Abstract
The phosphorylation state of the tumor suppressor protein BRCA1 is tightly associated with its functions including cell cycle control and DNA repair. Protein kinases involved in the DNA damage checkpoint control, such as ATM, ATR, and hCds1/Chk2, have been shown to phosphorylate and activate BRCA1 upon DNA damage. We reported previously that protein phosphatase 1α(PP1α) interacts with and dephosphorylates hCds1/Chk2-phosphorylated BRCA1. This study demonstrates the identification of a PP1-binding motif 898KVTF901 in BRCA1. Mutation or deletion of critical residues in this PP1-binding motif substantially reduces the interaction between BRCA1 and PP1α. PP1α can also dephosphorylate ATM and ATR phosphorylation sites in BRCA1 and may serve as a general regulator for BRCA1 phosphorylation. Unlike wild-type BRCA1, expression of the PP1 non-binding mutant BRCA1 protein in BRCA1-deficient cells failed to enhance survival after DNA damage. Taken together, these results suggest that interaction with PP1α is important for BRCA1 function.
Keywords: BRCA1, protein phosphatase 1, kinases, DNA damage
Introduction
The breast and ovarian tumor suppressor BRCA1 is a nuclear phosphoprotein consisting of 1863 amino acids with an apparent molecular weight of ∼220,000. Many studies indicate that BRCA1 plays an important role in fundamental cellular functions, such as transcriptional regulation, chromatin remodeling, cell cycle checkpoint control, DNA repair and recombination, as well as regulation of the centrosome function [1,2].
BRCA1 is expressed and phosphorylated in late G1 and S phases of the cell cycle [3]. BRCA1 is also phosphorylated by checkpoint kinases hCds1/Chk2, ATM and ATR in response to DNA damage induced by ionizing radiation (IR), UV, or chemicals. BRCA1 interacts with proteins involved in the repair of DNA double-strand breaks, such as BRCA2, Rad51, and the Rad50/MRE11/NBS complex, and may play a role as a sensor and/or an effector in the DNA damage repair pathway [1]. Phosphorylation of BRCA1 occurs primarily at multiple serine residues, such as S1524 and S1423 by ATM and ATR [4-8] and S988 by hCds1/Chk2 [9], and may be involved in cell cycle arrest and DNA repair. Failure of BRCA1 phosphorylation leads to hypersensitivity to IR as demonstrated in cultured cells [4,9]. Thus, phosphorylation state is important for BRCA1 functions.
BRCA1 is primarily hypophosphorylated in mitosis and G0/G1 phases of the cell cycle [10]. In addition, hypophosphorylated BRCA1 is associated with mitotic centrosomes through an interaction with γ-tubulin, an essential component of the centrosome [11]. Therefore, given that BRCA1 is phosphorylated at G1 and S, BRCA1 must undergo dephosphorylation during the G2/M progression. We have demonstrated that PP1α but not PP2A specifically dephosphorylates BRCA1, which is phosphorylated by hCds1/Chk2. PP1α catalytic subunit interacts with BRCA1 through binding to the BRCA1 fragment 4 (BF4), which contains amino acids 759-1064. In addition, BRCA1 inhibits PP1α activity in vitro [12].
PP1 is one of the four major types of serine/threonine phosphatases (PP1, PP2A, PP2B, and PP2C) which participate in cell cycle control and ensure correct chromosome segregation during mitosis by dephosphorylating critical target proteins. It has been reported that PP1, which contains three major subtypes, α, γ1 and δ, is involved in mitotic progression [13], checkpoint activation [14] and DNA repair [15], as well as recovery from DNA damage checkpoint arrest [16]. PP1α, γ1 and δ have distinct subcellular localization [17]. Interestingly, BRCA1 and PP1α are both localized in the nucleus and the centrosome [11,17]. The PP1 holoenzyme is composed of a catalytic subunit and regulatory subunits. The PP1 catalytic subunit interacts with numerous regulatory subunits, which target the catalytic subunit to specific subcellular localization. Regulatory subunits do not share obvious sequence similarities. However, they usually contain a consensus motif (R/K)(V/I)xF, which binds to a hydrophobic groove on the surface of the PP1 catalytic subunit. Mutation of this consensus motif, e.g. substitution of phenylalanine with alanine or deletion of the motif, greatly reduces the binding between regulatory subunits and PP1 catalytic subunit. Several substrates of PP1 also contain the consensus PP1-binding motif [13]. Given that BRCA1 is a substrate as well as a regulator of PP1α [12], it is possible that BRCA1 may have a PP1-binding motif. Here we sought to identify a PP1-binding site in BRCA1 and investigated the biological and functional significance of the interaction between BRCA1 and PP1α.
Materials and methods
Construction of vectors
GST-BF4F901A, GST-BF4DEL and GST-BF4S988E were constructed using the site-specific mutagenesis by overlap extension, a two-step PCR approach, to generate mutant BF4 DNA fragments, which were digested with BamHI and SalI, and then subcloned into pGEX-5X-3 (Amersham Biosciences, Piscataway, NJ). GST-BF4F901A was used as the template for PCR cloning of GST-BF4F901AS988E (GST-BF4FASE) using the same mutagenesis approach. pcDNABF4 and pcDNAPP1α were constructed by inserting PCR products amplified from the cDNA clones into pcDNA3.1(−)/Myc-His A (pcDNAmyc, Invitrogen, Carlsbad, CA). pcDNABF4F901A, pBRCA1GFPF901A, pBRCA1GFPDEL, pBRCA1GFPS988E and pBRCA1GFPF901AS988E (pBRCA1GFPFASE) were constructed by replacing a KpnI-ScaI fragment (∼830 bp) in pcDNABF4 or pBRCA1GFP [18] with that from GST-BF4F901A, GST-BF4DEL, GST-BF4S988E or GST-BF4FASE, which contained the mutations. All plasmid clones were sequence-verified.
GST pull-down assay
GST fusion proteins were expressed and purified as described previously [12,18]. COS-7 cells were lysed in NETN buffer (150 mM NaCl, 1mM EDTA, 20 mM Tris, pH 8.0, 0.5% NP40) with protease inhibitors. GST fusion protein (1-2 μg) was incubated with 1 mg of COS-7 cell lysate or 0.5 μl of recombinant PP1α catalytic subunit (2.5 U/μl, ∼15 units/ug; New England BioLabs, Ipswich, MA), and glutathione Sepharose beads in GST binding buffer for 2 h at 4°C, washed with L buffer (PBS with 0.1% NP40 and 0.1% Triton X-100), and subjected to SDS-PAGE as described [12,18]. Western blot analysis was performed using a goat polyclonal PP1α antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell culture and transfection
Cell lines were purchased from ATCC. COS-7 and 293T cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum, ES-2 cells were cultured in McCoy's 5A medium supplemented with 1.5 mM L-glutamine and 10% fetal bovine serum, and HCC1937 cells were grown in RPMI supplemented with 10% fetal bovine serum. Transfection was performed using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) for COS-7, 293T, and ES-2 cells, and the FuGENE 6 Transfection reagent (Roche, Indianapolis, IN) for HCC1937 cells according to the manufacturers' instructions. Cells were harvested for analysis 24 to 48 h after transfection.
Coimmunoprecipitation and Western analysis
COS-7 cells were lysed in L buffer supplemented with protease inhibitors, followed by brief sonication. Immunoprecipitation (IP) was performed using cleared cell extract containing 1-2 mg of protein and anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-GFP antibody (Clontech, Mountain View, CA), in the presence of protein G beads as described before [18]. Western blot analysis was performed using BRCA1 Ab-2 (Lab Vision/NeoMarkers, Fremont, CA) to detect BF4myc, BRCA1 antibody MS110 (Calbiochem, San Diego, CA) to detect BRCA1GFP and PP1α C-19 and anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA) to detect PP1αGFP and PP1αmyc. Quantitative analysis was performed using Quantity One software (BioRad, Hercules, CA)
Immunoprecipitation and phosphatase assay
293T cells were untreated, irradiated with 5 Gy of γ-radiation or treated with 1 mM of hydroxyurea (HU) for 1h, and then lysed in PP1 lysis buffer (1% TritonX-100, 150 mM NaCl, 10 mM Tris 7.5, 1 mM EDTA, 1 mM EGTA) supplemented with protease inhibitors, and phosphatase inhibitors (1 mM sodium orthovanadate, 50 mM sodium fluoride). Cleared lysates were incubated overnight at 4°C with BRCA1 Ab-1 (MS110) and Ab-3 antibodies (Calbiochem, San Diego, CA) in the presence of protein G beads. Protein G beads were then sequentially washed with PP1 lysis buffer and phosphatase reaction buffer and used for the phosphatase assay. Recombinant PP1α catalytic subunit or purified PP2A (Upstate, Temecula, CA) was added to the BRCA1 immunoprecipitates at 0.001 U/μl in a 25-μl reaction containing 50 mM Tris, pH 7.0, 0.1 mM Na2EDTA, 5 mM DTT, 0.01% Brij 35, and 1 mM MnCl2. The reactions were incubated for 30 min at 30°C, and then subjected to 4-12% SDS-PAGE and Western blot analysis using rabbit polyclonal phospho-specific BRCA1 antibodies for S1423 and S1524 (Calbiochem, San Diego, CA).
Cell survival assay
HCC1937 cells were transfected with the GFP control vector (pEGFP-N1) or pBRCA1GFP vectors (WT, F901A, DEL, S988E, or FASE) using the FuGENE 6 Transfection reagent (Roche, Indianapolis, IN), plated into 24-well plates 24 h after transfection, and were either untreated or irradiated with 4 Gy of γ-radiation one day afterwards. Cells were subjected to the MTT assay (Roche, Indianapolis, IN) one week after irradiation. Results were measured by optical density (OD) 550-650 nm, which has a linear correlation with viable cell number on a log scale. Relative cell survival was calculated as the OD ratio of IR-treated cells and untreated control cells. Cells were plated in triplicate. Two-sided t-tests were used to assess the significance of differential cell survival. P-values < 0.05 were considered statistically significant.
Results
BRCA1 contains a putative PP1-binding motif
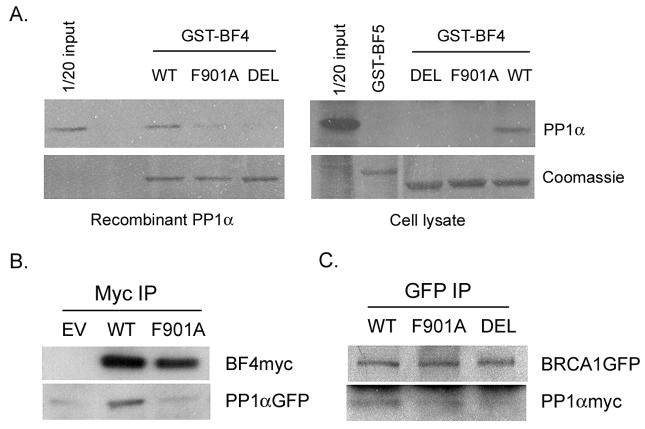
The BF4 region (amino acids 759-1064) of BRCA1 interacts with the catalytic subunit of PP1α [12]. Sequence search revealed that BF4 contains a putative PP1-binding motif 898KVTF901. Substitution of phenylalanine 901 with alanine (F901A) or deletion of the 898KVTF901 motif (DEL) in GST-BF4 was achieved by site-specific mutagenesis. The GST pull-down assay was performed by incubation of wild-type and the mutant GST-BF4 with either recombinant PP1α catalytic subunit or COS-7 cell lysate as a source for PP1α. As illustrated in Fig. 1A, F901A mutation or deletion of the 898KVTF901 motif substantially reduced the interaction between BF4 and PP1α, suggesting that 898KVTF901 may be a PP1-binding motif in BRCA1.
Fig. 1.
A PP1-binding motif 898KVTF901 in the BF4 region of BRCA1 is required for its interaction with PP1α. (A) The GST pull-down assay was performed using recombinant PP1α or COS-7 cell lysate, followed by Western analysis of PP1α. Coomassie staining of GST-fusion proteins on Western blots is shown in the bottom panels. GST-BF5 is a negative control. (B) COS-7 cells were cotransfected with pcDNABF4 (WT or F901A) and PP1αGFP, followed by IP and Western analysis. Wild-type but not the F901A mutant BF4myc coimmunoprecipitated PP1αGFP. EV, the empty vector pcDNAmyc. (C) COS-7 cells were cotransfected with pBRCA1GFP (WT, F901A, or DEL) and pcDNAPP1α. Coimmunoprecipitation between BRCA1GFP and PP1αmyc was significantly reduced when the PP1-binding site was mutated or deleted.
Mutation of the 898KVTF901 motif greatly reduces the interaction between BRCA1 and PP1α in vivo
Coimmunoprecipitation was performed to verify that the 898KVTF901 motif in BF4 was critical for its interaction with PP1α in vivo. COS-7 cells were cotransfected with pcDNAmyc, pcDNABF4 or pcDNABF4F901A and PP1αGFP [12] and harvested for IP using an anti-myc antibody to bring down BF4 proteins. Western analysis revealed that BF4 interacted with PP1αGFP in vivo, whereas substitution of phenylalanine 901 with alanine significantly reduced this interaction (Fig. 1B).
To test whether mutation of the 898KVTF901 motif in full-length BRCA1 protein also affected its binding to PP1α, COS-7 cells were cotransfected with pBRCA1GFP [18], pBRCA1GFPF901A, or pBRCA1GFPDEL and pcDNAPP1α, followed by IP using an anti-GFP antibody to bring down BRCA1 proteins. As shown in Fig. 1C, coimmunoprecipitation of PP1αmyc with BRCA1GFP was reduced to 35 or 10% respectively when the putative PP1-binding motif in BRCA1 was mutated (F901A) or deleted (DEL). Thus, both in vitro and in vivo data confirm that the 898KVTF901 motif in BRCA1 is a PP1-binding site. However, it remains a possibility that other minor PP1-binding sites with lower affinity [13] may be present in BRCA1.
PP1α dephosphorylates BRCA1 at multiple serine sites
BRCA1 is phosphorylated at multiple serine sites after DNA damage. We have reported previously that PP1α can dephosphorylate S988 in BRCA1 [12]. To determine whether PP1αcould dephosphorylate S1524 or S1423, hyperphosphorylated BRCA1 was immunoprecipitated from irradiated or HU-treated 293T cells, subjected to phosphatase assay, and followed by Western analysis using phospho-specific BRCA1 antibodies that recognize ATM and ATR phosphorylation sites, S1524 and S1423. As shown in Fig. 2A, recombinant PP1α, but not purified PP2A, efficiently dephosphorylated S1524 and S1423. Hyperphosphorylated and hypophosphorylated BRCA1 bands can be resolved on SDS-PAGE, with the hypophosphorylated band migrating more rapidly and the hyperphosphorylated band migrating slower. Western analysis using a BRCA1 antibody MS110 confirmed that more hypophosphorylated BRCA1 protein was present in the IP samples incubated with recombinant PP1α (Fig. 2A). Altogether, these results indicate that PP1α specifically dephosphorylates BRCA1 at multiple serine sites, including S988 [12], S1423, and S1524.
Fig. 2.
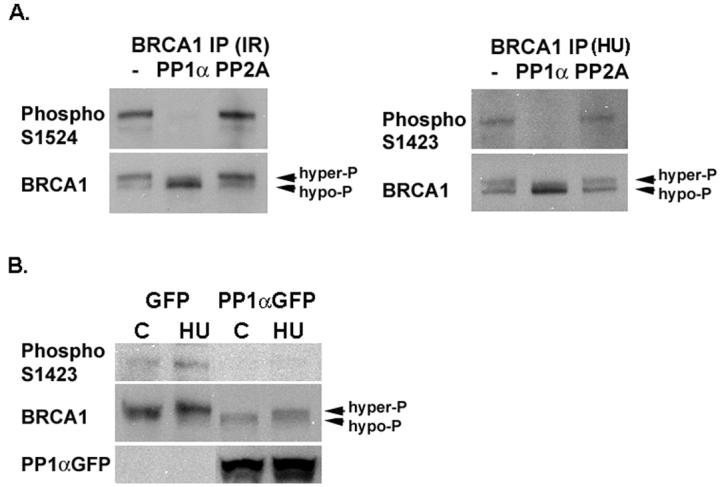
PP1α dephosphorylates BRCA1 at multiple serine residues. (A) Recombinant PP1α but not PP2A dephosphorylates S1524 and S1423 of BRCA1. Hyperphosphorylated BRCA1 was immunoprecipitated from 293T cells irradiated with 5 Gy of γ-radiation (IR) or treated with 1 mM of HU for 1h, and used for the phosphatase assay using buffer only (−), recombinant PP1α, or purified PP2A. (B) Overexpression of PP1αGFPdecreases BRCA1 S1423 phosphorylation both at the basal level (C) and after DNA damage induced by HU.
We reported previously that overexpression of PP1αGFP partially inhibited hyperphosphorylation of BRCA1 induced by IR in 293T cells as determined by mobility-shift [12]. To determine whether PP1α could dephosphorylate S1423 phosphorylation induced by HU, 293T cells were transfected with PP1αGFP expression vector or the GFP control vector pEGFP-N1, and either left untreated or treated with 1 mM of HU for 1 h two days after transfection. Western analysis using anti-phospho S1423 antibody indicated that overexpression of PP1αGFP reduced S1423 phosphorylation in both untreated and HU-treated cells compared to cells expressing GFP (Fig. 2B). When normalized with total BRCA1 protein detected by MS110 antibody, relative levels of S1423 phosphorylation in untreated GFP expressing cells, HU-treated GFP expressing cells, untreated PP1αGFP expressing cells and HU-treated PP1αGFP expressing cells were 1, 1.56, 0.32, and 0.33, respectively. Taken together, both in vitro and in vivo data indicate that PP1α dephosphorylates multiple serine sites in BRCA1 and may serve as a general regulator for BRCA1 phosphorylation.
Mutation of the PP1-binding motif affects phosphorylation of BRCA1
To determine whether the PP1 non-binding BRCA1 protein had a different phosphorylation pattern from wild-type protein, cells transfected with pBRCA1GFP or pBRCA1GFPDEL were incubated with or without 1 mM of HU for 1 h and harvested for Western analysis using anti-phospho S1423 and anti-BRCA1 MS110 antibodies. As illustrated in Fig. 3, S1423 phosphorylation of the PP1-non-binding DEL mutant protein was apparently increased compared to wild-type BRCA1GFP at the basal level and after HU treatment. This result further supports that PP1α regulates BRCA1 phosphorylation.
Fig. 3.
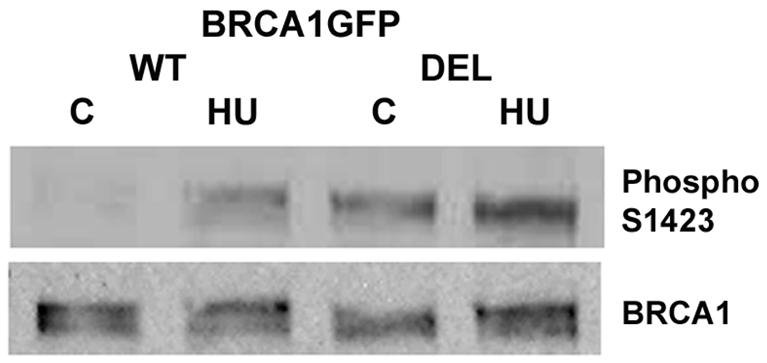
Phosphorylation of the PP1 non-binding mutant BRCA1 protein is increased at the basal level and after HU treatment. Similar results were obtained from several cell lines. An example from the ES-2 ovarian cancer cell line is shown.
PP1 non-binding mutant BRCA1 proteins fail to increase survival after DNA damage in HCC1937 cells
It has been reported that expression of wild-type BRCA1 protein protects the BRCA1-mutated HCC1937 cells from DNA damage induced by IR, whereas mutant BRCA1 proteins with critical serine residues changed to alanine, fail to confer resistance to γ-radiation in HCC1937 cells [4,9]. These findings demonstrate the importance of BRCA1 phosphorylation in the DNA damage response.
To determine if mutation or deletion of the PP1-binding 898KVTF901 motif affected BRCA1 function in cell survival following DNA damage, HCC1937 cells were transfected with the pEGFP-N1 control vector or pBRCA1GFP vectors (WT, F901A, or DEL), either left untreated or irradiated with 4 Gy of γ-radiation two days after transfection, and subjected to the MTT assay one week later. Relative cell survival was calculated as the OD ratio of IR-treated cells and untreated control cells. Consistent with reports by others [4,9], expression of wild-type BRCA1GFP increased relative cell survival when compared to the GFP control (P = 0.0062, t test, two-sided). In contrast F901A and DEL mutants did not increase cell survival (P = 0.00032 and 0.00037 respectively compared to wild-type BRCA1GFP; t test, two-sided)(Fig. 4). Although the increase in cell survival by wild-type BRCA1GFP was only 40% partly due to low transfection efficiency in HCC1937 cells (30-50%), the result was statistically significant and reproducible, suggesting that the PP1-binding domain is required for BRCA1 to restore survival of the BRCA1-mutated HCC1937 cells after DNA damage.
Fig. 4.
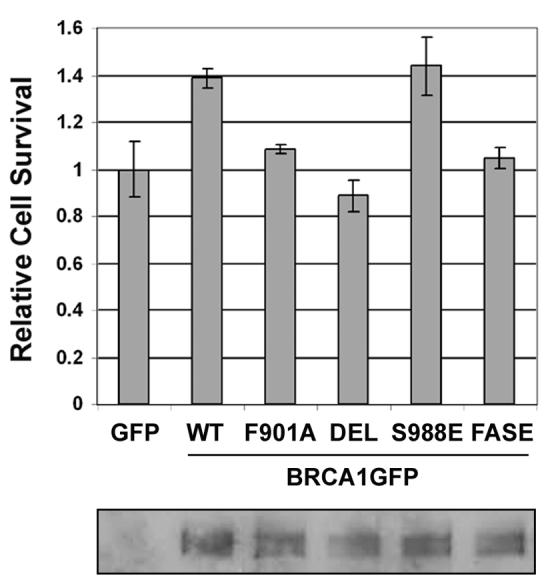
The PP1-binding domain is required for BRCA1 to protect cells from γ-radiation. The upper panel shows relative cell survival and the lower panel displays expression of BRCA1GFP proteins, which were immunoprecipitated by a GFP antibody followed by Western analysis using a BRCA1 antibody.
It has been demonstrated that BRCA1S988E (substitution of serine 988 with glutamic acid), which mimics S988 phosphorylation, restored cell survival in HCC1937 cells as efficient as wild-type BRCA1 [9]. Consistent with the report, expression of BRCA1GFPS988E enhanced cell survival the same as wild-type BRCA1GFP. However, expression of the F901AS988E (FASE) mutant BRCA1GFP protein failed to enhance cell survival in HCC1937 cells (P = 0.0066, S988E vs. FASA, t test, two-sided)(Fig. 4), indicating that F901A is dominant over S988E. This observation is consistent with the results shown above (Fig. 2) that PP1α regulates phosphorylation of multiple serine residues in BRCA1 and may have a broader effect on regulating BRCA1 function.
Discussion
The phosphorylation state of BRCA1 is important for its function. To date, studies have been focused on BRCA1 phosphorylation by protein kinases, while the involvement of protein phosphatases in the regulation of BRCA1 phosphorylation and function has not been extensively explored.
Our previous studies have revealed a novel finding regarding an interaction between PP1α and BRCA1 [12] Here we report the identification of a PP1-binding motif 898KVTF901 in BRCA1. The binding between PP1α and BRCA1 was significantly reduced when phenylalanine 901 was mutated or the 898KVTF901 motif was deleted as demonstrated by the GST pull-down assays and coimmunoprecipitation experiments (Fig. 1). Since the consensus PP1-binding motif is common to all PP1 isoforms [13], it is conceivable that BRCA1 may interact with other PP1 isoforms. Indeed, Winter et al. reported recently the interaction of all three major PP1 isoforms with BRCA1 and also identification of the 898KVTF901 motif in BRCA1 by a GST pull-down assay [19].
We have demonstrated that PP1αcandephosphorylate hCds1/Chk2 phosphorylation site S988 [12]. To extend this finding, we demonstrate here that PP1α can also dephosphorylate ATM and ATR phosphorylation sites S1423 and S1524 (Fig. 2). The mutant BRCA1 protein lacking a PP1-binding motif was highly phosphorylated at the basal level and after DNA damage (Fig. 3). Unlike the wild-type or S988E protein, expression of the PP1-non-binding F901A and DEL mutant BRCA1 proteins in the BRCA1-mutated HCC1937 cells failed to enhance survival after DNA damage (Fig. 4). Introduction of F901A into BRCA1S988E (glutamic acid mimics S988 phosphorylation), also failed to increase cell survival (Fig. 4), consistent with the finding that PP1α can dephosphorylate serine residues other than S988 in BRCA1 (Fig. 2). Thus, proper phosphorylation and dephosphorylation of multiple serine residues in BRCA1 is important for BRCA1 function.
A recent study revealed that S988 but not S1423/S1524 phosphorylation regulates the repair of DNA double-strand breaks by promoting error-free homologous recombination and inhibiting error-prone nonhomologous recombination [20]. This finding further emphasizes the functional significance of hCds1/Chk2 mediated phosphorylation of BRCA1 at S988 in DNA repair, while other evidence supports that phosphorylation of S1423 is important for the G2/M checkpoint control [21]. Expression of PP1-non-binding mutant BRCA1 proteins in HCC1937 cells may fail to enhance cell survival due to prolonged G2/M block and consequently growth arrest after DNA damage. Thus, dephosphorylation of BRCA1 by PP1 may be important in the DNA damage response by serving as a signal for completion of DNA repair, inactivating the G2 checkpoint, and playing a role in the G2/M progression. It is also likely that inhibitory phosphorylation sites may exist in BRCA1. Thus, disruption of PP1-BRCA1 interaction may lead to inactivation of BRCA1 function. Future study will dissect the underlying mechanisms.
We have reported that the PPP1CA gene encoding the catalytic subunit of PP1α and located on humn chromosome 11q13, is amplified and overexpressed in oral squamous cell carcinoma cell lines [22], suggesting a role of PP1α deregulation in tumor development and progression. Data presented here indicate that an appropriated cycle of phosphorylation and dephosphorylation is important for BRCA1 function. Given that BRCA1 mutation only accounts for a small percentage of breast and ovarian cancer cases, deregulation of PP1α and other PP1 isoforms may also contribute to breast and ovarian tumorigenesis.
Acknowledgments
The author thanks Dr. Jeff Holt for providing the BRCA1 cDNA, Dr. David Livingston for the GST-BRCA1 constructs, Dr. Hsien-Bin Huang for the PP1α cDNA, and Dr. David Virshup for helpful comments and suggestions. This study was supported by National Cancer Institute grant CA111436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 2.Deng C-X. Roles of BRCA1 in centrosome duplication. Oncogene. 2002;21:6222–6226. doi: 10.1038/sj.onc.1205713. [DOI] [PubMed] [Google Scholar]
- 3.Ruffner H, Verma IM. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc. Natl. Acad. Sci. U S A. 1997;94:7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of BRCA1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 5.Gatei M, Scott SP, Filippovitch I, Soronika N, Lavin MF, Weber B, Khanna KK. Role for ATM in DNA damage-induced phosphorylation of BRCA1. Cancer Res. 2000;60:3299–3304. [PubMed] [Google Scholar]
- 6.Chen J. Ataxia Telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60:5037–5039. [PubMed] [Google Scholar]
- 7.Tibbetts RS, Cortez D, Brumbaugh KM, Scully R, Livingston D, Elledge SJ, Abraham RT. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatei M, Zhou B-B, Hobson K, Scott S, Young D, Khanna KK. Ataxia Telangiectasia mutated (ATM) kinase and ATM and Rad3 related kinase mediate phosphorylation of BRCA1 at distinct and overlapping sites. J. Biol. Chem. 2001;276:17276–17280. doi: 10.1074/jbc.M011681200. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JE, Smith M, Tonkinson JL, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–809. [PubMed] [Google Scholar]
- 11.Hsu L-C, White RL. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. U S A. 1998;95:12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Virshup DM, White RL, Hsu L-C. Regulation of BRCA1 phosphorylation by interaction with protein phosphatase 1α. Cancer Res. 2002;62:6357–6361. [PubMed] [Google Scholar]
- 13.Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 14.Guo CY, Brautigan DL, Larner JM. Ionizing radiation activates nuclear protein phosphastase-1 by ATM-dependent dephosphorylation. J. Biol. Chem. 2002;277:41756–41761. doi: 10.1074/jbc.M207519200. [DOI] [PubMed] [Google Scholar]
- 15.Herman M, Ori Y, Chagnac A, Weinstein T, Korzets A, Zevin D, Malachi T, Gafter U. DNA repair in mononuclear cells: role of serine/threonine phosphatases. J. Lab. Clin. Med. 2002;140:255–262. doi: 10.1067/mlc.2002.127738. [DOI] [PubMed] [Google Scholar]
- 16.den Elzen NR, O'Connell MJ. Recovery from DNA damage checkpoint arrest by PP1-mediated inhibition of Chk1. EMBO J. 2004;23:908–918. doi: 10.1038/sj.emboj.7600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreassen PR, Lacroix FB, Villa-Moruzzi E, Margolis RL. Differential subcellular localization of protein phosphatase-1 α, γ1, and δ isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 1998;141:1207–1215. doi: 10.1083/jcb.141.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu L-C, Doan TP, White RL. Identification of a γ-tubulin-binding domain in BRCA1. Cancer Res. 2001;61:7713–7718. [PubMed] [Google Scholar]
- 19.Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. The interaction of PP1 with BRCA1 and analysis of their expression in breast tumors. BMC Cancer. 2007;7:85. doi: 10.1186/1471-2407-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, Chung JH, Powell SN, Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol. Cell. Biol. 2004;24:708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu B, Kim S-T, Kastan MB. Involvement of BRCA1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 2001;21:3445–3450. doi: 10.1128/MCB.21.10.3445-3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu L-C, Huang X, Seacholtz S, Potter DM, Gollin SM. Gene amplification and overexpression of protein phosphatase 1α in oral squamous cell carcinoma cell lines. Oncogene. 2006;25:5517–5526. doi: 10.1038/sj.onc.1209563. [DOI] [PubMed] [Google Scholar]