Abstract
Purpose
Adult T-cell leukemia/lymphoma induced by human T-cell leukemia virus type 1 (HTLV-1) is usually a fatal lymphoproliferative malignant disease. HTLV-1 Tax protein plays a critical role in HTLV-1-associated leukemogenesis and is an attractive target for vaccine development. Although HTLV-1Tax is the most dominant antigen for HTLV-1-specific CD8+ CTLs in HTLV-1-infected individuals, few epitopes recognized by CD4+ helper T lymphocytes in HTLV-1Tax protein have been described.The aim of the present study was to study T-helper-cell responses to HTLV-1 Tax and to identify naturally processed MHC class II – restricted epitopes that could be used for vaccine development.
Experimental Design
An MHC class II binding peptide algorithm was used to predict potential T-helper cell epitope peptides from HTLV-1 Tax. We assessed the ability of the corresponding peptides to elicit helper T-cell responses by in vitro vaccination of purified CD4+ T lymphocytes.
Results
Peptides Tax191–205 and Tax305–319 were effective in inducingT-helper-cell responses. Although Tax191–205 was restricted by the HLA-DR1 and DR9 alleles, responses to Tax305–319 were restricted by either DR15 or DQ9. Both these epitopes were found to be naturally processed by HTLV-1+ T-cell lymphoma cells and by autologous antigen-presenting cells that were pulsed with HTLV-1Tax+ tumor lysates. Notably, the two newly identified helper T-cell epitopes are found to lie proximal to known CTL epitopes, which will facilitate the development of prophylactic peptide – based vaccine capable of inducing simultaneous CTL andT-helper responses.
Conclusion
Our data suggest that HTLV-1 Tax protein could serve as tumor-associated antigen for CD4+ helper T cells and that the present epitopes might be used for T-cell-based immunotherapy against tumors expressing HTLV-1.
Human T-cell leukemia virus type 1 (HTLV-1) is a member of the mammalian type C oncovirus family and is the only known infectious agent etiologically associated with adult T-cell leukemia/lymphoma (ATLL; refs. 1, 2). Infection with this virus can also lead to a slowly progressive neurologic disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP; ref. 3). It is known that the majority of seropositive individuals remain throughout their lives as asymptomatic carriers due to diverse factors, such as genetic predisposition, the route of infection, and the presence of cytotoxic and helper T-lymphocyte responses that could play a role in the control of disease progression (4–10). Although exposure to this virus usually leads to a persistent infection, once ATLL develops, it progresses rapidly and becomes resistant to conventional chemotherapy, causing a high mortality rate (11). Therefore, it is felt that immunologic approaches, such as T-cell-based vaccines to treat or prevent the HTLV-1-associated malignancy, could be of value.
HTLV-1 possesses four main genomic regions—Gag, Pol, Env, and pX (12). The pX gene encodes the Tax 40-kDa transcriptional regulatory protein, which is known to interact with various cellular transcription factors promoting genetic mutations that inhibit apoptosis of infected host cell and lead to drive host cell proliferation and transformation. It has been reported that Tax is a dominant target antigen recognized by HTLV-1-specific CTLs from asymptomatic carriers, which are capable of killing HTLV-1+ leukemic cells (6, 13, 14). The low frequency of HTLV-1 Tax–specific CTLs observed in ATLL patients probably contributes to HTLV-1-induced leukemogenesis. On the other hand, host immune responses against HTLV-1 tend to be higher in HAM/TSP patients than in ATLL patients (6, 7, 15). Together, these observations suggest that the Tax is a promising tumor-associated antigen (TAA) for the development of prophylactic vaccines for HTLV-I and that augmentation of Tax-specific CTLs in pre-ATL patients could protect them from progressing into ATLL (16).
Both neutralizing antibody and CTL responses could be critical for viral clearance and in eliminating viral-infected and transformed cells (17–19). Because Tax is expressed early in the infection and is essential for the replication and persistence of the virus, vaccines to stimulate Tax-specific T-cell responses would be useful to inhibit both virus replication and viral-induced transformation. Consequently, a large number of MHC class I–restricted CTL epitopes derived from the Tax protein have been identified (6, 13, 14, 20). However, we believe that the CD4+ helper T lymphocytes (HTL) also play an important role in HTLV-1 infection because HTLs are required for clonal expansion of antibody-secreting B cells and induction and maintenance of optimal CTL responses (21, 22). Moreover, in some instances, HTLs can exhibit an effector function by directly recognizing and killing MHC class II+ virus-infected or tumor cells that present peptide epitopes on their surface (23–26). Thus, studies of HTL responses against HTLV-1 Tax could be of interest and elucidating the corresponding MHC class II–binding peptide epitopes will be necessary for designing more effective vaccines than those only inducing CTL responses.
In the present study, we describe two novel MHC class II–restricted epitopes, HTLV-1 Tax191–205 and HTLV-1 Tax305–319, both capable of stimulating in vitro CD4+ HTL responses from HTLV-1-naïve individuals. More importantly, the peptide-reactive HTLs were effective in directly recognizing HTLV-1-infected, MHC class II+ T-cell lymphoma cell lines. In addition, the Tax-specific HTL recognized naturally processed antigen in the form of cell lysates prepared from HTLV-1+ T-cell lymphoma cell lines or from lymphocytes from HAM/TSP patients presented by autologous antigen presenting cells (APC). Interestingly, our described HTL epitopes, Tax191–205 and Tax305–319, are located in close proximity to previously described HLA-B14 and HLA-A24-restricted CTL epitopes, respectively (15, 27, 28), which could facilitate the development of peptide vaccines capable of stimulating both CTLs and HTLs for the treatment/prevention of HTLV-1-associated ATLL.
Materials and Methods
Cell lines
EBV-transformed lymphoblastoid cells (EBV-LCL) were produced from peripheral blood mononuclear cells (PBMC) of HLA-typed volunteers using culture supernatant from the EBV-producing B95-8 cell line (American Type Culture Collection, Manassas, VA). Mouse fibroblast cell lines (L-cells) transfected and expressing individual human MHC class II molecules were kindly provided by Dr. Robert W. Karr (Pfizer Global R&D, New London, CT) and by Dr. Takehiko Sasazuki (Tokyo, Japan). The HTLV-I-infected T-cell lymphoma cell lines TL-Su, TCL-Kan, and HUT102 and T-cell leukemia cell line TL-Hir (HTLV-1 Tax negative) were supplied by the Cell Resource Center for Biomedical Research Institute of Development, Aging, and Cancer (Tohoku University, Sendai, Japan). The HTLV-1-infected T-cell lymphoma cell line OKM-2T was purchased from Dainippon Sumitomo Pharma (Osaka, Japan). The Jurkat T-cell lymphoma cell line (HTLV-I negative) was purchased from American Type Culture Collection. MT2 is an HTLV-I-transformed T cell line that was kindly provided by Dr. Y. Hinuma (Institute of Virus Research, Kyoto University, Kyoto, Japan; ref. 29).
Synthetic peptides
Potential HLA-DR-restricted CD4+ T-cell epitopes were selected from the amino acid sequence of the HTLV-I-Tax using algorithm tables for three HLA-DR alleles, DRB1*0101, DRB1*0401, and DRB1*0701 (30). The predicted peptide epitopes were synthesized by solid phase organic chemistry and purified by high-performance liquid chromatography. The purity (>80%) and identity of peptides were assessed by high-performance liquid chromatography and mass spectrometry, respectively.
In vitro induction of antigen-specific HTL lines with synthetic peptides
The procedure selected for the generation of HTLV-I-Tax-reactive HTL lines using peptide-stimulated PBMCs from five healthy donors whose MHC class II alleles were HLA-DR1/15 DQ5/6, HLA-DR4/15 DQ1/4, HLA-DR4/9 DQ7/8, HLA-DR9/14 DQ5/9, and HLA-DR9/14 DQ7/9. This procedure has been previously described in detail (31). Briefly, dendritic cells were produced in tissue culture from purified CD14+ monocytes (using antibody-coated magnetic microbeads from Miltenyi Biotech, Auburn CA) that were cultured for 7 days at 37°C in a humidified CO2 (5%) incubator in the presence of 50 ng/mL granulocyte macrophage colony-stimulating factor and 1,000 IU/mL interleukin-4. Peptide-pulsed dendritic cells (3 μg/mL for 2 hours at room temperature) were irradiated (4,200 rads) and cocultured with autologous purified CD4+ T cells (Miltenyi Biotech) in 96 round-bottomed-well culture plates. One week later, the CD4+ T cells were restimulated with peptide-pulsed irradiated autologous PBMCs, and 2 days later human recombinant interleukin-2 was added at a final concentration of 10 IU/mL. One week later, the T cells were tested for antigen reactivity using a cytokine release assay as described below. Those cultures exhibiting a significant response of cytokine release to peptide (at least 2.5-fold over background) were expanded in 24- or 48-well plates by weekly restimulation with peptides and irradiated autologous PBMC. Complete culture medium for all procedures consisted of AIM-V medium supplemented with 3% human male AB serum. All blood samples were obtained after the appropriate informed consent.
Measurement of antigen-specific responses with HTL lines
CD4+ T cells (3 × 104 per well) were mixed with irradiated APC in the presence of various concentrations of antigen (peptides, tumor lysates), in 96-well culture plates. APC consisted of either autologous PBMC (1 × 105 per well), HLA-DR-expressing L cells (3 × 104 per well), MHC-typed EBV-LCLs (3 × 104 per well), T-cell lymphoma cell lines (3 × 104 per well), or autologous dendritic cells (5 × 103 per well). The expression of HLA class II molecules on tumor cell lines was evaluated by flow cytometry using anti-HLA class II monoclonal antibody (mAb), TÜ 32, conjugated with fluorescein isothiocynate (BD Biosciences, San Jose, CA). Tumor cell lysates were prepared by three freeze-thaw cycles of 1 × 108 tumor cells that were resuspended in 1 mL serum-free RPMI 1640. Lysates were used as a source of antigen at 5 × 105 cell equivalents/mL. Culture supernatants were collected after 48 hours for measuring antigen-induced lymphokine (IFN-γ) production by the HTL using commercially available ELISA kits (PharMingen, San Diego, CA). To show antigen specificity and MHC restriction, blocking of the antigen-induced proliferative response was assessed by adding anti-HLA-DR mAb L243 (IgG2a, prepared from supernatants of the hybridoma HB-55 obtained from the American Type Culture Collection), anti-HLA-DQ mAb SPV-L3 (IgG2a, Beckman Coulter, Inc., Fullerton, CA), or anti-HLA-A, B, C mAb W6/32(IgG2a, American Type Culture Collection). The effect of antigen-specific antibodies on the response of HTL to HTLV-1 Tax protein was investigated by adding anti-p40 HTLV-1 Tax mAb Lt-4 (IgG3; ref. 32). All antibodies were used at a final concentration of 10 μg/mL throughout the 48-hour incubation period. All assessments of ELISA were carried out at least in triplicate and results correspond to the mean values with SD.
Western blot analysis
One million tumor cells were washed in PBS and lysed in Laemmli buffer. The cell lysate was subjected to electrophoresis in a 4% to 12% NuPage bis-Tris SDS-PAGE gel (Invitrogen, Carlsbad, CA) under reducing condition and then transferred to Immobilon-P (Millipore, Bedford, MA) membrane. The membrane was then blocked in PBS containing 0.01% Tween 20 and 5% nonfat dry milk for 1 hour at room temperature and incubated first with anti-p40 HTLV-1 Tax mAb Lt-4 at 1 μg/mL in blocker overnight at 4°C. After washing, the membrane was incubated with horseradish peroxidase–labeled goat anti-mouse IgG and subjected to the enhanced chemiluminescence assay using the ECL detection system (Amersham, Little Chalfont, Buckinghamshire, UK).
Results
Prediction and selection of potential HTL epitopes for HTLV-1 Tax protein
First, we used the MHC class II peptide binding algorithm developed by Southwood et al. (30) to select potential peptides from the Tax protein that would bind to HLA-DR1, HLA-DR4, and HLA-DR7. With this algorithm, we succeeded in defining the HTL epitopes from multiple TAAs such as proteins that are overexpressed by epithelial tumors, melanomas, and oncogenic viruses (23–25, 31, 33–37). In addition, the work by Southwood et al. (30) reported that some peptides that score high for the DR1, DR4, and DR7 algorithms also have the capacity to bind to additional MHC class II alleles such as DR9, DR13, DR15, and DR52, indicating that this algorithm is effective for identifying highly promiscuous MHC class II binders. In support of this, we reported that HTL responses induced by peptides predicted by this algorithm to be promiscuous MHC class II binders were restricted by DR9, DR15, DR16, DR52, DR53, DQ2, and DQ6. In this study, this prediction system could select eight peptide sequences from HTLV-1 Tax protein as potentially promiscuous MHC class II–restricted T-cell epitopes (data not shown). When examining the position that these peptides occupy within the Tax protein sequences, some peptide sequences were located near previously described CTL epitopes. Specifically, the predicted peptide Tax86–100 was found proximal to a previously described HLA-A2-restricted CTL epitope (Tax80–95), peptide Tax152–166 was found to overlap with another HLA-A2-restricted CTL epitope (Tax151–165), peptide Tax191–205 was found to lie proximal to an HLA-B44-restricted CTL epitope (Tax181–195), and peptide Tax305–319 was found to overlap with an HLA-A24- restricted CTL epitope (Tax301–315; refs. 27, 28, 38). Because it would be advantageous for a single peptide vaccine to simultaneously elicit effective CTL and HTL responses, we decided to focus our efforts in studying those potential HTL epitopes that contained proximal CTL epitopes.
Isolation of HTLV-1 Tax peptide–reactive T-helper cells from healthy individuals
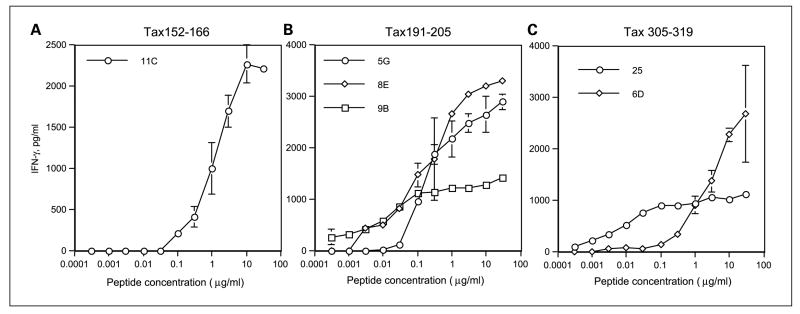
Peptides Tax86–100, Tax152–166, Tax191–205, and Tax305–319 were synthesized and evaluated for their ability to elicit T-helper responses by in vitro vaccination of PBMC from five healthy volunteers. Purified CD4+ T cells were stimulated in vitro with individual each peptide pulsed autologous dendritic cells. One week later, after restimulating with γ-irradiated autologous PBMCs and peptides, microcultures were tested for their ability to produce IFN-γ upon stimulation with peptide-loaded autologous PBMCs. Positive microcultures that exhibited at least a 2.5-fold increase in IFN-γ production to peptide compared with in the absence of peptide were expanded in 48- or 24-well plate for further analysis, and in some cases T-cell clones were also isolated by limiting dilution. As shown in Fig. 1, three of the four peptides (Tax152–166, Tax191–205, and Tax 305–319) were able to elicit peptide- specific HTLs that responded to their corresponding peptide in a dose-dependent manner as presented by autologous APCs.
Fig. 1.
Induction of HTL responses using predicted peptide epitopes derived from HTLV-1Tax. HTL clones induced with peptideTax152–166 (A; clone 11C from DR4/9, DQ7/8 donor), peptideTax191–205 (B; clone 5G from DR1/15, DQ5/6 donor; clone 8E from DR4/9, DQ7/8 donor; and clone 9B from DR9/14, DQ7/9 donor), or peptide Tax305–319 (C; clone 25 from DR4/15, DQ1/4 donor and clone 6D from DR9/14, DQ5/9 donor) were tested for their capacity to recognize autologous PBMC as APCs in the presence of various concentrations of peptide. Points, mean of triplicate determinations; bars, SD. Points without bars had SD <10% the value of the mean. Results are representative of at least two experiments that were done with the same samples.
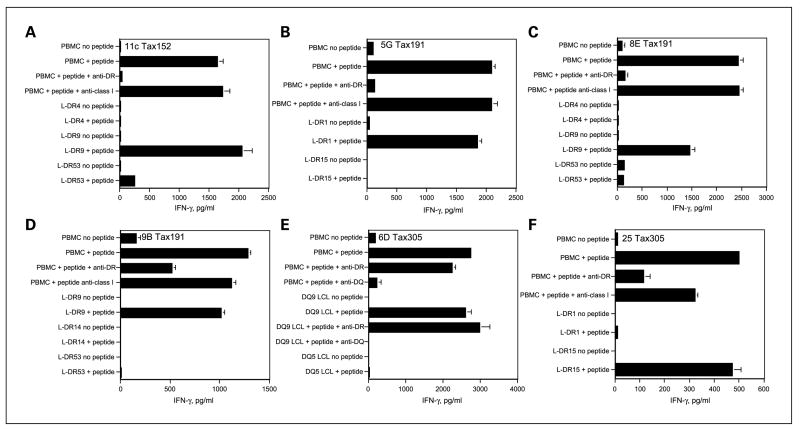
HLA restriction analysis of HTLV-1 Tax peptide–reactive HTLs
Peptide-reactive HTL clones were isolated by limiting dilution and were analyzed for their MHC class II restriction pattern. Peptide-induced lymphokine production was evaluated using a panel of HLA-DR-transfected mouse fibroblasts (L cells) or EBV-LCLs (homozygous for MHC class II), which were used as APCs. In addition, anti-HLA-DR (L243), anti-HLA-DQ (SPV-L3), or anti-HLA class I mAbs (W6/32) were used to inhibit the response of these HTL to antigen. The results presented in Fig. 2 show that peptide Tax152–166 could be presented to the T cells in the context of HLA-DR9 (HTL clone 11C), and the recognition of peptide Tax191–205 by the HTL clones was restricted by HLA-DR1 (for clone 5G) and HLA-DR9 (for clone 8E and 9B). HTL clones 6D and 25, which were elicited by peptide HTLV-1 Tax305–319, responded to peptide presented by HLA-DQ9 and DR15, respectively. These results indicate that at least two of the three peptides (Tax191–205 and Tax305–319) behave as promiscuous HTL epitopes because more than one MHC class II allele can present them.
Fig. 2.
MHC restriction analysis of peptide-reactive Tax152–166 HTL (A), peptide-reactiveTax191–205 HTL (B–D), and peptide-reactiveTax305–319 HTL (E and F). MHC class II restriction molecules were identified using antibody blocking: anti-DR L243, anti-DQ SPV-L3, or anti-class I W6/32 (negative control) all used at 10 μg/mL. Experiments were done using irradiated peptide-pulsed autologous PBMCs as APC. In addition, HTL responses were also evaluated using various MHC-typed APCs (L cells transfected with individual HLA-DR genes or allogeneic EBV-LCLs homozygous for DQ molecules) to define the restricting MHC class II alleles. A, peptideTax152–166-reactive HTL clone 11C recognized antigen in the context of HLA-DR9. B, peptideTax191–205-reactive HTL clone 5G recognized antigen in the context of HLA-DR1. C and D, peptideTax191–205-reactive HTL clone 8E and HTL clone 9B recognized antigen in the context of HLA-DR9. E, peptideTax305–319-reactive HTL clone 6D recognized antigen in the context of HLA-DQ9. F, peptideTax305–319-reactive HTL clone 25 recognized antigen in the context of HLA-DR15. Columns, mean of triplicate determinations; bars, SD. Columns without bars had SD <10% the value of the mean. Results are representative of two experiments that were done with the same samples.
Recognition of HTLV-1-infected T-cell lymphoma cells by peptide-reactive HTLs
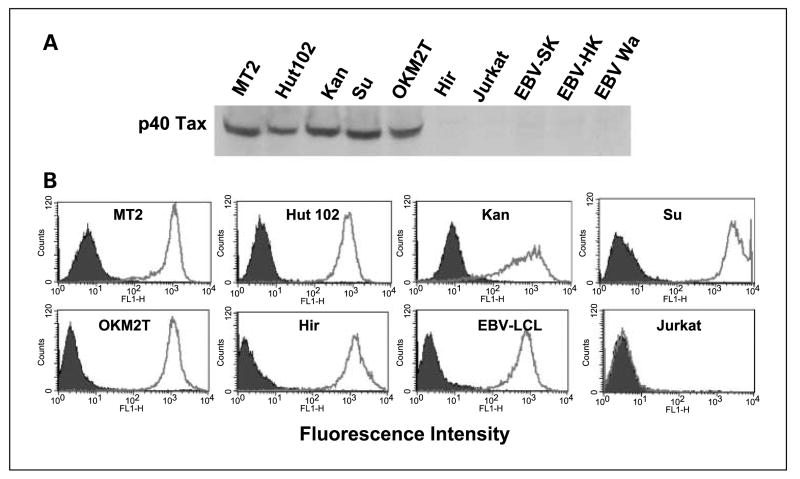
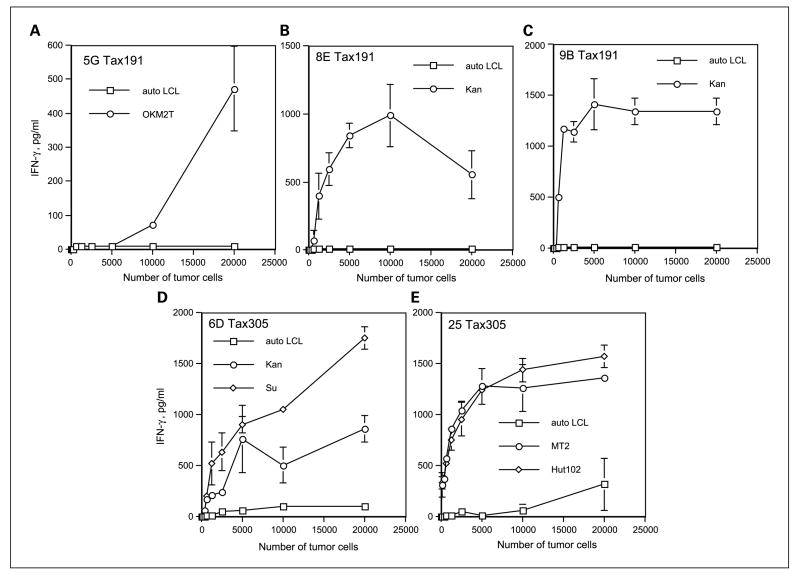
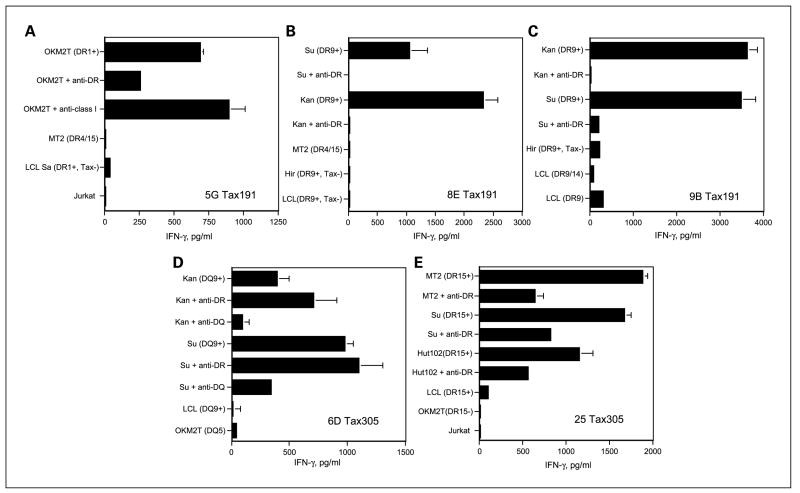
We proceeded to assess whether the peptide-reactive HTLs would be able to directly recognize intact HTLV-1-infected T-cell lymphoma cells that endogenously express the Tax gene product. This would signify that these peptide epitopes can be expressed on the MHC class II molecules of the tumor cells. Before performing these experiments, we first examined whether our HTLV-1-positive T-cell lymphoma cell lines expressed the Tax protein and cell surface MHC class II molecules. As shown in Fig. 3, all five HTLV-1-infected T-cell lymphoma cells, MT2 (DR4/15, DQ6/8), Hut102 (DR15, DQ6), Kan (DR4/9, DQ8/9), Su (DR9/15, DQ6/9), and OKM2T (DR1, DQ5) expressed the HTLV-1 Tax protein and surface HLA class II molecules. On the other hand, T-cell leukemia cell line Hir (DR4/9, DQ6/9) and the EBV-LCLs all expressed cell surface MHC class II molecules but did not express the Tax protein. The Jurkat T-cell lymphoma was negative for both HLA class II and Tax, allowing us to use some of these cell lines as negative control APCs. The data presented in Fig. 4 indicates that HTL clones reactive with peptides Tax191–205 and Tax305–319 were effective in directly recognizing the MHC class II matched, HTLV-1 Tax–expressing T-cell lymphoma cell lines. These HTL clones did not react with autologous EBV-LCLs, indicating that the response was antigen specific. Moreover, the recognition of HTLV-1+ T-cell lymphoma cells by these HTLs was inhibited by the corresponding anti-HLA-DR mAb (for HTLs 5G, 8E, 9B, and 25) or anti-HLA-DQ mAb (for HTL 6D), confirming that antigen recognition is through the presentation of peptide by MHC class II molecules (Fig. 5). However, our results showed that Tax152–166-reactive HTL clone 11C did not recognize HTLV-1-infected T-cell lymphoma cells, suggesting that this epitope is not processed and presented by intact HTLV-1+ tumor cells (data not shown).
Fig. 3.
Expression of HTLV-1Tax protein and cell surface expression of MHC class II molecules in human tumor cell lines. A, Western blot analysis was done using a HTLV-1Tax – specific mAb Lt-4 as described in Materials and Methods. HTLV-1Tax protein was detected in the indicated HTLV-1-infectedT-cell lymphoma cell lines but not in theT-cell leukemia cell lines Hir and Jurkat and in the EBV-LCLs. The HTLV-1Tax protein has a mass of 40 kDa. B, the expression of HLA class II molecules on tumor cells was evaluated by flow cytometry using anti-HLA class II mAb Tu 39 conjugated with fluorescein isothiocynate (thick-line open histograms). Staining with the isotype-negative control (filled histograms). All tumor cell lines, except JurkatT-cell lymphoma, expressed high levels of surface MHC class II molecules.
Fig. 4.
Antitumor reactivity of the peptideTax191–205-specific HTLs 5G, 8E, and 9B (A–C) and peptide Tax305–319-specific HTLs 6D and 25 (D and E). HTLs (3 × 10 per well) were tested at various APC numbers for their capacity to recognize the HTLV-1-positive T-cell lymphoma cells and autologous EBV-LCLs (HTLV-1negative). The peptide-specific HTLs secreted IFN-γ as the result of recognizing antigen in the MHC class II – matched HTLV-1+ T-cell lymphoma cell lines but not HTLV-1-negative autologous EBV-LCLs. Points, mean of triplicate samples; bars, SD. Points without bars had SD <10% the value of the mean. Results are representative of two experiments that were done under the same conditions.
Fig. 5.
Antigen specificity of direct tumor recognition by HTLV-1Tax – reactive HTLs. The peptideTax191–205-reactive HTLs 5G, 8E, and 9B (A–C) and peptide Tax305–319-reactive HTLs 6D and 25 (D and E) produced IFN-γ as the result of recognizing antigen on restricted HLA class II allele – positive HTLV-1-infected T-cell lymphoma cell line presenting the naturally processed epitope. Production of IFN-γ was inhibited by anti-HLA-DR mAb L243 (A–D) or anti-HLA-DQ mAb SPV-L3 (E) and could not be detected using HTLV-1-negative EBV-LCLs, HLA class II fully allogeneic HTLV-1+ T-cell lymphoma cell lines, and HTLV-1-negative HLA class II JurkatTcell lymphoma. Columns, means of triplicate determinations; bars, SD. Columns without bars had SD <10% the value of the mean. Representative of two experiments that were done under the same conditions.
Indirect recognition of naturally processed viral antigen by autologous dendritic cells
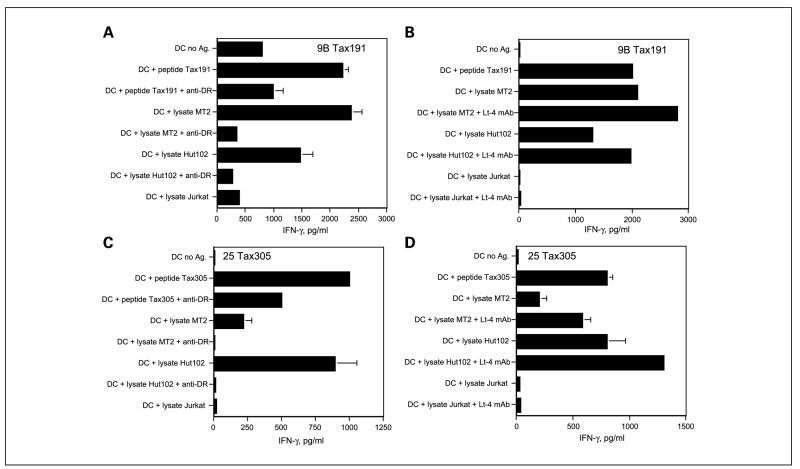
We have observed that some peptide-elicited HTLs can only respond with peptide-pulsed APCs but not with APCs that are fed protein or tumor lysates that requires antigen processing via the MHC class II endocytic pathway. Thus, we proceeded to evaluate whether the HTLV-1 Tax peptide-reactive HTLs would be able to recognize the naturally processed viral antigen. These experiments were done using autologous dendritic cells as APCs that were fed with freeze/thaw lysates derived from HTLV-1 Tax+ T-cell lymphomas. As shown in Fig. 6A and C, both the Tax191–205-reactive HTL line 9B and Tax305–319-reactive HTL clone 25 responded efficiently to autologous dendritic cells pulsed with lysates from HTLV-1+ T-cell lymphoma cells (MT2, Hut102) but not with dendritic cells pulsed with lysates from Jurkat cells (HTLV-I negative). In addition, these responses were inhibited by anti-HLA-DR mAb treatment, indicating that both Tax191–205 and Tax305–319 were presented by MHC class II surface molecules. On the other hand, Tax152–166-reactive HTL clone 11C, Tax191–205-reactive HTL clones 5G and 8E, and Tax305–319-reactive HTL clone 6D were not able to recognize the naturally processed antigen from tumor lysates presented by the autologous dendritic cells (data not shown). In these experiments, we observed that the T-cell responses to the tumor lysates could be substantially enhanced by the addition of anti-HTLV-1 Tax mAb, Lt-4 (Fig. 6B and D), presumably by increasing the delivery of antigen to the APCs via the Fc receptor–mediated endocytosis of immune complexes containing the relevant viral protein (39, 40). The enhancement by the anti-Tax mAbs was antigen specific because no effects were observed with lysates from Jurkat.
Fig. 6.
HTLV-1Tax – reactive HTL recognize naturally processed exogenous antigen presented by autologous dendritic cells.The peptideTax191–205-reactive HTL line 9B (A) and peptide Tax305–319-reactive HTL clone 25 (C) were able to recognize the HTLV-1-positive T-cell lymphoma cell lysates (MT2, Hut102) but not HTLV-1-negative JurkatT-cell lymphoma cell lysate presented by autologous dendritic cells. In addition, antigen recognition in all cases was inhibited by anti-DR mAb L243 at 10 μg/mL. The responses of these HTLs to the naturally processed antigen derived from tumor lysate were enhanced by adding HTLV-1Tax – specific mAb Lt-4 at10 μg/mL using tumor lysates from HTLV-1+ MT2 or Hut102 but not HTLV-1-negative Jurkat lysate (B and D). Columns, mean of triplicate determinations; bars, SD. Columns without bars had SD <10% the value of the mean. Representative of two experiments that were done under the same conditions and with the same samples.
Recognition of naturally processed viral epitope from lysates prepared from HAM/TSP patient’s PBMC by autologous dendritic cells
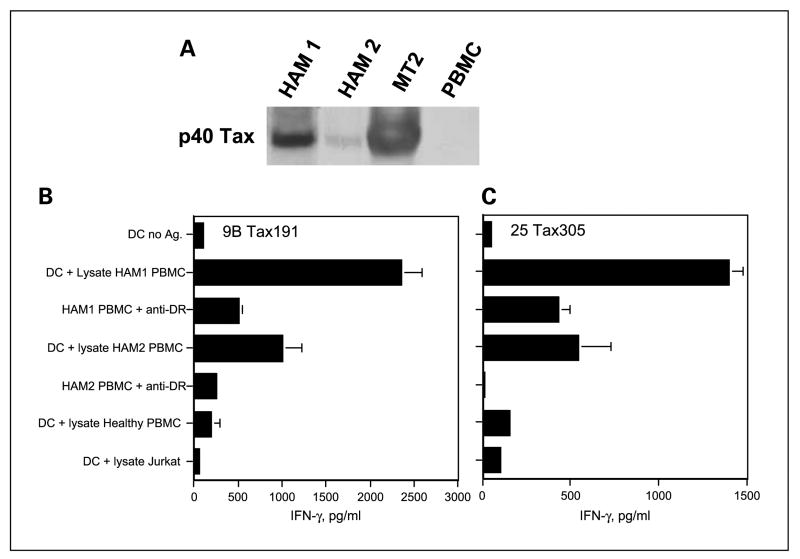
The results presented above, demonstrating indirect recognition of naturally processed antigens via autologous dendritic cells, were obtained using lysates derived from HTLV-1-infected cultured cell lines. We also examined whether T-cell epitopes Tax191–205 and Tax305–319 could be generated using lysates derived from “primary HTLV-1+ PBMC” as source of antigen. It has been noted that expression of HTLV-1 viral antigens, including Tax in HTLV-1 infected cells, could increase by in vitro culture (10, 26, 41). Moreover, it is known that the expression of Tax mRNA in PBMCs isolated from HAM patients reaches a maximum at 24 hours after in vitro culture (41). Thus, we cultured PBMCs of patients with HAM for 24 hours before preparing the lysates. First, we confirmed by Western blot analysis that the lysates from PBMC derived from two HAM patients (HAM1, HAM2) expressed the HTLV-1 Tax protein (Fig. 7A). On the other hand, the Tax protein was not detected in lysates derived from PBMC of HTLV-1 naïve individuals. The HTLV-1+ MT2 cells served as a positive control. Next, we assessed whether the Tax191–205 and Tax305–319-specific HTLs would be able to recognize the autologous dendritic cells pulsed with lysates prepared from HAM patient’s PBMCs. Figure 7B and C shows that the autologous dendritic cells that were fed with PBMC lysates from both HAM1 and HAM2 patients were effective in stimulating the responses of the HTLs in an antigen-specific manner.
Fig. 7.
Recognition of naturally processed HTLV-1 Tax protein derived from HAM patient’s PBMC. A, HTLV-1Tax protein content was evaluated by Western blot analysis using antibody Lt-4 specific for HTLV-1Tax to determine whether the HTLV-1 Tax protein was present in PBMCs from two HAM patients (HAM1and HAM2).The HTLV-1Tax protein was detected in cell lysates prepared from both HAM patient’s PBMC and MT2 cells (positive control HTLV-1+ lymphoma) but not in PBMC from a healthy individual. B and C, the peptideTax191–205-specific HTL line 9B and peptide Tax305–319-reactive HTL clone 25 recognized the lysates prepared from HAM patient’s PBMC and the response was inhibited by anti-DR mAb L243 at 10 μg/mL. Results correspond to triplicate samples. Columns without bars had SD <10% the value of the mean. Representative of two experiments that were done with the same samples.
Discussion
CTLs have been proposed to be main effector cells against many pathogenic viruses, including HTLV-1. Thus, a large number of CTL epitopes derived from components of HTLV-1, such as the Tax, Env, Gag, and Pol proteins, have been identified and some of these are being considered as potential subunit peptide-based vaccine candidates (6, 13, 14, 20, 42). However, recent findings indicate that the presence of antigen-specific CD4+ helper T lymphocytes is necessary for the optimal induction and maintenance of antigen-specific CTL and for the development of effective antibody responses. Using an experimental animal model for HTLV-1, rats that were inoculated with HTLV-1-infected T-cell lymphoma cells exhibited antiviral CD4+ T helper responses, indicating that the HTLV-1-infected tumor cells were taken up by APCs and that the naturally processed epitopes were effectively processed and presented via the MHC class II pathway (21). Moreover, it has been reported that HTLV-1-reactive CD4+ T cells are present in HAM/TSP patients and the T-helper cells produce Th1 cytokines (interleukin-2, IFN-γ) in responses to antigen stimulation (10, 26). In view of the above, it is clear that the identification of MHC class II–restricted tumor and viral HTL epitopes in addition to CTL epitopes will be a critical step in the development of effective vaccines for this virus. As a key to the above problem, we previously showed that three HTLV-1 Env peptides—Env196–210, Env317–331, and Env384–398—induced HTLs that recognized intact HTLV-1+ T-cell lymphoma cells in vitro and that some HTLs were able to directly recognize and lyse HTLV-1+ T-cell lymphoma cells (35). Interestingly, HTL epitopes Env196–210 and Env384–398 are located closely to previously described antibody epitopes (17, 18, 43).
In the present study, we identified the two naturally processed MHC class II–restricted HTL epitopes from the HTLV-1 Tax protein (Tax191–205 and Tax305–319). A number of groups have presented evidence that HTLV-1 Tax is a major target for HTLV-1-specific CTLs (6, 13). Yamano et al. (44) indicated that CTL activity against Tax is predominantly detected in HAM/TSP patients and in some patients, the frequency of Tax-specific CTLs can be as high as 30% of all CD8+ T cells in peripheral blood. Moreover, recent work by Harashima et al. (28) reported that after successful treatment by hematopoietic stem cell transplantation of an ATLL patient, most of CTL activity (>60%) was toward Tax. In general, although HAM/TSP patients suffer from chronic neurologic disorders, those patients exhibiting high CTL responses against HTLV-1 Tax rarely progress to ATLL. On the other hand, the low levels of Tax-specific CTLs observed in ATLL patients and the observation that these CTL are capable of killing leukemic cells in vitro led us to hypothesize that the absence of Tax-reactive CTLs in some ATLL patients may be associated with leukemogenesis (5–7). Furthermore, a recent report showed that when HTLV-1-specific CTLs were induced in ATLL patients, it was difficult to expand them in vitro, suggesting the presence of an immunosuppression or tolerance effect. Alternatively, the cause could be a lack of CD4+ T helper cells, which are required for effective CTL expansion (45). It should be mentioned that although CD4+ T cells are main viral reservoir of HTLV-1, some reports indicate that HTLV-1-specific CD4+ T cells in HAM/TSP patients are not themselves infected with transcriptionally active HTLV-1 virus, implying that they would not be targeted by HTLV-1-specific CTL (26).
Recognition of naturally processed antigen has become the hallmark that a predicted T-cell epitope will be relevant for vaccine/immunotherapy development. Here, we have shown that T-cell responses induced with peptide Tax191–205 by the DR1-restricted HTL 5G and the DR9-restricted HTL 8E were accompanied by effective direct recognition of HTLV-1-expressing T-cell lymphoma cell lines. However, both of these HTLs were not able to recognize autologous APCs that were fed with tumor cell lysates. In contrast, the DR9-restricted HTL 9B, also specific for peptide Tax191–205, was able to respond with both HTLV-1-positive T-cell lymphoma cells and professional APCs that exogenously captured tumor lysate. Similarly, although the Tax305–319-specific HTL 25 could recognize both HTLV-1 Tax– expressing T-cell lymphoma directly and antigen processed by autologous dendritic cells, the HTL 6D, which is specific for the same epitope, was able to react only with the HTLV-1 Tax–expressing tumor cell lines and not with the lysate-fed APCs. These apparently contradictory findings could be explained by differences in the affinity for antigen (Fig. 1B and C). Regardless, these results show that both Tax191-205 and Tax305–319 HTL epitopes can be processed endogenously by tumor cells and exogenously by professional APCs but that the recognition pattern may differ among various HTLs.
We showed that adding HTLV-1 Tax–specific mAb (Lt-4) with tumor lysates enhanced T-cell responses (Fig. 6). Dendritic cells express several receptors for the Fc portion of IgG (FcγR), which mediate internalization of antigen-IgG complexes and promote efficient MHC class II–restricted antigen presentation. In the past, we observed that HTL responses to hepatitis B virus antigen was enhanced by adding antibodies due to an enhancement of antigen delivery to APC by FcR-mediated endocytosis (39). It is known that antibodies to HTLV-1 Tax protein are detected in most HTLV-1-infected subjects (46). The production of specific antibodies could induce formation of immune complexes between antigens derived from virus-infected cells (or tumor cells), which could be efficiently taken up by FcR on APCs to more efficiently activate CD4+ T cells in vivo.
Early studies by Ohashi et al. (47, 48) showed effective antitumor effects induced by HTLV-1 Tax–encoded DNA vaccination in a rat model system. More recently, the same group reported that immunization with oligopeptides from HTLV-1 Tax elicited CTL responses that could eradicate fatal HTLV-1-infected T-cell lymphomas (21). In these studies, it was shown that HTLV-1-specific CD4+ helper T lymphocytes and CD8+ CTLs collaborated to eradicate HTLV-1+ T-cell lymphomas, indicating that CD4+ helper T-cell responses are necessary for induction of effective CTL responses against HTLV-1-associated tumor (21). Because peptides are relatively easy to synthesize and safe to administer, a peptide-based T-cell vaccination strategy would be an attractive approach against HTLV-1-associated malignancies. One would expect that vaccines capable of activating both CTLs and HTLs should be more effective than vaccines that only target CTL responses. Because the two HTL epitopes described here, Tax191–205 and Tax305–319 lie proximal to previously described CTL epitopes (27, 28, 38), one could envision the possibility of using a single peptide of relatively small size (<25 to ~30 residues) to stimulate both CTL and HTL responses in individuals expressing the appropriate MHC class I and class II alleles.
Acknowledgments
Grant support: NIH grants R01CA80782, P50CA91956, and R01CA103921 (E. Celis) and Ministry of Education, Sports, and Culture of Japan grant-in-aid 18590360 (H. Kobayashi).
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JM, Harrod R, Franchini G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus type-1 (HTLV-1) Int J Exp Pathol. 2001;82:135–47. doi: 10.1046/j.1365-2613.2001.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Usuku K, Sonoda S, Osame M, et al. HLA haplo-type-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23(Suppl):S143–50. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 5.Kannagi M, Sugamura K, Kinoshita K, Uchino H, Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killerTcell line derived from an adult T cell leukemia/lymphoma patient. J Immunol. 1984;133:1037–41. [PubMed] [Google Scholar]
- 6.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–8. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 7.Parker CE, Daenke S, Nightingale S, Bangham CR. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found inhealthy seropositives as well as in patients with tropical spastic paraparesis. Virology. 1992;188:628–36. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- 8.Kannagi M, Matsushita S, Harada S. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int J Cancer. 1993;54:582–8. doi: 10.1002/ijc.2910540411. [DOI] [PubMed] [Google Scholar]
- 9.Manca F, Li Pira G, Fenoglio D, et al. Recognition of humanT-leukemia virus (HTLV-1) envelope by human CD4+ T-cell lines from HTLV-1seronegative individuals: specificity and clonal heterogeneity. Blood. 1995;85:1547–54. [PubMed] [Google Scholar]
- 10.Goon PK, Igakura T, Hanon E, et al. Human T cell lymphotropic virus type I (HTLV-I)-specific CD4+ T cells: immunodominance hierarchy and preferential infection with HTLV-I. JImmunol. 2004;172:1735–43. doi: 10.4049/jimmunol.172.3.1735. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Tomonaga M, Fukuda H, et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol. 2001;113:375–82. doi: 10.1046/j.1365-2141.2001.02737.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Greene WC. Molecular biology of the type I humanT-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J Clin Invest. 1991;87:761–6. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannagi M, Harada S, Maruyama I, et al. Predominant recognition of humanTcell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int Immunol. 1991;3:761–7. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- 14.Elovaara I, Koenig S, Brewah AY, et al. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567–73. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannagi M, Matsushita S, Shida H, Harada S. CytotoxicTcell response and expression of the target antigen in HTLV-I infection. Leukemia. 1994;8(Suppl 1):S54 –9. [PubMed] [Google Scholar]
- 16.Kannagi M, Harashima N, Kurihara K, et al. Adult T-cell leukemia: future prophylaxis and immunotherapy. Expert RevAnticancerTher. 2004;4:369–76. doi: 10.1586/14737140.4.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Baba E, Nakamura M, Tanaka Y, et al. Multiple neutralizing B-cell epitopes of humanT-cell leukemia virus type 1 (HTLV-1) identified by human monoclonal antibodies. A basis for the design of an HTLV-1 peptide vaccine. J Immunol. 1993;151:1013–24. [PubMed] [Google Scholar]
- 18.Baba E, Nakamura M, Ohkuma K, et al. A peptide-based human T cell leukemia virus type I vaccine containing T and B cell epitopes that induces high titers of neutralizing antibodies. J Immunol. 1995;154:399–412. [PubMed] [Google Scholar]
- 19.Lairmore MD, DiGeorge AM, Conrad SF, et al. HumanT-lymphotropic virus type 1peptides in chimeric and multivalent constructs with promiscuous T-cell epitopes enhance immunogenicity and overcome genetic restriction. J Virol. 1995;69:6077–89. doi: 10.1128/jvi.69.10.6077-6089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pique C, Ureta-Vidal A, Gessain A, et al. Evidence for the chronic in vivo production of humanTcell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J Exp Med. 2000;191:567–72. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanabuchi S, Ohashi T, Koya Y, et al. Regression of humanT-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. JNatl Cancer Inst. 2001;93:1775–83. doi: 10.1093/jnci/93.23.1775. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara K, Harashima N, Hanabuchi S, et al. Potential immunogenicity of adult Tcell leukemia cells in vivo. Int J Cancer. 2005;114:257–67. doi: 10.1002/ijc.20737. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Lu J, Celis E. Identification of helper T-cell epitopes that encompass or lie proximal to cytotoxic T-cell epitopes in the gp100 melanoma tumor antigen. Cancer Res. 2001;61:7577–84. [PubMed] [Google Scholar]
- 24.Kobayashi H, Song Y, Hoon DS, Appella E, Celis E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 2001;61:4773–8. [PubMed] [Google Scholar]
- 25.Omiya R, Buteau C, Kobayashi H, Paya CV, Celis E. Inhibition of EBV-induced lymphoproliferation by CD4(+) Tcells specific for an MHC class II promiscuous epitope. J Immunol. 2002;169:2172–9. doi: 10.4049/jimmunol.169.4.2172. [DOI] [PubMed] [Google Scholar]
- 26.Goon PK, Hanon E, Igakura T, et al. High frequencies ofTh1-type CD4(+) Tcells specific to HTLV-1Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood. 2002;99:3335–41. doi: 10.1182/blood.v99.9.3335. [DOI] [PubMed] [Google Scholar]
- 27.Parker CE, Nightingale S, Taylor GP, Weber J, Bangham CR. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–8. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harashima N, Kurihara K, Utsunomiya A, et al. Graft-versus-Tax response in adult T-cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res. 2004;64:391–9. doi: 10.1158/0008-5472.can-03-1452. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi I, Kubonishi I, Yoshimoto S, et al. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemicTcells. Nature. 1981;294:770–1. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 30.Southwood S, Sidney J, Kondo A, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363–73. [PubMed] [Google Scholar]
- 31.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60:5228–36. [PubMed] [Google Scholar]
- 32.Tanaka Y, Yoshida A, Tozawa H, et al. Production of a recombinant human T-cell leukemia virus type-I trans-activator (tax1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax1 antigen. Int J Cancer. 1991;48:623–30. doi: 10.1002/ijc.2910480423. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Omiya R, Ruiz M, et al. Identification of an antigenic epitope for helperT lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8:3219–25. [PubMed] [Google Scholar]
- 34.Kobayashi H, Omiya R, Sodey B, et al. Identification of naturally processed helper T-cell epitopes from prostate-specific membrane antigen using peptide-based in vitro stimulation. Clin Cancer Res. 2003;9:5386–93. [PubMed] [Google Scholar]
- 35.Kobayashi H, Nagato T, Yanai M, et al. Recognition of adult T-cell leukemia/lymphoma cells by CD4+ helper T lymphocytes specific for human T-cell leukemia virus type I envelope protein. Clin Cancer Res. 2004;10:7053–62. doi: 10.1158/1078-0432.CCR-04-0897. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi H, Nagato T, Oikawa K, et al. Recognition of prostate and breast tumor cells by helper T lymphocytes specific for a prostate and breast tumor-associated antigen, TARP. Clin Cancer Res. 2005;11:3869–78. doi: 10.1158/1078-0432.CCR-04-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi H, Nagato T, Aoki N, et al. Defining MHC class II T helper epitopes for WT1 tumor antigen. Cancer Immunol Immunother. 2006;55:850–60. doi: 10.1007/s00262-005-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kannagi M, Shida H, Igarashi H, et al. Target epitope in theTax protein of human T-cell leukemia virus type I recognized by class I major histocompatibility complex-restricted cytotoxic T cells. J Virol. 1992;66:2928–33. doi: 10.1128/jvi.66.5.2928-2933.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celis E, Chang TW. Antibodies to hepatitis B surface antigen potentiate the response of humanT lymphocyte clones to the same antigen. Science. 1984;224:297–9. doi: 10.1126/science.6231724. [DOI] [PubMed] [Google Scholar]
- 40.Regnault A, Lankar D, Lacabanne V, et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. JExp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanon E, Hall S, Taylor GP, et al. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95:1386–92. [PubMed] [Google Scholar]
- 42.Sundaram R, Sun Y, Walker CM, et al. A novel multivalent human CTL peptide construct elicits robust cellular immune responses in HLA-A*0201 transgenic mice: implications for HTLV-1vaccine design. Vaccine. 2003;21:2767–81. doi: 10.1016/s0264-410x(03)00179-8. [DOI] [PubMed] [Google Scholar]
- 43.Palker TJ, Riggs ER, Spragion DE, et al. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J Virol. 1992;66:5879–89. doi: 10.1128/jvi.66.10.5879-5889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamano Y, Nagai M, Brennan M, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) Tcells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- 45.Arnulf B, Thorel M, Poirot Y, et al. Loss of the ex vivo but not the reinducible CD8+ T-cell response toTax in human T-cell leukemia virus type 1-infected patients with adult T-cell leukemia/lymphoma. Leukemia. 2004;18:126–32. doi: 10.1038/sj.leu.2403176. [DOI] [PubMed] [Google Scholar]
- 46.Rudolph DL, Coligan JE, Lal RB. Detection of antibodies to trans-activator protein (p40taxI) of human T-cell lymphotropic virus type I by a synthetic peptide-based assay. Clin Diagn Lab Immunol. 1994;1:176–81. doi: 10.1128/cdli.1.2.176-181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohashi T, Hanabuchi S, Kato H, et al. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J Virol. 1999;73:6031–40. doi: 10.1128/jvi.73.7.6031-6040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohashi T, Hanabuchi S, Kato H, et al. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J Virol. 2000;74:9610–6. doi: 10.1128/jvi.74.20.9610-9616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]