABA), but significantly potentiates responses elicited by stationary application of a low concentration of GABA (1 μM), believed to reflect ambient levels in the CNS (Yang et al., 2002). In contrast, at α2-containing receptors, 17α-MeT enhances peak current, slows deactivation and diminishes desensitization of responses elicited by application of mM GABA, but is without effect on responses elicited by tonic application of 1 μM GABA (Yang et al., 2005). Gamma subunit composition (γ1 versus γ2) does not impart a significant effect on modulation by 17α-MeT of recombinant (α1β3γx or α2β3γx) receptors (Clark et al., 2004), however, δ and ε subunit composition has a dramatic impact on AAS modulation. Specifically, substitution of a δ for the γ subunit abrogates the ability of 17α-MeT to potentiate currents elicited by either brief applications of mM or slow perfusion of μM GABA to recombinant α1β3δ receptors (Yang et al., 2005). In contrast to γ or δ-containing receptors, 17α-MeT acts as a negative steroid modulator of both spontaneous (i.e., unliganded) and GABA-evoked currents from recombinant α2β3ε receptors (Jones et al., 2006). For GABA-evoked responses, this AAS inhibited phasic and tonic currents, accelerating desensitization and slowing paired-pulse response recovery. Kinetic modeling suggests that the inhibition of α2β3ε receptors by 17α-MeT occurs by an allosteric block in which the AAS interacts preferentially with and promotes accumulation in a closed state (Jones et al., 2006).
4. Neurosteroid and anabolic androgenic steroid levels in the CNS
Concentrations of neurosteroids in the brain have been shown to vary significantly during different developmental epochs, between the sexes, among different brain regions, as a function of hormonal state, and during acute stress (for review, Henderson and Jorge, 2004). For example, concentrations of both pregnenolone sulfate and DHEAS have been detected in human brain tissues at concentrations that are several fold higher than in plasma and are present at different levels in women than in men (Lacroix et al., 1987; Lanthier et al., 1986; Herrington, 1998). In addition, concentrations of allopregnanolone and 5α-DHP (5α-pregnane-3,20-dione), show significant differences during the menstrual cycle and among brain regions (Bixo et al., 1997).
For rats, the preponderance of studies has used radioimmunoassays (RIAs) to determine neurosteroid levels. Using this methodology, allopregnanolone concentrations in cortical homogenates were estimated to be ~1-2 ng/g in juvenile, ~1 ng/g in diestrous, 4-6 ng/g in estrous, and 4-8 ng/g in proestrous females, with levels reaching ~10-30 ng/g during pregnancy (Paul and Purdy, 1992; Corpéchot et al., 1993; Concas et al., 1998; Kellogg and Frye, 1999). These assessments have been used to estimate that allopregnanolone levels are diestrus females (Paul and Purdy, 1992), but can reach ~100 nM during the late stages of pregnancy (see for example; Brussaard et al., 1997; Lambert et al., 2003). The androgenic neurosteroid, 3α-diol, also showed cycle-dependent variation in cortical homogenates, with levels being lowest in diestrus I (0.08 ng/g) and rising steadily as the cycle progresses to a maximum of 0.33 ng/g during estrus (Kellogg and Frye, 1999). With respect to the hypothalamus, allopregnanolone levels were also shown to vary across the estrous cycle, but, in contrast to cortex, the lowest levels were reported on the evening of proestrus (~40 pg/mg protein) and the highest levels during the morning of diestrus I (~95 pg/mg protein) (Genazzani et al., 1995). The differences in concentrations of progestin- and testosterone-derived neurosteroids as a function of hormonal state contrasts with that for THDOC which shows only a small increase from ~1.5 ng/g during estrus to ~ 3ng/g in late pregnancy (Concas et al., 1998).
For adult male rats, neurosteroid values assessed by RIA are variable. Corpéchot et al. (1993) reported that allopregnanolone was below the limit of detection in the adult male rat brain. In contrast, Kellogg and Frye (1999) reported levels of allopregnanolone in brain extracts from adult males of 3.3 ng/g; a concentration only slightly lower than that they reported for estrous females and comparable to that which they found for 3α-diol in males (3.2 ng/g). For the negative neurosteroid modulators, Robel and Baulieu (1995) estimated levels of ~1-5 ng/g for DHEAS in the brains of the adult male rat and various strains of mice, while Kazihnitková et al., 2004 provided somewhat higher values for DHEAS of ~6.2 and 8.8 ng/g in male rat subcortex and cortex, respectively.
While widely used, a number of reports have questioned the accuracy of RIA assessments, noting that while RIAs provided highly sensitive assays, cross-reactivity of antibodies, interference with antibody binding by contaminating substances and limitations imposed by extraction procedures are confounds that are likely to underlie the appreciable variation in neurosteroid concentrations reported even within a given sex and species (for discussion, see Cheney et al., 1995; Liu et al., 2003; Liere et al., 2000; 2004). Two alternative approaches, enzyme-linked immunosorbent (ELISA) assays performed following separation of cross-reactive steroids, and liquid or gas chromatography coupled with mass spectrometry or mass fragmentographic analyses, have provided revised and appreciably lower estimates of brain neurosteroid concentrations. Specifically, Cheney et al. (1995) reported levels of ~ 1 ng/g for allopregnanolone in adult male rat brain homogenates, and levels of DHEAS and pregnenolone sulfate have been estimated to be either extremely low or below the level of detection in the adult male or unstaged female rat brain (Higashi et al., 2003; Liu et al., 2003; Liere et al., 2004). To my knowledge, comparable approaches have not yet been used for analysis of neurosteroids in the brain of cycling or pregnant females. Despite the controversy raised in estimating absolute levels of neurosteroids by different methodological approaches, it is worth noting that the relative variations found using any single technique, for example across the cycle or with pregnancy, should still hold true.
Will significant modulation of GABAA receptors be elicited by these concentrations in the intact animal? This remains a somewhat open question, especially in juvenile or diestrus females and adult males, since the estimates obtained with RIAs may be artefactually high and EC50 values, for example, for allopregnanolone potentiation of different isoforms of recombinant GABAA receptors activated by an EC10 concentration of GABA, range from ~75 to 560 nM (Lambert et al., 2003). While these data might suggest minimal potentiation by allopregnanolone and other neurosteroids except during very restricted hormonal states, such as pregnancy, such generalization need to be made with caution since estimates made for large brain regions, even by improved chromatography/spectrometry techniques, do not reflect neurosteroid concentrations in microdomains, such as synapses, where synthesis in nearby glial cells and restricted diffusion/clearance may produce greatly elevated concentrations in the local environments of the GABAA receptors.
With regard to the AAS, humans illicitly administer these steroids in sophisticated and complex regimes of doses and patterns typified by the concurrent use of multiple AAS (stacking) and a pattern of on-drug and off-drug use (cycling) and pyramiding; the process of administering first escalating then declining doses during the cycle, with typical cycles lasting 6-18 weeks (Gallaway, 1997; Brower, 2002). During a cycle, adult men are reported to self-administer AAS at concentrations that reflect 10-100× therapeutic replacement doses of testosterone (therapeutic doses: 10-40 mg/day) (for review, Clark et al., 2006). Although assessments of circulating levels of AAS in chronic steroid users is hindered by the lack of reagents to detect AAS metabolites as well as the inability to accurately monitor illicit users, cerebrospinal levels of 17α-MeT from adult male volunteers given a brief (3 day) administration of a moderate dose (240 mg/day) of this AAS were measured to be as high as 898 nmol/L (Daly et al., 2001). Adolescents and women are reported to take AAS at levels equivalent to or even exceeding those administered by men (Franke and Berendonk, 1997). Since the endogenous levels of plasma testosterone in adult females and in prepubertal adolescents are markedly lower than in men (< 2ng/L; Wu, 1997), the same self-administered doses that produce 10- to 100-fold higher than normal levels of endogenous androgens in males can yield circulating levels of androgens that are orders of magnitude higher still than normal concentrations in adolescents and women.
5. Forebrain and hypothalamic regions regulating the expression of reproductive behaviors
The neural substrates of the hypothalamus/basal forebrain required for the expression of sexual behaviors in rodents have been well documented by lesion studies, anatomical tracer studies, and assessments of c-fos expression associated with hormone treatment and mating behaviors (for review, Meisel and Sachs, 1994; Pfaff et al., 1994; Paredes and Baum, 1997; Blaustein and Erskine, 2002; Hull et al., 2002). For females, activity within the VMN and the midbrain central grey (MCG) facilitates the expression of lordosis, while activity in the olfactory bulbs, the mPOA, and the LS antagonize this behavior. Neural activity within the arcuate nucleus, the medial amygdala (MeA) and the bed nucleus of the stria terminalis (BNST) are also implicated in hormone-mediated control of female mating behaviors. In males, activity in the olfactory bulbs, the MeA, the BNST, and the mPOA is critical for expression of copulatory behavior. Regions inhibiting male sexual behavior have not been noted. While the role of the VMN in males has been less well studied, McGinnis and colleagues have shown that activity within subregions of this nucleus is also required for hormone-dependent copulatory behaviors in males (McGinnis et al., 1996; Harding and McGinnis, 2004). In both sexes, the mPOA is a key site for the integration of sensory input and the regulation of sexual performance (for review, Meisel and Sachs, 1994; Pfaff et al., 1994; Paredes and Baum, 1997; Blaustein and Erskine, 2002; Hull et al., 2002), as well as partner preference and motivation (Baum, 2006; Guarraci and Clark, 2006).
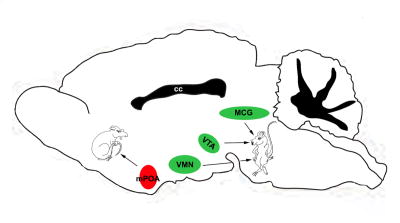
While neuronal activity within the VMN facilitates and activity within the mPOA antagonizes lordosis, GABAA receptor agonists, counter-intuitively, have similar effects: facilitating lordosis in the VMN and MCG while antagonizing this behavior in the mPOA (McCarthy et al., 1990; 1991a,b) (Figure 2), suggesting disinhibitory actions of GABA though multisynaptic pathways within these regions. GABAA receptor activity within the mPOA inhibits male copulatory behavior (Fernandez-Guasti et al., 1986); however, the role of GABAergic transmission in the VMN on male sex behavior has not been assessed.
Figure 2. Anatomical correlates of female reproductive behaviors.
Schematic representation of a midline-sagittal section and hypothalamic/basal forebrain or downstream midbrain regions in which GABAergic transmission facilitates (green) or antagonize (red) lordosis in rodents. mPOA: medial preoptic nucleus, VMN: ventromedial nucleus of the hypothalamus, VTA: ventral tegmental area, MCG: midbrain central gray; cc: corpus callosum.
Within the mPOA/anterior basal hypothalamus, neural control of the hypothalamic-pituitary-gonadal (HPG) axis is under the command of a small number (~800-1200) of sparsely distributed GnRH neurons (Witkin et al., 1982; Malik et al., 1991). GABAergic control of GnRH pulsatility from these neurons is essential for pubertal onset and reproductive maturation. Specifically, changes in synaptic regulation at puberty (both excitatory, via glutamate and the peptide, kisspeptin, and inhibitory, via GABA) leads to activation of the hypothalamic pulse generator, which promotes enhanced, pulsatile GnRH release that, in turn, regulates the secretion of luteinizing hormone (LH) from the pituitary (for review, Freeman, 1994; Ojeda and Urbanski, 1994; Ojeda et al., 2006). In adult subjects, inhibitory GABAergic inputs onto GnRH neurons promotes diminished action potential bursting activity and subsequent pulsatile GnRH and LH release (Sim et al., 2000; Nunemaker et al., 2003; Han et al., 2004; for review, Herbison et al., 1991; Moenter et al., 2003). A very interesting facet with respect to GABAergic control of GnRH neuron activity is that the developmental change in the chloride reversal potential (ECl), which causes GABA to switch from imparting depolarizing to hyperpolarizing effects, is dramatically delayed in GnRH neurons. Han et al. (2002) report that this developmental change does not occur until approximately the time of pubertal onset (postnatal day 31; PN31), while DeFazio et al. (2002) do not observe a developmental shift at all and believe that GABA continues to depolarize GnRH neurons even into adulthood. While the discrepancy between these two reports might result from sampling differences as there is a rostral-caudal gradient in the expression of the potassium-chloride cotransporter KCC2 in female mice (Leupen et al., 2003), the common finding is that GABA continues to depolarize GnRH neurons well beyond the age (~PN14) where GABA switches to a hyperpolarizing action in non-GnRH neurons in the hypothalamus/basal forebrain (Han et al., 2002; Defazio et al., 2002). Thus, steroid allosteric modulators that enhance GABAA receptor-mediated responses may have a paradoxical excitatory effect on GnRH neuron firing well into adolescence and perhaps beyond.
While reproductive competence and a successful repertoire of sexual behaviors are under the command of the GnRH neurons in the mPOA/anterior hypothalamus, magnocellular (oxytocin-containing) neurons of the SON and the PVN are the hypothalamic generals for the regulation of maternity, including pregnancy, parturition and lactation. In brief, oxytocin neurons are relatively quiescent, except at parturition and during lactation when their firing increases dramatically, often in intermittent bursts, and results in pulsatile secretion of high concentrations of oxytocin from the posterior pituitary. This oxytocin release is required for the coordinate uterine contractions that expel the fetus and for subsequent milk ejection during lactation (for review, Russell et al., 2003; Kiss and Mikkelsen, 2005). While published studies examining neurosteroid modulation of GABAergic inputs onto oxytocin neurons have focused on the role of this modulation during parturition and lactation, it is noted that oxytocin also plays an important role in the expression of both male and female sexual and affiliative behaviors (for review, Gimpl and Farhenholz, 2001). Thus, neurosteroid modulation of this hypothalamic system may also have significant effects on those behaviors that both precede and promote pregnancy and parturition.
6. Steroid modulation of GABAergic transmission in hypothalamus/basal forebrain
Although neurosteroid modulation of GABAA receptor-mediated responses was first noted in the early 1980s (for review, Lambert et al., 2003), steroid modulation of neurons in neuroendocrine control regions has been assessed only during the past decade. Initial studies concentrated on modulation of GABAA receptor-mediated responses in dissociated embryonic hypothalamic neurons, while more recent reports have examined effects of steroid modulators in defined regions/nuclei of acutely isolated slices. In 1996, Dayanithi and Tapia-Arancibia demonstrated that allopregnanolone induces a dose-dependent increase in [Ca2+]in (EC50 = 10 nM) in cultured embryonic hypothalamic neurons through potentiating effects at depolarizing GABAA receptors; an effect that did not require G protein activation. The lack of a requirement for G protein activity in these embryonic hypothalamic neurons in culture contrasts with the demonstrated role of G proteins in mediating the effects of allopregnanolone on synaptic currents in SON neurons isolated from juvenile and adult rats and mice (see this section, below). Poisbeau et al. (1997) reported that allopregnanolone, at low nanomolar concentrations, has not only postsynaptic, but also presynaptic effects on rat embryonic hypothalamic neurons grown in co-culture with pituitary intermediate lobe cells; augmenting GABAA receptor-mediated postsynaptic current (PSC) amplitude and increasing miniature PSC (mPSC) frequency. Pregnenolone sulfate was found to antagonize PSCs in this in vitro system by progressive reduction in average time constant of decay, but only at a high (10 μM) concentration. Wetzel et al. (1999) reported that THDOC also potentiates PSCs formed between embryonic hypothalamic neurons, although the mechanism reported here is by prolongation of synaptic current decay, rather than a potentiation of peak amplitude. Interestingly, when GABA at 1 μM, a concentration that reflects in situ ambient levels present at extrasynaptic receptors, was applied directly to these embryonic hypothalamic neurons, THDOC had a bidirectional effect, potentiating current amplitudes at higher concentrations (100 nM - 1 μM) but suppressing them at a low concentration (10 nM). Results from these studies suggest that the action of an individual neurosteroid is not monolithic, but may vary with the concentration of the neurosteroid and of GABA. Heterogeneity in the action of negative neurosteroids has also been reported for GABAA receptors in postsynaptic pituitary cells where the inhibitory effects of DHEAS, and its apparent dissociation constants, vary by 3 orders of magnitude (Hansen et al., 1999).
In studies in which the mPOA was specifically identified in brain slices of young (prepubertal) male or female rats, applications of 300 nM or 1 μM allopregnanolone were shown to augment the amplitude of responses elicited by 20 μM GABA (Huang and Dillon, 2002). Allopregnanolone was also shown to prolong miniature inhibitory postsynaptic current (mIPSC) decay with no effect on mIPSC amplitudes in mPOA neurons acutely dissociated with adherent presynaptic terminals (Haage and Johansson, 1999: 50 nM; Uchida et al., 2002: 10 nM) or sIPSC amplitudes in dissociated medial preoptic nucleus (MPN) neurons (Haage et al., 2005; 1 μM). Allopregnanolone was also reported by Haage and colleagues (Haage and Johansson, 1999; 2 μM;Haage et al., 2005) and Uchida et al. (2002; 10 nM) to increase mIPSC and sIPSC frequency in dissociated mPOA/MPN neurons. In contrast to the lack of effect of allopregnanolone on PSC amplitudes noted in these studies, Jorge-Rivera et al. (2000) reported that allopregnanolone and 3α-diol (both at 1 μM) potentiated the peak amplitude of spontaneous IPSCs (sIPSCs) recorded from the mPOA and the VMN of neonatal female rats. It is possible that acutely dissociated neurons with adherent presynaptic terminals respond differently to neurosteroid modulation (Haage and Johansson, 1999; Uchida et al., 2002; Haage et al., 2005) than do neurons in the intact slice (Jorge-Rivera et al., 2000).
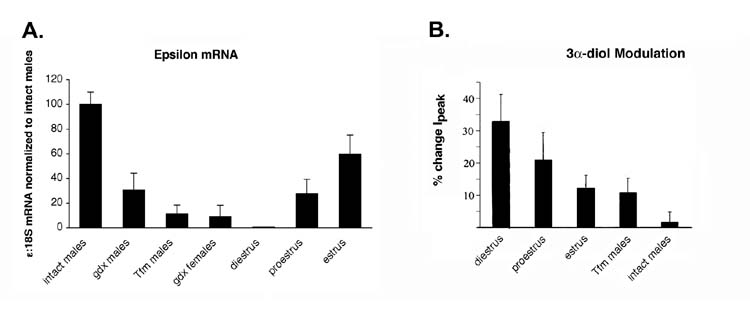
Similar to results reported for mPOA neurons from neonatal female rats (Jorge-Rivera et al., 2000), 1 μM 3α-diol also potentiated the peak amplitude and prolonged decay of both sIPSCs and mIPSCs in mPOA neurons from adult female mice (Jorge et al., 2002). Of particular interest to steroid modulation of neuroendocrine control, the extent of modulation induced by 3α-diol, a steroid implicated in estrus termination (Erskine, 1987), varied significantly with the stage of the estrous cycle, being highest at diestrus (Jorge et al., 2002) (Figure 3). Sensitivity to 3α-diol also varied significantly between the androgen receptor-null mutant (Tfm) and wildtype male mice, indicting that there may be androgen receptor-dependent organizational imprinting of 3α-diol sensitivity in the mPOA of males (Figure 3). Semi-quantitative RT-PCR revealed an inverse correlation between the levels of ε subunit mRNA expression in the mPOA and the extent of 3α-diol potentiation among subjects of different hormonal states (Jorge et al., 2002) (Figure 3); a finding that is consistent with the correlation between insensitivity to neurosteroid modulation and ε subunit composition of recombinant receptors (Section 3.2).
Figure 3.
(A) Levels of ε subunit mRNA in the mPOA as assessed by standard semi-quantitative RT-PCR. Data are normalized to values from adult, gonadally-intact C57Bl/6 males (100%). (B) Percent potentiation in average peak current (Ipeak) over control elicited by 3α-diol.
A role for the ε subunit in imparting insensitivity to neurosteroid modulation is further indicated by studies of GnRH neurons within the mPOA/anterior forebrain, which express high levels of the ε subunit (Moragues et al., 2003): a very high concentration of allopregnanolone (5 μM) elicited only modest increases in mPSC amplitudes and prolongation of decay time in these neurons, while DHEAS (5 μM) moderately diminished peak amplitude with no change in decay kinetics (Sullivan and Moenter, 2003). It is interesting to speculate that GnRH neurons, which need to be exquisitely sensitive to changes in gonadal steroids, may be buffered from the influences of neurosteroids (whose concentrations will also vary with hormonal state) by expressing GABAA receptors whose subunit composition limits neurosteroid responsiveness. In contrast, Calogero et al. (1998) have shown that GnRH release from intact hypothalamic hemi-slices of adult male rats is diminished by a low concentration of allopregnanolone (10 nM), suggesting that modulation of GABAA receptor- mediated synapses upstream of the GnRH neurons may be sensitive to physiological concentrations of endogenous neurosteroids and that these steroids may nonetheless modulate control of the HPG axis.
While the modest sensitivity of GnRH neurons to neurosteroid modulation reported by Sullivan and Moenter (2003), as well as the sex- and cycle-dependent differences in neurosteroid modulation reported by Jorge et al. (2002) are consistent with the hypothesis that high levels of ε subunit expression in native neurons confers diminished neurosteroid responsiveness, a recent study demonstrating that potentiation elicited by pregnane neurosteriods in the hippocampus also varies as a function of the estrous cycle and is highest in diestrous females (Maguire et al., 2005), suggests that other contributing factors, such as the posttranslational status of the receptor, must be considered as critical regulators of steroid modulation, since the hippocampus does not express the ε subunit.
Differences within the hypothalamus/basal forebrain as a function of sex or hormonal state in the expression of other GABAA receptor subunits (Clark et al., 1998; Nett et al., 1999; Penatti et al., 2005), in the numbers of GABAA receptors and in their binding affinities (O’Connor et al., 1988; Lasaga et al. 1988; Schumacher et al., 1989; Juptner and Hiemke, 1990) have also been reported. It is likely that these differences play significant roles with respect to the expression of sexual and reproductive behaviors, but their impact on allosteric modulation of GABAA receptors in these brain regions or the potential outcome of such modulation on the expression of sexual or reproductive behaviors is currently not known.
As with the neurosteroids, the AAS allosterically modulate sIPSCs and mIPSCs in the mPOA and the VMN. Three different and commonly abused AAS, stanozolol, nandrolone, and 17α-MeT (at 1 μM: a concentration that reflects levels of these synthetic steroids in AAS users), were found to augment peak sIPSC amplitude and prolong current decay in VMN neurons in slices acutely isolated from neonatal female rats (Jorge-Rivera et al., 2000). The AAS, 17α-MeT, was also shown to increase the probability of single channels residing in open configurations in acutely isolated neonatal VMN neurons (Clark et al., 2006). In contrast to the VMN, all three AAS diminished sIPSC peak amplitudes with no change in current decay kinetics in mPOA neurons from neonatal female rats (Jorge-Rivera et al., 2000). No effect on IPSC frequency was evoked by AAS exposure in either region. Moreover, individual mPOA neurons that responded with negative modulation to acute application of an AAS were nonetheless potentiated by endogenous neurosteroids (Jorge-Rivera et al., 2000). These findings highlight the different fundamental mechanisms by which these synthetic steroids and the endogenous steroid modulators impart their effects at the GABAA receptor.
Fáncsik et al. (2000) have shown that allopregnanolone (1 μM) increases both the fast and the slow time constant of current decay of sIPSCs recorded in slices of the SON acutely isolated from juvenile male rats No significant change in sIPSC amplitude or frequency was observed for the population of responses as a whole in this study, although some individual cells showed changes in both for within individual cell comparisons. Intriguingly, blocking the activity of either G proteins or protein kinase C (PKC) abrogated the potentiating action of allopregnanolone on sIPSC decay indicating that neurosteroid binding to and modulation of the GABAA receptor requires the receptor to be phosphorylated via a G protein- and PKC-dependent mechanism.
Brussaard and colleagues (1997) have shown that that high concentrations of allopregnanolone (1 -10 μM) prolong sIPSC decay and diminish action potential firing in magnocellular neurons from the SON of juvenile female rats and that this modulation also depends upon a G protein/PKC-dependent mechanism. Remarkably, in contrast to juvenile females, the potentiating effect of allopregnanolone on GABAergic sIPSCs and its inhibitory effect on action potential firing were found to be absent or markedly reduced in parturient or postpartum females (Brussaard et al., 1997). The change in allopregnanolone at the time of parturition was attributed to enhanced PKC activation and phosphorylation of postsynaptic GABAA receptors arising from oxytocin receptor-mediated signaling (Brussaard et al., 2000). While these results also demonstrate that PKC phosphorylation is critical for allopregnanolone modulation of GABAA receptor-mediated responses in the SON, in contrast to the results described by Fáncsik et al. (2000) for juvenile males, this phosphorylation in parturient or postpartum females renders GABAA receptors in the SON insensitive to modulation by allopregnanolone. The diminished effects of allopregnanolone, in turn, result in diminished GABAergic inhibitory tone, enhanced electrical activity and increased oxytocin release (Brussaard et al., 2000; Koksma et al., 2003).
It is tempting to speculate, although the idea is untested, that the differences in the results reported by these two laboratories may reflect either age- or sex-specific differences in the basal level of PKC-dependent phosphorylation of the receptor. In this regard, Widmer et al. (2003) have demonstrated that the ability of allopregnanolone to augment oxytocin release from the SON in female rats is indeed age-dependent: a relatively brief (10-15 min) application of 100 nM allopregnanolone evokes release of ~375 pg from the SON of postnatal day 9 (PN9) subjects, but only ~60 pg in adult animals older than 32 weeks (basal release ~30 pg). The authors show that the actions of allopregnanolone are dependent upon GABAA receptor activation and attribute the diminished effect of allopregnanolone with age to a shift in the actions of GABA from depolarizing in the youngest animals (tested at PN9) to hyperpolarizing in adults. It should be noted, however, that they report values of oxytocin release from the SON of PN34 rats of >300 pg; a value comparable to that from PN9 neonates. This late adolescent age is well beyond the expected developmental shift in ECl in non-GnRH hypothalamic neurons (Han et al., 2002; DeFazio et al., 2002), and suggests that other factors, such as the posttranslational status of the GABAA receptor, are also likely to contribute to age-specific changes in allopregnanolone sensitivity.
Few studies have addressed neurosteroid modulation in the PVN. Womack et al. (2006), studying parvocellular PVN neurons that project to spinal sympathetic centers, have shown that THDOC (EC50 = 67 nM) prolongs macroscopic current decay with no change in single channel burst or open duration kinetics in neurons from juvenile rats of either sex. This enhanced GABAergic transmission was also shown to underlie the ability of THDOC to inhibit action potential frequency in these cells.
7. Steroid modulation of reproductive behaviors
The effects of intrahypothalamic injection/implantation of a number of A-ring reduced progesterone derivatives into estrogen-primed female rats on female sexual behavior was first examined by Beyer and colleagues (Rodgrígues-Manzo et al., 1986; Beyer et al., 1989). Crystallized 5β,3β-pregnanolone was found to induce intense lordosis when implanted into the mPOA, a result the authors attribute to a depression of mPOA neuronal firing (Rodgrígues-Manzo et al., 1986). A later study examining the effects of a panel of A-ring reduced neurosteroids (5 μg) injected in oil indicated that a number of neurosteroids, including allopregnanolone, could facilitate lordosis when injected into the mPOA (Beyer et al., 1989). Similar to its action in the mPOA, 5β,3β-pregnanolone also induced lordosis when injected into the VMN, although allopregnanolone was ineffective in this region (Beyer et al., 1989). McCarthy and colleagues (McCarthy et al., 1995) have shown that infusion of allopregnanolone (in propylene glycol) into the MCG; the midbrain component of the lordosis circuit directly downstream of the VMN (Pfaff et al., 1994), also induces rapid and dose-dependent facilitation of lordosis in ovarectomized and estradiol benzoate-treated rats. Over the past decade, Frye and colleagues have published a large number of reports indicating that both 3α-diol and allopregnanolone can induce rapid effects on proceptive and receptive sex behaviors in female rodents via allosteric modulation of GABAA receptors. Specifically, in their initial studies Frye et al. (1996a,b) demonstrated that 3α-diol infused in to the mPOA could rapidly inhibit lordosis and also rapidly facilitate lordosis when infused in the VMN. However, these investigators found that 3α-diol could also inhibit lordosis when applied to either the VMN or the mPOA if circulating (systemic) levels of steroids and/or neurosteroids were altered concomitantly in these animals. In addition to these hypothalamic/basal forebrain regions, Frye and colleagues have gone on to show that allopregnanolone elicits rapid facilitation of lordosis when infused into the ventral tegmental area (VTA) and have implicated both G protein and adenylyl cyclase activity in this action (Frye et al., 2006a,b; for review, Frye et al., 2006c). While it is clear that rapid actions of neurosteroids do indeed influence the expression of female sexual behaviors, there does not appear to be general agreement as to an immutable role (facilitating or inhibitory) for any given neurosteroid on the expression of these behaviors; variability that is likely to reflect differences in the endogenous hormonal state of test subjects, in hormone treatments accompanying or preceding neurosteroid exposure, the route of neurosteroid exposure, the anatomical site of exposure, and the time increment between exposure to the steroid and the time of the assays. That neurosteroid effects on GABAA receptor-mediated reproductive behaviors will be influenced by the endogenous steroid milieu is supported by biochemical assays that indicate modulatory potency varies as a function of sex and hormonal state (Wilson and Biscardi, 1997; Finn and Gee, 1993). Neither the acute actions of the positive neurosteroid modulators on male sexual behaviors nor the acute actions of either the negative neurosteroid modulators or the AAS on reproductive behaviors in either sex have been reported.
With respect to other measures of reproductive function, Genazzani et al. (1995) report that intracerebroventricular (i.c.v.) injection of two doses of allopregnanolone (20 μg, on diestrus II and the morning of proestrus) decreases the number of oocytes retrieved at estrus, and that female rats injected i.c.v. with either a single dose at proestrus or a double dose at diestrus II and proestrus of an antiserum against allopreganolone produce more oocytes at estrus and display enhanced lordosis intensity (a measurement that encompassed both proceptive and receptive behaviors). Taken together, these results suggest that enhancement of GABAergic activity results in diminished reproductive competence in female rats. These data contrast with those of Brann et al. (1990) in which intraperitoneal (i.p.) injection of allopregnanolone, acting at the GABAA receptor, elicit LH secretion in estrogen-primed ovariectomized juvenile or adult female rats. Both studies are complicated by the fact that neither i.p. nor i.c.v injections localize allopregnanolone infusion to specific brain sites.
Perhaps the best-characterized system that ties together cellular events that alter steroid modulation of GABAA receptors and resulting changes in reproductive behaviors is that of allopregnanolone on the activity of oxytocin-containing magnocellular neurons within the dorsal SON in female rats. Initial reports indicated that a shift in the relative expression levels of GABAA receptor α1 and α2 subunit mRNAs in these neurons as female rodents transit from late pregnancy through parturition and to a lactating state results in a concomitant decrease in the sensitivity of these neurons to modulation by allopregnanolone, thus promoting disinhibition and enhanced firing of oxytocin neurons (Fénelon and Herbison, 1996a; 2000; Brussaard et al., 1997; Brussaard et al., 1999; for review Brussaard and Herbison, 2000; Herbison, 2001). While effects of antisense knockdown of α2 subunit expression in neocortical slices demonstrated the expected acceleration of synaptic current decay kinetics (Brussaard et al., 1997), SON slices were not tested with anti-sense constructs, nor were antisense-treated cortical slices assayed for differences in the sensitivity to allosteric modulation by allopregnanolone to test directly if changes in α subunit expression resulted in concomitant changes in allopregnanolone potentiation. More recently, assessment of allopregnanolone sensitivity in α1-/- mice indicates that the absence of the α1 subunit does not alter the efficacy of allopregnanolone modulation (Koksma et al., 2003); a result consistent with a number of studies from recombinant GABAA receptors and native receptors in other brain regions indicating that α1 vs. α2 subunit composition has only a modest correlation with allosteric modulation of recombinant GABAA receptors elicited by the positive neurosteroids and no correlation with allopregnanolone modulation in primary neurons (Section 3.2). More recently, a surprising report by Koksma et al. (2005) appears to refute all of the previous studies and suggests that there is no change in α1 or α2 mRNAs levels during pregnancy and parturition, but that there appears to be an increase in α2-containing receptors due to selective insertion and clustering of α2-containing receptors.
While it is unclear to what extent α subunit-switching in oxytocin neurons as a function of hormonal state occurs and what the functional importance of this switch might be, as indicated above (Section 6), our current understanding suggests that a G protein-mediated change in receptor phosphorylation may be the key regulator of GABAA receptor sensitivity to allopregnanolone in SON/PVN neurons during, pregnancy, parturition and lactation. Specifically, Koksma et al. (2003) hypothesize that low levels of oxytocin in early pregnancy result in minimal oxytocin receptor signaling and low levels of [Ca2+]in in SON neurons; a signaling milieu which favors the activity of serine/threonine phosphatases over PKC and a predominance of dephosphorylated GABAA receptors. These dephosphorylated receptors are more sensitive to the enhancing effects of allopregnanolone. At parturition and in lactating females, oxytocin levels rise dramatically, shifting the balance to PKC over phosphatase activity in SON neurons and promoting phosphorylation of GABAA receptors. This posttranslational modification renders receptors less sensitive to the enhancing effects of allopregnanolone which results in decreased inhibitory tone and increased firing of oxytocin neurons.
A recent report indicates that the pheromone, androstenol (5α-androst-16-en-3α-ol) (Figure 1), like the positive neurosteroids, can potentiate currents from both native and recombinant receptors and elicit anxiolytic behavioral effects (Kaminski et al., 2006). While the actions of androstenol on hypothalamic neurons or on reproductive/sexual behaviors in rodents has not been assessed, this pheromone has been shown to decrease LH pulse frequency during the follicular phase and may contribute to the menstrual synchrony in human females (Morofushi et al., 2000; Shinohara et al., 2000). This novel capacity of pheromones adds an intriguing new wrinkle in understanding the complexities of the role of allosteric modulation of GABAA receptors on the expression of reproductive behaviors.
8. Future directions
Nearly all studies to date have focused on the effects of allosteric modulators on synaptic responses in the hypothalamus and the potential consequences of allosteric modulation of these responses may have for neuroendocrine control. Of unexplored, but potentially high significance in understanding modulation of GABAergic tone in the hypothalamus is the impact steroid modulators may have on tonic currents mediated by low concentrations of ambient GABA through high affinity extrasynaptic receptors. Such receptors have been studied extensively in the hippocampus and cerebellum where they have been shown to play a critical role in regulating action potential firing and input-output relationships in neurons (for review, Semyanov et al., 2004). Recently, tonic currents in oxytocin and vasopressin neurons of the SON have been thoroughly characterized. While allosteric modulation of this tonic conductance was not assessed, receptors mediating the tonic current were shown to be pharmacologically distinct from those mediating the synaptic responses, and this tonic current had a significant effect on action potential firing in these cells (Park et al., 2006). Moreover these authors postulate that the extrasynaptic conductance may arise from α5βxδ-containing receptors (Park et al., 2006), which would be expected to be highly sensitive to modulation by positive neurosteroids (Bianchi et al., 2002; 2004; Wohlfarth et al., 2002). Thus, it is tempting to postulate that allopregnanolone modulation of these extrasynaptic, rather than synaptic, conductances may be the critical factor in mediating the changes in oxytocin neuronal firing that occur during late pregnancy and parturition.
Delta-containing receptors are known to give rise to most tonic, extrasynaptic conductances in other brain regions, however, given that δ subunit expression in the hypothalamus/basal forebrain is debatable (Fritschy and Mohler, 1995; Pirker et al., 2000) and expression of ε in this brain region is marked (Whiting et al., 1997; Sinkkonen et al., 2000; McIntyre et al., 2002), most notably in the GnRH neurons (Moragues et al., 2003), an intriguing possibility is that ε-containing receptors may play a comparable role in regulating tonic GABAergic conductances and allosteric modulation to both the endogenous neurosteroids and the AAS. The AAS, 17α-MeT, inhibits tonic and spontaneous currents in GnRH neurons (Jones et al., 2006) and can both potentiate and inhibit sIPSCs in these neurons (Carlos Penatti, unpublished data), suggesting that ε-containing receptors comprise an appreciable proportion of the extrasynaptic receptor population and may have mixed synaptic expression in these cells (Figure 4). Thus, while inclusion of the δ subunit may confer exquisite sensitivity to positive neurosteroid modulation within structures such as the hippocampus, inclusion of the ε subunit may render neurons in neuroendocrine control regions, especially GnRH neurons, relatively insensitive to the actions of the endogenous neurosteroids and result in GABAergic currents that are paradoxically antagonized by the allosteric actions of the AAS.
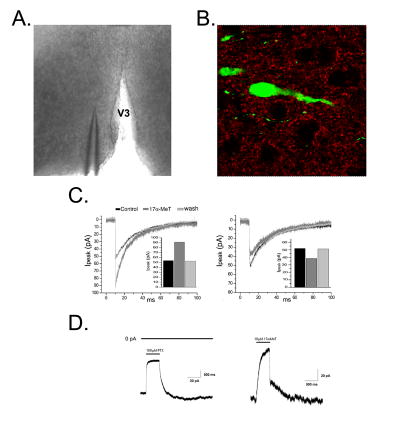
Figure 4.
A) Representative micrograph of an acutely isolated slice from a female mouse at the level of the mPOA and a patch electrode. B) Micrograph of the mPOA processed for synaptophysin immunocytochemistry (red) from the transgenic line of mice in which GFP is expressed in GnRH neurons (green) amidst a number of non-GnRH cell bodies (black). Mice were kindly provided by Dr. Sue Moenter (University of Virginia). C) Representative sIPSCs recorded from an identified GFP-GnRH neuron demonstrating reversible potentiation (left) and inhibition (right) of peak current amplitude by 1 μM 17α-MeT. D) Representative whole-cell currents recorded in the absence of GABA from an acutely dissociated GFP-GnRH neuron. Left: current arising from spontaneously active GABAA receptors. No exogenous GABA was applied; spontaneous current is antagonized by the noncompetitive GABAA receptor antagonist, picrotoxin. Right: spontaneous current from the same GFP-GnRH neuron demonstrating reversible inhibition by a maximally effective (10 μM) concentration of 17α-MeT. V3: Third Ventricle. Data are courtesy of Dr. Carlos Penatti, Dr. Brian Jones and Ms. Sandra Pahl.
Does phosphorylation of the GABAA receptor change with hormonal state? Certainly the studies by Brussaard et al. (2000), Fáncsik et al. (2000) and Koksma et al. (2003) described above strongly suggest this, but it has not been directly tested. Multiple subunits within the receptor complex are substrates for a host of different kinases and phosphatases (for review, Brandon et al., 2002), and while the effects of phosphorylation may be variable, depending on differences in receptor subunit composition, in receptor-associated proteins that may themselves be the substrates, in the endogenous state of receptor phosphorylation, in the activity of kinases/phosphatases in the cell signaling milieu (for review, Brandon et al., 2002; Song and Messing, 2005), and in temperature-dependent effects (thus species-specific effects) on enzyme activity (Machu et al., 2006). While both γ and β subunits of the GABAA receptor can be phosphorylated, the activities of a number of kinases, including PKC, converge on conserved serine residues in the β subunits (for review, Brandon et al., 2002; Song and Messing, 2005) that result in changes in deactivation, desensitization and may underlie the phosphorylation-dependent changes in sensitivity to a number of allosteric modulators, including the neurosteroids (Leidenheimer et al., 1992; Leidenheimer and Chapell, 1997; for review, Song and Messing, 2005). Thus, these β subunit residues seem to be likely targets for hormonal state-dependent changes in receptor phosphorylation. It will be important to determine not only if changes in phosphorylation of the β subunits correspond to the changes in neurosteroid sensitivity in SON neurons over pregnancy, but what role receptor phosphorylation may play in modulating GABAergic transmission and sensitivity to steroid modulation in the mPOA and other brain regions that are key for the expression of sexual behaviors.
In addition to structural considerations, one must also consider the temporal aspects of phosphorylation and neurosteroid sensitivity. Specifically, while neurosteroid modulation of both native hypothalamic and recombinant receptors can occur quite rapidly, Fáncsik et al., (2000) report that continuous (~10 minute) exposure to allopregnanolone was required to first detect significant enhancement of sIPSC decay in magnocellular neurons of the SON. Moreover, this effect increased not only during continued allopregnanolone exposure, but also continued to increase for many minutes even after the steroid was removed from the bath solution. A recent report by Wegner et al., (2006) on recombinant receptors also finds a significant effect of prolonged exposure on neurosteroid modulation. In this case, it was found that brief, but repetitive exposures of receptors to the positive neurosteroids resulted in increasingly enhanced potentiation of submaximal GABA-evoked currents and this ramped-up potentiation required receptor phosphorylation. Taken in conjunction with results with both neurosteroids (Wetzel et al., 1999) and the AAS (Yang et al., 2002; 2005) that indicate that the modulation of responses depends upon both the steroid concentration and on the concentration of GABA, one is presented with a fascinating, but complex system, in which steroid modulation may be a much more malleable and intricate regulatory system than previously thought. Specifically, the modulatory actions of these steroids at the GABAA receptors involved in mediating reproductive behaviors are likely to depend not only on subunit composition of the receptor, but also on the phosphorylation state of the receptor or it associated proteins, the concentration of the steroid modulator(s), the concentration of GABA, whether GABA is acting at high affinity extrasynaptic or lower affinity synaptic sites and the temporal characteristics of steroid exposure. Moreover, these different variables are also likely to be dependent upon one another. Finally, it is noted that activation of hypothalamic GABAA receptors has been shown to inhibit the activity of the steroidogenic enzymes, 3β-hydroxysteroid dehydrogenase and P450C17 (17α-hydroxylase) and neurosteroid production in the hypothalamus, suggesting that neurosteroid potentiation of hypothalamic GABAergic transmission may be self-limiting (Do-Rego et al., 2000).
Acknowledgments
This work was supported by the NIH (DA/NS14137 and DA18255). I thank Drs. Ann S. Clark, Carlos Penatti and Beth Costine for their review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. Journal of Physiology. 2001;532.2:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Kiyama H, Tohyama M. The GABAA receptor γ1 subunit is expressed by distinct neuronal populations. Molecular Brain Research. 1992;15:121–132. doi: 10.1016/0169-328x(92)90159-9. [DOI] [PubMed] [Google Scholar]
- Araki T, Kiyama H, Maeno H, Tohyama M. Differential immunocytochemical localization of GABAA receptor γ1 and γ2 subunits in the rat brain. Molecular Brain Research. 1993;20:263–266. doi: 10.1016/0169-328x(93)90050-y. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Mammalian animal models of psychosexual differentiation: When it ‘translation’ to the human situation possible? Hormones and Behavior. 2006;50:579–588. doi: 10.1016/j.yhbeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanism of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Reviews. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Beyer C, González-Mariscal G, Eguíbar JR, Gómora P. Lordosis facilitation in estrogen primed rats by intrabrain injection of pregnanes. Pharmacology Biochemistry & Behavior. 1989;31:919–926. doi: 10.1016/0091-3057(88)90405-4. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. Journal of Neuroscience. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroid modulation of δ subunit-containing GABAA receptor channels. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. CRC Press; Boca Raton, FL: 2004. pp. 77–94. [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Research. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Academic Press; Orlando: 2002. pp. 139–213. [Google Scholar]
- Brandon NJ, Jovanovic JN, Moss SJ. Multiple roles of protein kinases in the modulation of γ-aminobutyric acidA receptor function and cell surface expression. Pharmacology & Therapeutics. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brann DW, Putnam CD, Mahesh VB. γ-Aminobutyric acidA receptors mediate 3α-hydroxy-5α-pregnan-20-one-induced gonadotropin secretion. Endocrinology. 1990;126:1854–1859. doi: 10.1210/endo-126-4-1854. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Current Psychiatry Reports. 2002;4:377–387. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. British Journal of Pharmacology. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. Journal of Physiology (London) 1999;516.2:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends in Neuroscience. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Wossink J, Lodder JC, Kits KS. Progesterone-metabolite prevents protein kinase C-dependent modulation of γ-aminobutyric acid type A receptors in oxytocin neurons. Proceedings of the National Academy of Science USA. 2000;97:3625–3630. doi: 10.1073/pnas.050424697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero AE, Palumbo MA, Bosboom AMJ, Burrello N, Ferrara E, Palumbo G, Petraglia F, D’Agata R. The neuroactive steroid allopregnanolone suppresses hypothalamic gonadotropin-releasing hormone release through a mechanism mediated by the gamma-aminobutyric acidA receptor. Journal of Endocrinology. 1998;158:121–125. doi: 10.1677/joe.0.1580121. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Uzunov D, Costa E, Guidoti A. Gas chromatographic-mass fragmentographic quantitation of 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) and its precursors in the blood and brain of adrenalectomized and castrated rats. Journal of Neuroscience. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CAA, Porter DM, Yang P, Henderson LP. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Research. 2006 doi: 10.1016/j.brainres.2006.08.081. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neuroscience and Biobehavioral Reviews. 2003;27:413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Clark AS, Jones BL, Yang P, Henderson LP. Anabolic androgenic steroids and the brain: novel actions at the GABAA receptor and on GABAA receptor-mediated behaviors. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. CRC Press; Boca Raton, FL: 2004. pp. 119–141. [Google Scholar]
- Clark AS, Myers M, Robinson S, Chang P, Henderson LP. Hormone-dependent regulation of GABAA receptor γ subunit mRNAs in sexually dimorphic regions of the rat brain. Proceedings of the Royal Society (London, Series B) 1998;265:1853–1859. doi: 10.1098/rspb.1998.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barvaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proceedings of the National Academy of Science USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpéchot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VVK, Banner C, Sjövall J, Baulieu EE, Robel P. Neurosteroids: 3α-hydroxy-5α-pregnan-20--one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Course JF, Korach KS. Exploring the role of sex steroids through studies of receptor deficient mice. Journal of Molecular Medicine. 1998;76:497–511. doi: 10.1007/s001090050244. [DOI] [PubMed] [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Archives of General Psychiatry. 2001;58:172–177. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Evidence for the formation of functionally distinct αβγε GABAA receptors. Journal of Physiology (London) 2001;537(Pt 1):101–113. doi: 10.1111/j.1469-7793.2001.0101k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Penschuck S, Fritschy J-M, McCarthy MM. Developmental switch in the expression of GABAA receptor subunits α1 and α2 in the hypothalamus and limbic system of the rat. Developmental Brain Research. 2000;119:127–138. doi: 10.1016/s0165-3806(99)00150-9. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Tapia-Arancibia L. Rise in intracellular calcium via a nongenomic effect of allopregnanolone in fetal rat hypothalamic neurons. Journal of Neuroscience. 1996;16:130–136. doi: 10.1523/JNEUROSCI.16-01-00130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Molecular Endocrinology. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- Do-Rego JL, Mensah-Nyagan GA, Beaujean D, Vaudry D, Sieghart W, Luu-The V, Pelletier G, Vaudry H. γ-aminobutyric acid, acting through γ-aminobutyric acid type A receptors, inhibits the biosynthesis of neurosteroids in the frog hypothalamus. Proceedings of the National Academy of Science USA. 2000;97:13925–13930. doi: 10.1073/pnas.240269897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS. Serum 5α-androstane-3α,17β-diol increases in response to paced coital stimulation in cycling female rats. Biology of Reproduction. 1987;37:1139–1148. doi: 10.1095/biolreprod37.5.1139. [DOI] [PubMed] [Google Scholar]
- Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. Journal of Neuroscience. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénelon VS, Herbison AE. Plasticity in GABAA receptor subunit mRNA expression by hypothalamic magnocellular neurons in the adult rat. Journal of Neuroscience. 1996a;16:4872–4880. doi: 10.1523/JNEUROSCI.16-16-04872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénelon VS, Herbison AE. In vivo regulation of specific GABAA receptor subunit messenger RNAs by increased GABA concentrations in rat brain. Neuroscience. 1996b;71:661–670. doi: 10.1016/0306-4522(95)00492-0. [DOI] [PubMed] [Google Scholar]
- Fénelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64:1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Larsson K, Beyer C. GABAergic control of masculine sexual behavior. Pharmacology, Biochemistry and Behavior. 1986;24:1065–1070. doi: 10.1016/0091-3057(86)90456-9. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The influence of estrus cycle on neurosteroid potency at the γ-aminobutyric acidA receptor complex. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1374–1379. [PubMed] [Google Scholar]
- Finn DA, Purdy RH, Koob GF. Animal models of anxiety and stress-induced behavior: Effects of neuroactive steroids. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. CRC Press; Boca Raton, FL: 2004. pp. 317–338. [Google Scholar]
- Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clinical Chemistry. 1997;43:1262–1279. [PubMed] [Google Scholar]
- Freeman MC. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Vol. 2. Raven Press Ltd; New York: 1994. pp. 613–658. [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Möhler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. Journal of Neuroscience. 1994;14:5302–24. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J-M, Möhler H. GABAA-receptor heterogeneity in the adult rat brain: Differential regional and cellular distribution of seven major subunits. Journal of Comparative Neurology. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duncan JE, Basham M, Erskine MS. Behavioral effects of 3α-androstanediol II: Hypothalamic and preoptic actions via a GABAergic mechanism. Behavioural Brain Research. 1996a;79:119–130. doi: 10.1016/0166-4328(96)00005-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006c;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Rao PN, Erskine MS. Progesterone and 3α-androstanediol conjugated to bovine serum albumin affects estrous behavior when applied to the MBH and the POA. Behavioral Neuroscience. 1996b;110:603–612. doi: 10.1037//0735-7044.110.3.603. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Petralia SM. Progestin facilitation of lordosis in rodents involves adenylyl cyclase activity in the ventral tegmental area. Hormones and Behavior. 2006a;50:237–244. doi: 10.1016/j.yhbeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Petralia SM. Progestins’ effects on sexual behaviour of female rats and hamsters involving D1 and GABAA receptors in the ventral tegmental area by be G-protein-dependent. Behavioural Brain Research. 2006b;172:286–293. doi: 10.1016/j.bbr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gallaway S. The Steroid Bible. Third edition. Belle International; Honolulu, HI: 1997. [Google Scholar]
- Genazzani AR, Palumbo MA, de Micheroux AA, Artini PG, Criscuolo M, Ficarra G, Guo AL, Benelli A, Bertolini A, Petraglia F, Purdy RH. Evidence for a role for the neurosteroid allopregnanolone in the modulation of reproductive function in female rats. European Journal of Endocrinology. 1995;133:375–380. doi: 10.1530/eje.0.1330375. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function and regulation. Physiological Reviews. 2001;81:630–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Clark AS. Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain Research. 2006;1076:163–170. doi: 10.1016/j.brainres.2005.12.120. [DOI] [PubMed] [Google Scholar]
- Haage D, Bäckström T, Johansson S. Interaction between allopregnanolone and pregnenolone sulfate in modulating GABA-mediated synaptic currents in neurons from the rat medial preoptic nucleus. Brain Research. 2005;1033:58–67. doi: 10.1016/j.brainres.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Haage D, Johansson S. Neurosteroid modulation of synaptic and GABA-evoked currents in neurons from the rat medial preoptic nucleus. Journal of Neurophysiology. 1999;82:143–151. doi: 10.1152/jn.1999.82.1.143. [DOI] [PubMed] [Google Scholar]
- Han S-K, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- Han S-K, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Fjalland B, Jackson MB. Differential blockade of γ-aminobutyric acid type A receptors by the neuroactive steroid dehydroepiandrosterone sulfate in posterior and intermediate pituitary. Molecular Pharmacology. 1999;55:489–496. [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiology & Behavior. 2004;81:671–680. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Henderson LP, Jorge JC. Steroid modulation of GABAA receptors: from molecular mechanisms to CNS roles in reproduction, dysfunction and drug abuse. Advances in Molecular and Cell Biology. 2004;32:217–249. [Google Scholar]
- Herbison AE. Physiological roles for the neurosteroid allopregnanolone in the modulation of brain function during pregnancy and parturition. Progress in Brain Research. 2001;133:39–47. doi: 10.1016/s0079-6123(01)33003-0. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Fénelon VS. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. Journal of Neuroscience. 1995;15:2328–2337. doi: 10.1523/JNEUROSCI.15-03-02328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR, Simonian SX, Skynner MJ, Sim JA. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Molecular and Cellular Endocrinology. 2001;185:185–194. doi: 10.1016/s0303-7207(01)00618-9. [DOI] [PubMed] [Google Scholar]
- Herrington DM. DHEA: a biological conundrum. Journal of Laboratory and Clinical Medicine. 1998;131:292–294. doi: 10.1016/s0022-2143(98)90176-7. [DOI] [PubMed] [Google Scholar]
- Higashi T, Daifu Y, Ikeshima T, Yagi T, Shimada K. Studies on neurosteroids XV. Development of enzyme-linked immunosorbet assay for examining whether pregnenolone sulfate is a veritable neurosteroid. Journal of Pharmaceutical and Biomedical Analysis. 2003;30:1907–1917. doi: 10.1016/s0731-7085(02)00534-4. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Huang R-Q, Dillon GH. Functional characterization of GABAA receptors in neonatal hypothalamic brain slice. Journal of Physiology. 2002;88:1655–1663. doi: 10.1152/jn.2002.88.4.1655. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Orlando, FL: Academic Press; 2002. pp. 3–137. [Google Scholar]
- Jones BL, Whiting PJ, Henderson LP. Mechanisms of anabolic androgenic steroid inhibition of ε-containing GABAA receptors. Journal of Physiology (London) 2006;573.3:571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends in Neuroscience. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Jorge JC, McIntyre KL, Henderson LP. The function and the expression of forebrain GABAA receptors change with hormonal state in the adult mouse. Journal of Neurobiology. 2002;50:137–149. doi: 10.1002/neu.10021. [DOI] [PubMed] [Google Scholar]
- Jorge-Rivera J-C, McIntyre KL, Henderson LP. Anabolic steroids induce region- and subunit-specific rapid modulation of GABAA receptor-mediated currents in the rat forebrain. Journal of Neurophysiology. 2000;83:3299–3309. doi: 10.1152/jn.2000.83.6.3299. [DOI] [PubMed] [Google Scholar]
- Juptner M, Hiemke C. Sex differences in GABAA receptor binding in rat brain measured by an improved in vitro binding assay. Experimental Brain Research. 1990;81:297–302. doi: 10.1007/BF00228119. [DOI] [PubMed] [Google Scholar]
- Kazihnitková H, Tejkalová H, Benesová, Bicíková M, Hill M, Hampl R. Simultaneous determination of dehyroepiandrosterone, its 7-hydroxylated metabolites, and their sulfates in rat brain tissue. Steroids. 2004;69:667–674. doi: 10.1016/j.steroids.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Frye CA. Endogenous levels of 5 alpha-reduced progestins and androgens in fetal and adult rat brains. Developmental Brain Research. 1999;115:17–24. doi: 10.1016/s0165-3806(99)00041-3. [DOI] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin--anatomy and functional assignments: a minireview. Endocrine Regulations. 2005;39:97–105. [PubMed] [Google Scholar]
- Koksma J-J, van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Lüddens H, Brussaard AB. Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. Journal of Neuroscience. 2003;23:788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksma J-J, Fritschy J-M, Mack V, van Kesteren RE, Brussaard AB. Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Molecular and Cellular Neuroscience. 2005;28:128–140. doi: 10.1016/j.mcn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Kaminski M, Marini H, Ortinski PI, Vicini S, Rogawski MA. The pheromone androstenol (5α-androst-16-en-3α-ol) is a neurosteroid positive modulator of GABAA receptors. Journal of Pharmacology and Experimental Therapeutics. 2006;317:694–703. doi: 10.1124/jpet.105.098319. [DOI] [PubMed] [Google Scholar]
- Lacroix C, Fiet J, Benais JP, Gueux B, Bonete R, Villette JM, Gourmel B, Dreux C. Simultaneous radioimmunoassay of progesterone, androst-4-enedione, pregnenolone, dehydroepiandrosterone and 17-hydroxyprogesterone in specific regions of human brain. Journal of Steroid Biochemistry. 1987;28:317–325. doi: 10.1016/0022-4731(87)91025-9. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Progress in Neurobiology. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends in Pharmacological Science. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lanthier A, Patwardhan VV. Sex steroids and 5-en-3 beta-hydroxysteroids in specific regions of the human brain and cranial nerves. Journal of Steroid Biochemistry. 1986;25:445–449. doi: 10.1016/0022-4731(86)90259-1. [DOI] [PubMed] [Google Scholar]
- Lasaga M, Duvilanski B, Seilicovich A, Afione S, Debeljuk L. Effects of sex steroids on GABA receptors in rat hypothalamus and anterior pituitary gland. European Journal of Pharmacology. 1988;155:163–166. doi: 10.1016/0014-2999(88)90416-5. [DOI] [PubMed] [Google Scholar]
- Leidenheimer NJ, McQuilkin SJ, Hahner LD, Whiting PJ, Harris RA. Activation of protein kinase C selectively inhibits the γ-aminobutyric acidA receptor: role of desensitization. Molecular Pharmacology. 1992;41:1116–1123. [PubMed] [Google Scholar]
- Leidenheimer NJ, Chapell R. Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABAA receptors. Molecular Brain Research. 1997;52:173–181. doi: 10.1016/s0169-328x(97)00255-6. [DOI] [PubMed] [Google Scholar]
- Leupen SM, Tobet SA, Crowley WF, jr, Kaila K. Heterogeneous expression of the potassium-chloride cotransporter, KCC2 in gonadotropin-releasing hormone neurons of the adult mouse. Endocrinology. 2003;144:3031–3036. doi: 10.1210/en.2002-220995. [DOI] [PubMed] [Google Scholar]
- Liere P, Akwa Y, Weill-Engerer S, Eychenne B, Pianos A, Robel P, Sjövall J, Schumacher M, Baulieu EE. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. Journal of Chromatography B. 2000;739:301–312. doi: 10.1016/s0378-4347(99)00563-0. [DOI] [PubMed] [Google Scholar]
- Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjövall J, Baulieu E-E. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. Journal of Lipid Research. 2004;45:2287–2302. doi: 10.1194/jlr.M400244-JLR200. [DOI] [PubMed] [Google Scholar]
- Liu S, Sjovall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation, and analysis ob nanoscale liquid chromatography-electrospray mass spectrometry. Analytical Chemistry. 2003;75:5835–5846. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- Machu TK, Dillon GH, Huang R, Lovinger DM, Leidenheimer NJ. Temperature: an important experimental variable in studying PKC modulation of ligand-gated ion channels. Brain Research. 2006;1086:1–8. doi: 10.1016/j.brainres.2006.01.091. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neuroscience. 2005;8:798–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maksay G, Thompson SA, Wafford KA. The pharmacology of spontaneously open α1β3ε GABAA receptor-ionophores. Neuropharmacology. 2003;44:994–1002. doi: 10.1016/s0028-3908(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Malik KF, Silverman AJ, Morrell JI. Gonadotropin releasing hormone mRNA in the rat: distribution and neuronal content over the estrous cycle and after castration in males. The Anatomical Record. 1991;231:457–466. doi: 10.1002/ar.1092310408. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Hormones and Behavior. 1995;29:279–295. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Malik KF, Feder HF. Increased GABAergic transmission in the medial hypothalamus facilitates lordosis but has the opposite effect in preoptic area. Brain Research. 1990;507:40–44. doi: 10.1016/0006-8993(90)90519-h. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Masters DB, Fiber JM, López-Colomé A-M, Beyer C, Komisaruk BR, Feder HH. GABAergic control of receptivity in the female rat. Neuroendocrinology. 1991a;53:473–479. doi: 10.1159/000125760. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Pfaff DW, Schartz-Giblin S. Midbrain central gray GABAA receptor activation enhances, and blockade reduces, sexual behavior in the female rat. Experimental Brain Research. 1991b;86:108–116. doi: 10.1007/BF00231045. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Warner Williams G, Lumia AR. Inhibition of male sex behavior by androgen receptor blockade in preoptic area or hypothalamus, but not amygdala or septum. Physiology & Behavior. 1996;60:783–789. doi: 10.1016/0031-9384(96)00088-1. [DOI] [PubMed] [Google Scholar]
- McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex- and dose-dependent changes in GABAA receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43:634–645. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 2. New York, NY: Raven Press; 1994. pp. 3–106. [Google Scholar]
- Mienville JM, Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Research. 1989;489:190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretions. Frontiers in Neuroendocrinology. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor ε subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Research. 2003;967:285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- Morofushi M, Shinohara K, Funabashi T, Kimura F. Positive relationship between menstrual synchrony and ability to smell 5α-androst-16-en-3α-ol. Chemical Senses. 2000;25:407–411. doi: 10.1093/chemse/25.4.407. [DOI] [PubMed] [Google Scholar]
- Nett ST, Jorge-Rivera J-C, Myers M, Clark AS, Henderson LP. Properties and sex-specific differences of GABAA receptors in neurons expressing γ1 subunit mRNA in the preoptic area of the rat. Journal of Neurophysiology. 1999;81:192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144:823–831. doi: 10.1210/en.2002-220585. [DOI] [PubMed] [Google Scholar]
- O’Connor L, Nock B, McEwen BS. Regional specificiy of gamma-aminobutyric acid receptor regulation by estradiol. Neuroendocrinology. 1988;47:473–481. doi: 10.1159/000124958. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent A-S, Matagne V, Mungenast AE. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Vol. 2. Raven Press Ltd; New York: 1994. pp. 363–410. [Google Scholar]
- Paredes RG, Baum MJ. Role of the medial preoptic area/anterior hypothalamus in the control of masculine sexual behavior. Annual Review of Sex Research. 1997;8:68–101. [PubMed] [Google Scholar]
- Park JB, Skalaska S, Stern JE. Characterization of a novel tonic γ-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB Journal. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Penatti CAA, Porter DM, Jones BL, Henderson LP. Sex-specific differences in the effects of chronic anabolic androgenic steroid treatment on GABAA receptor expression and function in adolescent mice. Neuroscience. 2005;135:533–543. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow L-M. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 2. New York, NY: Raven Press; 1994. pp. 107–220. [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlicter R. Modulation of GABAA receptor-mediated IPSCs by neuroactive steroid in a rat hypothalamic-hypophyseal coculture model. Journal of Physiology (London) 1997;500.2:475–485. doi: 10.1113/jphysiol.1997.sp022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors and Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- Rahman M, Lindblad C, Johansson I-M, Bäckström t, Wang M-D. Neurosteroid modulation of recombinant rat α5β2γ2L and α1β2γ2L GABAA receptors in Xenopus oocytes. European Journal of Pharmacology. 2006;547:37–44. doi: 10.1016/j.ejphar.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Robel P, Baulieu EE. Dehydroepiandrosterone (DHEA) is a neuroactive steroid. Annals of the New York Academy of Sciences. 1995;774:82–110. doi: 10.1111/j.1749-6632.1995.tb17374.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzo G, Cruz ML, Beyer C. Facilitation of lordosis behavior in ovariectomized estrogen-primed rats by medial preoptic implantation of 5β, 3β, pregnanolone: A ring A reduced progesterone metabolite. Physiology & Behavior. 1986;36:277–281. doi: 10.1016/0031-9384(86)90016-8. [DOI] [PubMed] [Google Scholar]
- Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Frontiers in Neuroendocrinology. 2003;24:27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, McEwen B. Regulation of high-affinity GABAA receptors in specific brain regions by ovarian hormones. Neuroendocrinology. 1989;50:315–320. doi: 10.1159/000125239. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining tone. Trends in Neuroscience. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clinical Therapeutics. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. Journal of Neuroscience. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Morofushi M, Funabashi T, Mitsushima D, Kimura F. Effects of 5α-androst-16-en-3α-ol on the pulsatile secretion of luteinizing hormone in human females. Chemical Senses. 2000;25:465–467. doi: 10.1093/chemse/25.4.465. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacological Reviews. 1995;47:181–234. [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape J-R, Herbison AE. Late postnatal reorganization of GABAA receptor signaling in native GnRH neurons. European Journal of Neuroscience. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Hanna MC, Kirkness EF, Korpi ER. GABAA receptor ε and θ subunits display unusual structural variation between species and are enriched in the rat locus ceruleus. Journal of Neuroscience. 2000;20:3588–3595. doi: 10.1523/JNEUROSCI.20-10-03588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS. Withdrawal effects of a neuroactive steroid as a model of PMS: Synaptic physiology to behavior. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. CRC Press; Boca Raton, FL: 2004. pp. 143–172. [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell and Molecular Life Sciences. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Neurosteroids alter γ-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology. 2003;144:4366–4375. doi: 10.1210/en.2003-0634. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Bonnert TP, Whiting PJ, Wafford KA. Functional characteristics of recombinant human GABAA receptors containing the ε-subunit. Toxicology Letters. 1998;100-101:233–238. doi: 10.1016/s0378-4274(98)00190-8. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Bonnert TP, Cagetti E, Whiting PJ, Wafford KA. Overexpression of GABAA receptor ε subunit results in insensitivity to anaesthetics. Neuropharmacology. 2002;43:662–668. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- Uchida S, Noda E, Kakazu Y, Mizoguchi Y, Akaike N, Nabekura J. Allopregnanolone enhancement of GABAergic transmission in rat medial preoptic area neurons. American Journal of Physiology- Endocrinology and Metabolism. 2002;283:E1257–1265. doi: 10.1152/ajpendo.00049.2002. [DOI] [PubMed] [Google Scholar]
- Vicini S. THDOC and the GABAA receptor. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. CRC Press; Boca Raton, FL: 2004. pp. 63–75. [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng C-M, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. 3β-hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. Journal of Neuroscience. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner F, Rassler C, Allgaier C, Strecker K, Wohlfarth K. Auto-modulation of neuroactive steroids on GABAA receptors: A novel pharmacological effect. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.09.009. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wetzel CHR, Vedder H, Holsboer F, Zieglgänsberger W, Deisz RA. bidirectional effects of the neuroactive steroid tetrahydrocorticosterone on GABA-activated Cl- currents in cultured rat hypothalamic neurons. British Journal of Pharmacology. 1999;127:863–868. doi: 10.1038/sj.bjp.0702597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O’Donnell R, Rigby MR, Sirinathsinghji DJS, Marshall G, Thompson SA, Wafford KA. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties. Journal of Neuroscience. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H, Ludwig M, Bancel F, Leng G, Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. The Journal of Physiology (London) 2003;48.1:233–244. doi: 10.1113/jphysiol.2002.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sciences. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. Journal of Neuroscience. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JW, Paden CM, Silverman AJ. The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology. 1982;35:429–438. doi: 10.1159/000123419. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the delta subunit. Journal of Neuroscience. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Pyner S, Barrett-Jolley R. Inhibition by α-tetradhyrodeoxycorticosterone (THDOC) of pre-sympathetic parvocellular neurones in the paraventricular nucleus of rat hypothalamus. British Journal of Pharmacology. 2006;149:600–607. doi: 10.1038/sj.bjp.0706911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FC. Endocrine aspects of anabolic steroids. Clinical Chemistry. 1997;43:1289–1292. [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Mechanisms of anabolic androgenic steroid modulation of α1β3γ2L GABAA receptors. Neuropharmacology. 2002;43:619–633. doi: 10.1016/s0028-3908(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Role of the α subunit in the modulation of GABAA receptors by anabolic androgenic steroids. Neuropharmacology. 2005;49:300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ymer S, Draguhn A, Wisden W, Werner P, Keinänen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH. Structural and functional characterization of the γ1 subunit of GABAA /benzodiazepine receptors. EMBO Journal. 1990;9:3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]