Abstract
We postulate a novel and general mechanism in which the redox-active sulfur donor group of cyst(e)ine confers oxidoreductive characteristics on stable zinc sites in proteins. Thus, the present, an earlier, and accompanying manuscripts [Maret, W., Larsen, K. S. & Vallee, B. L. (1997) Proc. Natl. Acad. Sci. USA 94, 2233–2237; Jiang, L.-J., Maret, W. & Vallee, B. L. (1998) Proc. Natl. Acad. Sci. USA 95, 3483–3488; and Jacob, C., Maret, W. & Vallee, B. L. (1998) Proc. Natl. Acad. Sci. USA 95, 3489–3494] demonstrate that the interactive network featuring multiple zinc/sulfur bonds as found in the clusters of metallothionein (MT) constitutes a coordination unit critical for the concurrent oxidation of cysteine ligands and the ensuing release of zinc. The low position of MT (<−366 mV) on a scale of redox reagents allows its effective oxidation by relatively mild cellular oxidants, in particular disulfides. When MT is exposed to an excess of dithiodipyridine, all of its 20 cysteines are oxidized within 1 hr with the concomitant release of all 7 zinc atoms; similarly, the thiol/disulfide oxidoreductase DsbA reacts stoichiometrically with MT to release zinc. Zinc and sulfur ligands in the clusters are in a spatial arrangement that seemingly favors disulfide bond formation. Jointly, this and the above-mentioned manuscripts conclude that the control of cellular zinc distribution as a function of the energy state of the cell is the long sought role of MT. This specific MT function renders dubious the widely held belief that MT primarily scavenges radicals or detoxifies metals and is consistent with the frequent use of cysteine as a zinc ligand in proteins as a means of both tight and weak zinc binding of thiols and disulfides, respectively. Thus, we relate changes in the reducing power of the cell to the stability of the zinc/sulfur network in MT and the relative mobility of zinc and its control.
Metallothionein (MT) is a unique metalloprotein in which cysteine constitutes one-third of its amino acids and histidine and aromatic amino acids all are completely absent. All 20 cysteines bind seven zinc atoms such that each metal atom has a complement of four cysteine ligands. It is important to emphasize that the by now well known multinuclear cluster network, identified by both x-ray diffraction and NMR spectroscopy only less than a decade ago (1,2), shows the exceptional structural arrangement that was hitherto unknown in zinc/sulfur chemistry and has thus far been encountered solely in biological material such as MT. The zinc/cysteinyl interactions in the two clusters are of two different types: they are either bridging or terminal cysteine thiolates. In the β-domain cluster, three bridging and six terminal cysteine thiolates provide a coordination environment that is formally identical for each of the three zinc atoms. In the α-domain cluster, there are two different zinc sites; two of them have one terminal ligand and three bridging ligands, respectively, while the other two have two terminal and two bridging ligands.
We have performed and advocated experiments to relate the structure of MT to its possible function(s) on the basis of the nature of zinc coordination in MT (3–5). We have suggested that the characteristics of the cluster motif might be the key to the mode of cellular zinc distribution (6). MT binds zinc with high thermodynamic stability [Kd = 1.4 × 10−13 M for human MT at pH 7.0 (7)] while simultaneously providing a mechanism for kinetic lability whereby zinc can be released at rates that are orders of magnitude greater than those observed for zinc metalloenzymes. Zinc, as well as cadmium, is known to undergo rapid inter- and intracluster exchange (8, 9).
MT apparently binds zinc with higher affinity than do many other proteins. For MT to serve as a source for the distribution of zinc, mechanisms would be required that could regulate the binding and release of the metal. It has been shown that an interaction of MT with glutathione disulfide (GSSG) or other biological disulfides releases zinc (10, 11) and that the combination of reduced glutathione (GSH) and GSSG enhances transfer of zinc from MT to an apoenzyme (12). This has led us to infer that the reactivity and redox behavior of the sulfur ligands in the MT clusters are crucial for the dynamic state of zinc. We proposed that the zinc–sulfur cluster chemistry might be sensitive to changes of the cellular redox state and that oxidizing conditions induce the transfer of zinc from its binding sites in MT to those of lower affinity in other proteins.
We here provide additional support for this concept and show that a number of compounds, including some of potential biological importance, can oxidize the thiolate ligands of zinc in MT with concomitant release of zinc. We conclude that the redox properties of MT and their effect on zinc in the clusters are crucial to its function, which is further affected by additional interactions (12, 13).
MATERIALS AND METHODS
Materials.
Charge-separable cadmium and zinc MT-1 and -2 isoforms were prepared from rabbit or human liver according to established procedures (14). DsbA, a protein disulfide isomerase from Escherichia coli, was from Boehringer Mannheim. The sources of other chemicals have been described (9).
Preparation of MT Isoforms.
MT was reduced and reconstituted with zinc to obtain species that have (i) a defined content of 20 sulfhydryls as determined by spectrophotometric titrations with 2,2′-dithiodipyridine (ɛ343 = 7,600 M−1⋅cm−1) and (ii) a stoichiometry of seven zinc atoms per molecule as determined by atomic absorption spectrophotometry with a Perkin–Elmer Model 2280 instrument. Protein concentrations of MT were determined by spectrophotometry [ɛ220 = 155,000 M−1⋅cm−1 (15)].
Spectrophotometric Assays.
Reactions between MT and oxidizing agents were monitored by two types of assays. One assay is based on measuring zinc release from MT with the zinc-complexing dyes 4-(2-pyridylazo)resorcinol (PAR), ɛ500 = 65,000 M−1⋅cm−1 (16, 17) or 2-carboxy-2′-hydroxy-5′-sulfoformazylbenzene (zincon), ɛ620 = 17,500 M−1⋅cm−1. The other assay is based on the reduction of oxidizing agents with suitable chromophoric properties. Redox reactions were measured at 25°C with a Cary 1E spectrophotometer (Varian) by following the reduction of the oxidizing agent at its absorption maximum.
RESULTS AND DISCUSSION
The Zn–S Bond of MT Clusters Underlies Their Functions.
Classically, zinc has not been thought to be associated with biological redox reactions, because the metal is not readily oxidizable or reducible in solution. However, it is a distinct possibility that a change in the redox status of a donor atom of a zinc compound might alter the overall oxidoreductive properties of the complex and hence its biological reactivity. In MT, four thiolates interact with each of the seven zinc ions to form two clusters. The stability of zinc binding in these clusters could be altered selectively through interactions with cellular oxidoreductants. As a consequence, individual zinc atoms in the clusters could be differentially affected and become particularly reactive for transfer to other proteins. It is unlikely that such possibilities would have been considered prior to the determination of the MT structure, nor would one have been led to postulate or anticipate that a cluster structure might be a chemical basis for storage, release, or exchange of zinc. Indeed, we are unaware that the oxidoreductive state of the donor atom(s) of a zinc complex—be it sulfur, nitrogen, or oxygen—has been postulated to feature in biological zinc complexes of proteins and to affect function. This hypothesis suggests an important general principle that could underlie similar interactions in other biological compounds whose presumable kinetic and thermodynamic behavior would also not be predictable. The MT cluster structure, which allows zinc to bind tightly and stably but to become mobile through donor atom modification, could have far-reaching implications. The manner and extent of such changes, brought about in metabolism, would become the subject of intensive investigations in numerous enzymatic and molecular and cellular biological systems now known. We ourselves are examining a number of these to define as far as possible redox partners for the zinc–sulfur cluster components and the number and identity of the zinc atoms in the clusters involved. In the following we present examples of the current state of our investigations. Others will be described in the accompanying (12, 13) and subsequent manuscripts.
The Reaction of Oxidizing Agents with MT and Definition of Properties of MT as a Redox-Active Protein.
Table 1 shows that MT is oxidized by a large number of agents whose redox potentials cover a wide range. Iron(III) (ferricyanide, cytochrome c, ferricinium ion) and copper(II) compounds, dehydroascorbate, and disulfides are examples of such redox couples. The agents are tabulated in terms of their suitability to (i) estimate the redox potential of MT, (ii) determine the lowest redox potential with which MT could be oxidized, and (iii) serve as possible partners that might oxidize MT in vivo.
Table 1.
Reactions of MT with oxidizing agents
| Redox reagent | n* | Redox potential,† mV | Reaction with MT‡ | Ref(s). |
|---|---|---|---|---|
| H2O2/H2O | 2 | +1776 Eo | ++ | 21 |
| HO2/H2O2 | 1 | +1495 Eo | ++ | 22 |
| HNO2/NO | 1 | +983 Eo | ++ | 23 |
| Ferricinium/ferrocene | 1 | +400 Eo | ++ | Here |
| Ferricyanide/ferrocyanide | 1 | +358 E′o | ++ | 24, here |
| Azurin (Pseudomonas aeruginosa) | ||||
| [Cu(II)/Cu(I)] | 1 | +330 E′o | + | Here |
| Wurster’s blue§ | 1 | +260 E′o | + | Here |
| Cytochrome c | ||||
| [Fe(III)/Fe(II)] | 1 | +254 E′o | + | 25, here |
| Dehydroascorbate/ascorbate | 1 or 2 | +58 E′o | + | Here |
| Selenate/selenite | 2 | +50 Eo | ++ | Here |
| DsbAox/DsbAred (E. coli) | 2 | −125 E′o | + | Here |
| Dithionitrobenzoic acid/ thionitrobenzoic acid | 2 | −150 E′o | ++ | 26 |
| GSSG/GSH | 2 | −240 E′o | + | 10, 11 |
| CoA disulfide/CoA | 2 | −248 E′o | + | 10, 11 |
| Cystamine/cysteamine | 2 | −251 E′o | + | 10, 11 |
| Selenite/selenium | 4 | −366 Eo | ++ | Here |
| Metallothionein | — | <−366 E′o | — | Here |
| Methylviologen | 1 or 2 | −400 E′o | No | Here |
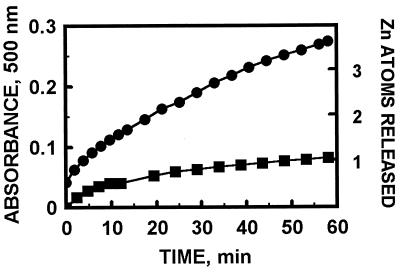
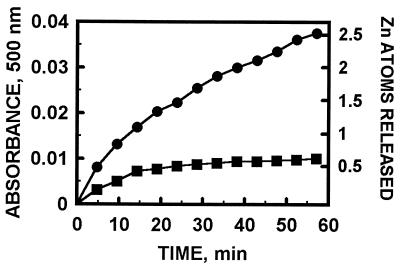
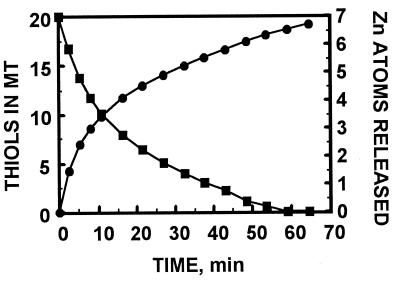
We have so far examined the reaction of MT with disulfides and cytochrome c in particular detail. GSH inhibits zinc release from MT (12), but it enhances the GSSG-induced release of zinc from MT (Fig. 1). This behavior is in agreement with our data that show modulation of zinc transfer from MT to zinc-depleted sorbitol dehydrogenase by GSH and GSSG (12). On the basis of changes of the Soret band of ferricytochrome c, it has been concluded recently that rabbit liver Cd,Zn-MT reduces cytochrome c (25), but it was unknown whether zinc is released in this process. Indeed, human liver MT-2 is oxidized by cytochrome c with concomitant release of zinc (Fig. 2). In some cases it is possible to monitor both reduction of the oxidant and zinc transfer simultaneously to ascertain that redox reactions are indeed coupled with the release of zinc. Thus, when MT reacts with the disulfide dithiodipyridine, all thiols of MT are oxidized after 1 hr, and concomitantly seven zinc ions are released as detected by reaction with zincon (Fig. 3).†
Figure 1.
Kinetics of zinc release from MT-2 induced by the glutathione redox couple. MT (1.3 μM) was incubated with PAR (100 μM) in 0.2 M Tris⋅HCl, pH 7.4, in the absence of glutathione (▪) or in the presence of 1.5 mM GSH and 3 mM GSSG (•). Zinc release was followed by measuring the formation of Zn(PAR)2 at 500 nm.
Figure 2.
Kinetics of zinc release from MT-2 by horse heart cytochrome c. MT (0.43 μM) was incubated with PAR (60 μM) in 0.2 M Tris⋅HCl, pH 7.4 (▪), or in the presence of 100 μM cytochrome c (•). Zinc release was measured as described in the legend of Fig. 1.
Figure 3.
Kinetics of zinc release from MT by dithiodipyridine and concomitant sulfhydryl oxidation. MT (0.5 μM) was dissolved in degassed, 20 mM Hepes, pH 7.5, and incubated with 100 μM zincon (to measure zinc release; right ordinate, •) and 50 μM dithiodipyridine (to measure thiol oxidation; left ordinate, ▪).
Thus far disulfides and selenite have proved to have the lowest redox potentials to oxidize MT. These findings provide an upper limit for the redox potential of MT, which must be below −366 mV—i.e., sufficiently low to allow cellular oxidants to react with MT and release zinc. A redox potential of MT <−366 mV would also be consistent with direct electrochemical studies on MT (27), in which cyclic voltammetry has revealed oxidation/reduction waves of MT around −380 mV (−600 mV against Ag/AgCl). Because in biology the differences of potentials between redox couples are relatively small, disulfides and selenite are the most likely candidates for the oxidation of MT in vivo. The potentials given in Table 1 are midpoint potentials in which the concentration of the oxidant is equal to that of the reductant, a situation rarely encountered in the cell. In fact, it is not uncommon for energy-dependent mechanisms to hold redox couples such as thiol/disulfide pairs in a nonequilibrium state (20). Thus, for the glutathione state, the redox potential derived from measured GSH/GSSG ratios of 30:1 to 100:1 in the cytosol has been calculated to fall between −221 and −236 mV (28). In the endoplasmic reticulum, however, the GSH/GSSG ratio is in the range from 1:1 to 3:1 (28), corresponding to redox potentials of −133 to −165 mV, respectively (for a total glutathione concentration of 1 mM). Thus, an oxidizing power stronger than indicated by the midpoint potential can be attained, particularly in a compartmentalized redox environment.
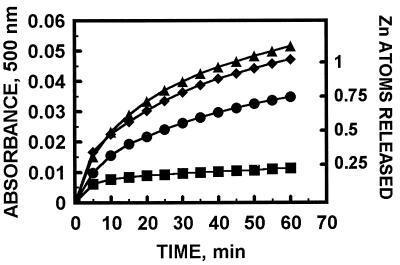
The capacity of biological disulfides to release zinc from MT varies considerably. Cystamine, e.g., releases 93% of zinc from MT in 1 hr, whereas in the same time and at the same concentration GSSG releases only 20% (10, 11). Yet the identity of biological disulfides that might react with MT in vivo is not known with certainty. We speculated that “the physiological reactant could well be a disulfide other than GSSG or a particular disulfide of a given protein or proteins” and that “if GSSG indeed is the cellular disulfide involved, then its reaction with MT might be enzyme catalyzed” (10). Since writing this speculation we have examined protein disulfides as possible candidates. The family of thiol/disulfide oxidoreductases includes thioredoxins and protein disulfide isomerases; we have selected DsbA for study as it has the highest redox potential (−125 mV) (29). Indeed, stoichiometric amounts of this enzyme react with MT and increase zinc release (Fig. 4). This experiment suggests that release of zinc from MT in the cell also could occur through a specific process generating a protein disulfide, presumably as a result of a local signaling event, and obviating associated concerns regarding overall redox changes in the whole cell. While a considerable change of the cellular redox state could affect many other processes, the localized production of a protein disulfide would provide the specificity that would be needed for zinc release. MT has been detected both in the cytosol and in the nucleus. Therefore, its cellular site of action requires further investigation.
Figure 4.
Kinetics of the reaction of MT-2 with protein disulfide isomerase (DsbA). MT (0.75 μM) was incubated with the indicated amounts of DsbA and PAR (90 μM) in 40 mM Hepes, pH 7.4; ▪, MT control; •, one equivalent of DsbA; ⧫, two equivalents of DsbA; ▴, three equivalents of DsbA. Zinc release was measured as described in the legend of Fig. 1.
Redox Reactions of Zinc-Coordinated Thiolate Ligands and the State of Sulfur in Oxidized MT.
One-, two-, and four-electron oxidants react with MT (Table 1). Disulfides; thiyl radicals, which can dimerize to disulfides; or, in the presence of oxygen, sulfenic acids (–SOH), sulfinic acids (–SO2H), and sulfonic acids (–SO3H) are possible oxidation products of thiols. The last have been generated by the oxidation of coordinated thiolate ligands (30), though we are unaware that such oxidation products, including disulfides, can act as metal ligands in a biological zinc complex. Sulfenic acid derivatives of cysteine, however, have been identified in a few enzymes either as stable or functional residues (31). Their stabilization requires the absence of other vicinal thiols. The presence of 20 thiols in MT, therefore, would not favor their existence. Formation of thio acids would change the overall charge of MT. We have found no gel electrophoretic evidence for altered charges in oxidized MT (data not shown). MT oxidation products generated with the hydroxyl radical or superoxide anions can be fully reduced to the native protein (22). This is generally taken as proof that oxidation states higher than disulfides have not formed, though this assumption is in need of reexamination and extension in the light of the present considerations. Moreover, MT reacts with nitric oxide to form disulfides, as demonstrated directly by resonance Raman spectroscopy (23). Mixed disulfides and intra- and intermolecular disulfides are thought to be the product of oxidation of MT with disulfides such as Ellman’s reagent (26) or with ferricyanide (24). Also, the reaction of selenite with thiols results in the production of disulfides (32). Only hydrogen peroxide oxidizes thiols in MT to levels higher than disulfides (21). Thus, most agents listed in Table 1 oxidize thiolates in MT to disulfides. On the basis of current knowledge this oxidation state would seem to be the most attractive biologically, because it is readily reversible to the thiol state. Two structural features of MT strongly favor the formation of disulfides—i.e., the Cys-Cys and Cys-Xaa-Xaa-Cys motifs in the linear protein sequence and the juxtaposition of thiols in the three-dimensional structure. The cluster structure could thereby contain the blueprint for the formation of specific disulfides that might influence the specificity of zinc release. The magnitude and number of interrelationships between such potential disulfides formed among 20 cysteine-containing segments cannot be predicted from the linear sequence of MT. Their establishment and possible interaction in MT would almost certainly turn out to be a formidable experimental task by techniques now feasible. Certainly the reactivities of different thiol/disulfide couples in MT would vary significantly, because their stability is influenced to a large extent by steric effects that can lead to differences of eleven orders of magnitude in equilibrium constants, equivalent to differences of 330 mV in their redox potentials (20).
Corollaries of the Redox Properties of MT.
Establishing the redox potential of MT that allows it to be readily oxidized by cellular constituents not only provides a different approach to determine its function, but beyond that, gives a new perspective to its nature. MT is known to react with radicals and reactive species and has been thought to be an antioxidant (33). This potential is entirely consistent with its ranking as a relatively strong reducing agent on the redox scale (Table 1). Indeed, MT is a chemically reactive molecule and should interact with a large number of agents. If scavenging of reactive species were a physiological function of MT, its capacity to release zinc and the subsequent cellular effects must be accounted for. We believe that MT has specific redox properties for a purpose that selectively controls the release and uptake of zinc rather than being a nonspecific antioxidant that releases the metal randomly and sporadically. Interestingly, the redox properties of MT also raise significant questions about the putative role of MT as a detoxifier of heavy metals. The relatively high reactivity of MT can also cause the dissociation of heavy metals, as has now been shown for nitric oxide-induced release of cadmium from MT (34). This observation must be somewhat disturbing to advocates of the concept that its primary function is to protect living matter against the consequences of metal pollution. Such findings can hardly be considered as support for the belief that MT constitutes a safe repository against pollution with unwelcome heavy metals.
Retrospects and Prospects.
The complete absence of aromatic amino acids or any other components with prominent spectroscopic features, including the optically silent cadmium and zinc atoms, hampered the identification of MT as a protein; its isolation, characterization, and localization; and recognition of its metabolic role(s). More than 30 years passed between its discovery (35) and the establishment of its three-dimensional structure (1, 2). Even the latter achievement failed to provide parameters to guide the experimental search for its function.
It was during these 30 years that the indispensability of zinc to development, growth, and differentiation in all phyla and species gained prominence together with the recognition of zinc-binding signature sequences in enzymes and other proteins that underscore zinc’s biochemical and functional diversity (36). The identification of MT as an oxidoreductive component of a zinc storage and distribution system simultaneously raises questions of how it might operate to ensure homeostasis and fine-tuning. Much as the important and potentially far-reaching role of MT in this regard has just been recognized, the emerging knowledge of zinc metabolism is provocative and timely and suggests the existence of a corollary metabolic system at the intersections of protein, nucleic acid, and zinc chemistry. Clearly, the distribution of zinc and the priorities of its delivery and transfer require as careful an orchestration and coordination as that of the macromolecules to which it lends specificity. It seems reasonable to postulate the existence of a regulatory system at these interfaces designed to achieve fine-tuning of the relevant processes.
The awareness of the importance of zinc in metabolism is counterbalanced by the large gaps in knowledge of the laws that regulate its participation and how they relate to MT. Clearly, zinc binds quite tightly to proteins and hence, free zinc is not readily available in the cell. Rather, a network of transporters seems to coordinate its transmembrane traffic (37–42) at considerable expenditure of energy, apparently ensuring and safeguarding its availability, and to provide it when needed. If zinc is handled almost exclusively while complexed to proteins, how is it then passed from one protein to another and how is it distributed and allocated? Answers to such questions are not obvious, because zinc traffic does not seem to be driven solely by thermodynamic gradients: flux of zinc does not necessarily move it from a site of low binding affinity to other sites of higher binding affinity. Here MT exemplifies how zinc is handled. In MT, zinc is bound quite tightly, with one of the tightest binding constants of zinc in the cell. How then can zinc be transferred from MT to binding sites of lower binding affinity? Our results suggest how this could be achieved.
The mechanisms by which zinc is mobilized from its biological binding sites and distributed among proteins define an important aspect of inorganic biochemistry. While thiol redox chemistry apparently controls the flux of redox-inert zinc, the strong binding of zinc to thiol ligands also suggests that redox-inert zinc might help to control the thiol/disulfide redox state, much as this influence of zinc on redox equilibria has not been widely appreciated. The gap in knowledge has apparently persisted owing to the fact that the properties of zinc have held little attraction for experimentalists because the metal lacks accessible spectroscopic properties and because much of the zinc in the typical adult is contained in MT, a protein whose function was not apparent.
We have reached the threshold of a new chapter in zinc chemistry and its impact on life processes. It now presents us with problems whose time for solution has come.
Acknowledgments
We are greatly indebted to Dr. C. Jacob, Dr. L. J. Jiang, Iwei Yeh for their assistance and participation in the experiments. This work was supported by the Endowment for Research in Human Biology, Inc.
ABBREVIATIONS
- MT
metallothionein
- PAR
4-(2-pyridylazo)resorcinol
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- DsbA
protein disulfide isomerase from E. coli
Footnotes
A commentary on this article begins on page 3333.
Zincon (Kd(Zn) = 1.26 × 10−5 M) is the only suitable chromophoric reagent known to us for the purpose, because it does not react with MT and, hence, does not affect the reactivity of thiols in MT.
References
- 1.Robbins A H, McRee D E, Williamson M, Collett S A, Xuong N H, Furey W F, Wang B C, Stout C D. J Mol Biol. 1991;221:1269–1293. [PubMed] [Google Scholar]
- 2.Arseniev A, Schultze P, Wörgötter E, Braun W, Wagner G, Vašák M, Kägi J H R, Wüthrich K. J Mol Biol. 1988;201:637–657. doi: 10.1016/0022-2836(88)90644-4. [DOI] [PubMed] [Google Scholar]
- 3.Vallee B L. Experientia Suppl. 1979;34:19–40. doi: 10.1007/978-3-0348-6493-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Vallee B L. Experientia Suppl. 1987;52:5–16. doi: 10.1007/978-3-0348-6784-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Vallee B L, Maret W. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 1–27. [Google Scholar]
- 6.Vallee B L. Methods Enzymol. 1991;205:3–7. doi: 10.1016/0076-6879(91)05077-9. [DOI] [PubMed] [Google Scholar]
- 7.Kägi J H R. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 29–55. [Google Scholar]
- 8.Otvos J D, Liu X, Li H, Shen G, Basti M. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 57–74. [Google Scholar]
- 9.Maret W, Larsen K S, Vallee B L. Proc Natl Acad Sci USA. 1997;94:2233–2237. doi: 10.1073/pnas.94.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maret W. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maret W. Neurochem Int. 1995;27:111–117. doi: 10.1016/0197-0186(94)00173-r. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L J, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob C, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vašák M. Methods Enzymol. 1991;205:41–44. doi: 10.1016/0076-6879(91)05082-7. [DOI] [PubMed] [Google Scholar]
- 15.Schäffer A. Methods Enzymol. 1991;205:529–540. doi: 10.1016/0076-6879(91)05137-k. [DOI] [PubMed] [Google Scholar]
- 16.Shaw C F, III, Laib J E, Savas M, Petering D H. Inorg Chem. 1990;29:403–411. [Google Scholar]
- 17.Fliss H, Ménard M. Arch Biochem Biophys. 1992;293:195–199. doi: 10.1016/0003-9861(92)90384-9. [DOI] [PubMed] [Google Scholar]
- 18.Weast R C, editor. Handbook of Chemistry and Physics. 61st Ed. Boca Raton, FL: CRC; 1980. pp. D-155–D-157. [Google Scholar]
- 19.Fasman G D, editor. Handbook of Biochemistry and Molecular Biology: Physical and Chemical Data. 3rd Ed. Vol. 1. Boca Raton, FL: CRC; 1976. pp. 122–130. [Google Scholar]
- 20.Gilbert H F. Methods Enzymol. 1995;251:8–28. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- 21.Quesada A R, Byrnes R W, Krezoski S O, Petering D H. Arch Biochem Biophys. 1996;334:241–250. doi: 10.1006/abbi.1996.0452. [DOI] [PubMed] [Google Scholar]
- 22.Thornalley P J, Vašák M. Biochim Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 23.Kröncke K-D, Fehsel K, Schmid T, Zenke F T, Dasting I, Wesener J R, Bettermann H, Breunig K D, Kolb-Bachofen V. Biochem Biophys Res Commun. 1994;200:1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Onana P, Shaw C F, III, Petering D H. Biochem J. 1996;317:389–394. doi: 10.1042/bj3170389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpkins C, Eudaric P, Torrence C, Yang Z. Life Sci. 1993;53:1975–1980. doi: 10.1016/0024-3205(93)90019-y. [DOI] [PubMed] [Google Scholar]
- 26.Savas M M, Shaw C F, III, Petering D H. J Inorg Biochem. 1993;52:235–249. doi: 10.1016/0162-0134(93)80028-8. [DOI] [PubMed] [Google Scholar]
- 27.Olafson R W. Bioelectrochem Bioenerg. 1988;19:111–125. [Google Scholar]
- 28.Hwang C, Sinskey A J, Lodish H F. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 29.Åslund F, Berndt K D, Holmgren A. J Biol Chem. 1997;272:30780–30786. doi: 10.1074/jbc.272.49.30780. [DOI] [PubMed] [Google Scholar]
- 30.Kuehn C G, Isied S S. Prog Inorg Chem. 1980;27:153–221. [Google Scholar]
- 31.Claiborne A, Miller H, Parsonage D, Ross R P. FASEB J. 1993;7:1483–1490. doi: 10.1096/fasebj.7.15.8262333. [DOI] [PubMed] [Google Scholar]
- 32.Gopalakrishna R, Gundimeda U, Chen Z H. Arch Biochem Biophys. 1997;348:25–36. doi: 10.1006/abbi.1997.0334. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Bremner I. Free Radical Biol Med. 1993;14:325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 34.Misra R R, Hochadel J F, Smith G T, Cook J C, Waalkes M P, Wink D A. Chem Res Toxicol. 1996;9:326–332. doi: 10.1021/tx950109y. [DOI] [PubMed] [Google Scholar]
- 35.Margoshes M, Vallee B L. J Am Chem Soc. 1957;79:4813. [Google Scholar]
- 36.Vallee B L, Falchuk K H. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 37.Palmiter R D, Findley S D. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmiter R D, Cole T B, Findley S D. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 39.Palmiter R D, Cole T B, Quaife C J, Findley S D. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eide D. Curr Opin Cell Biol. 1996;9:573–577. doi: 10.1016/s0955-0674(97)80036-1. [DOI] [PubMed] [Google Scholar]
- 41.Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Nature (London) 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 42.Huang L, Gitschier J. Nat Genet. 1998;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]