Abstract
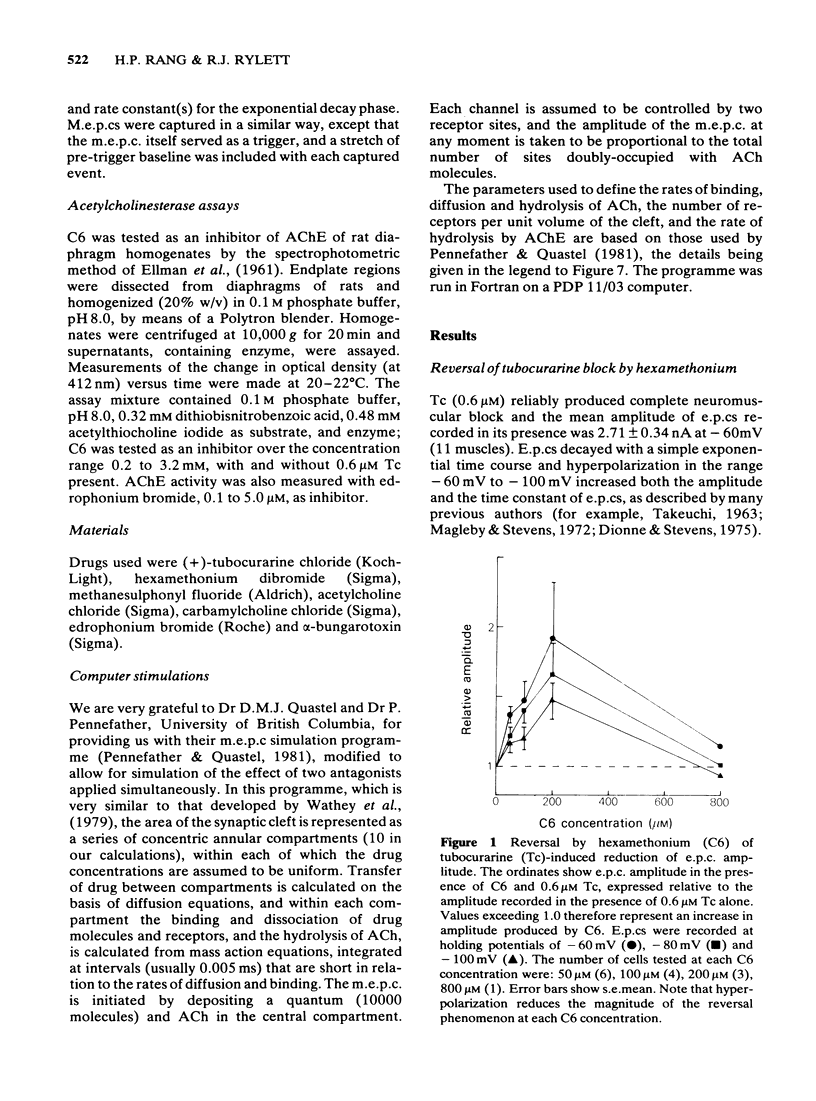
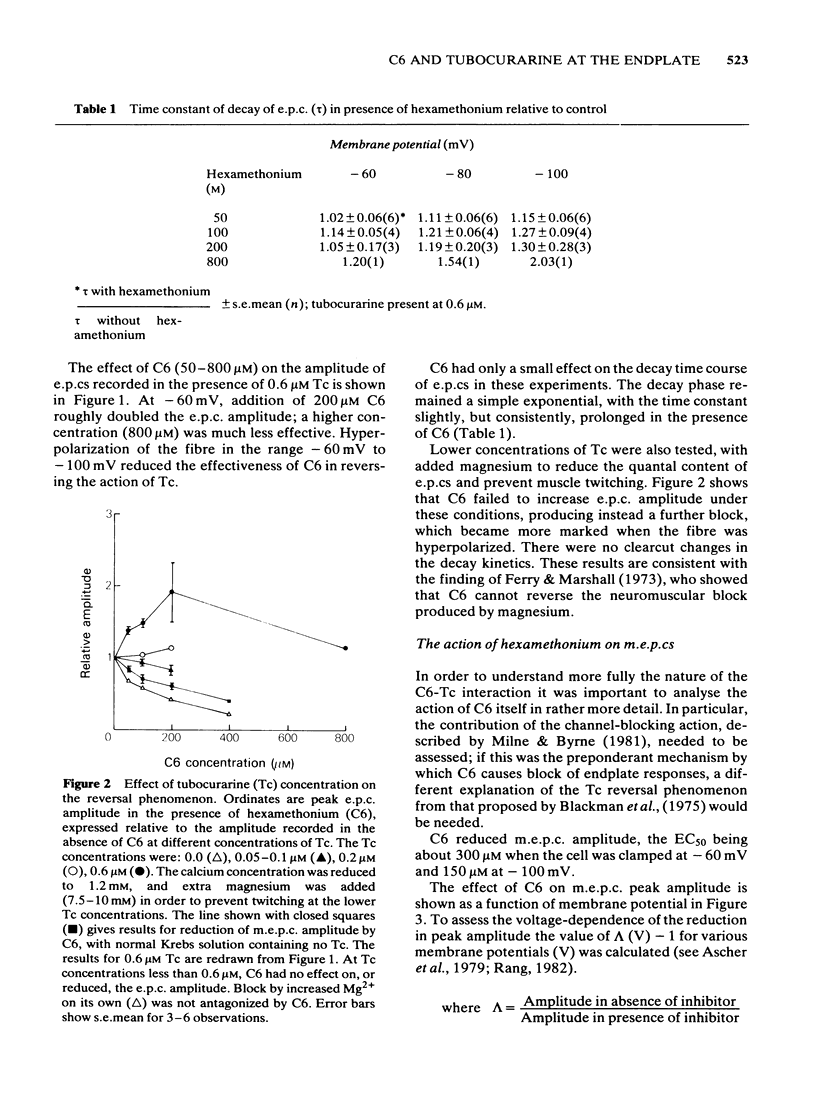
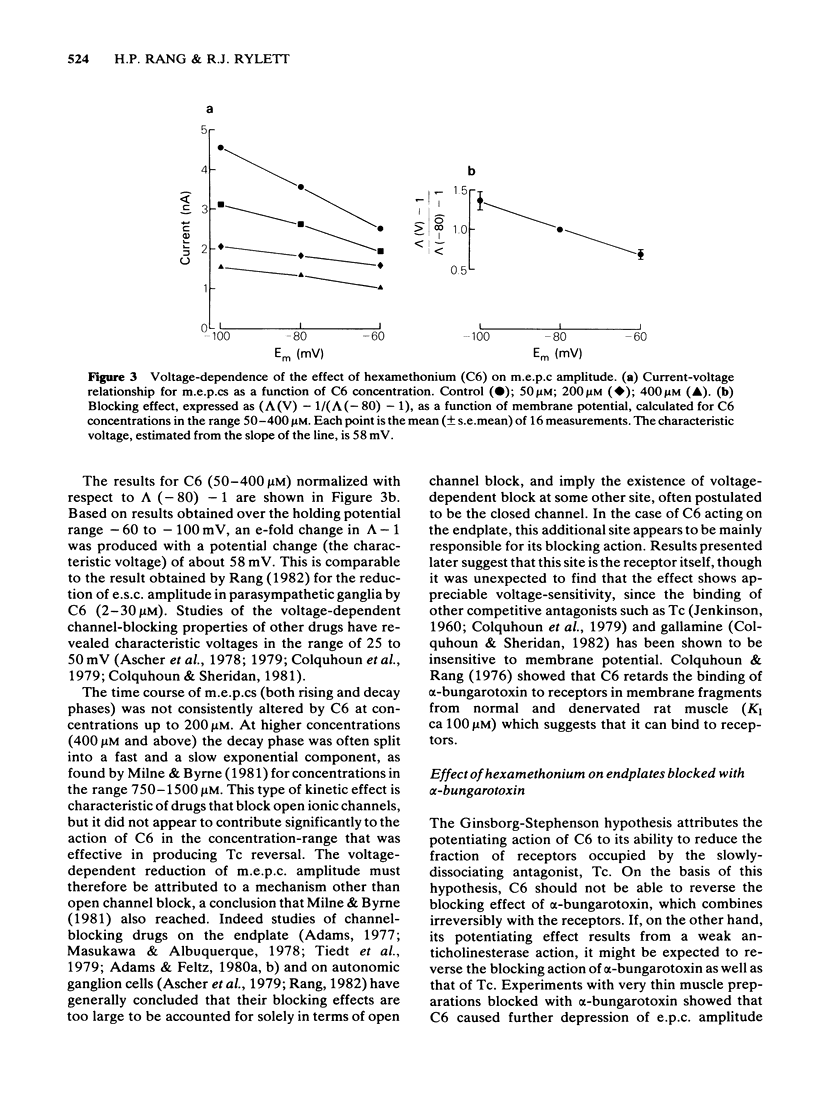
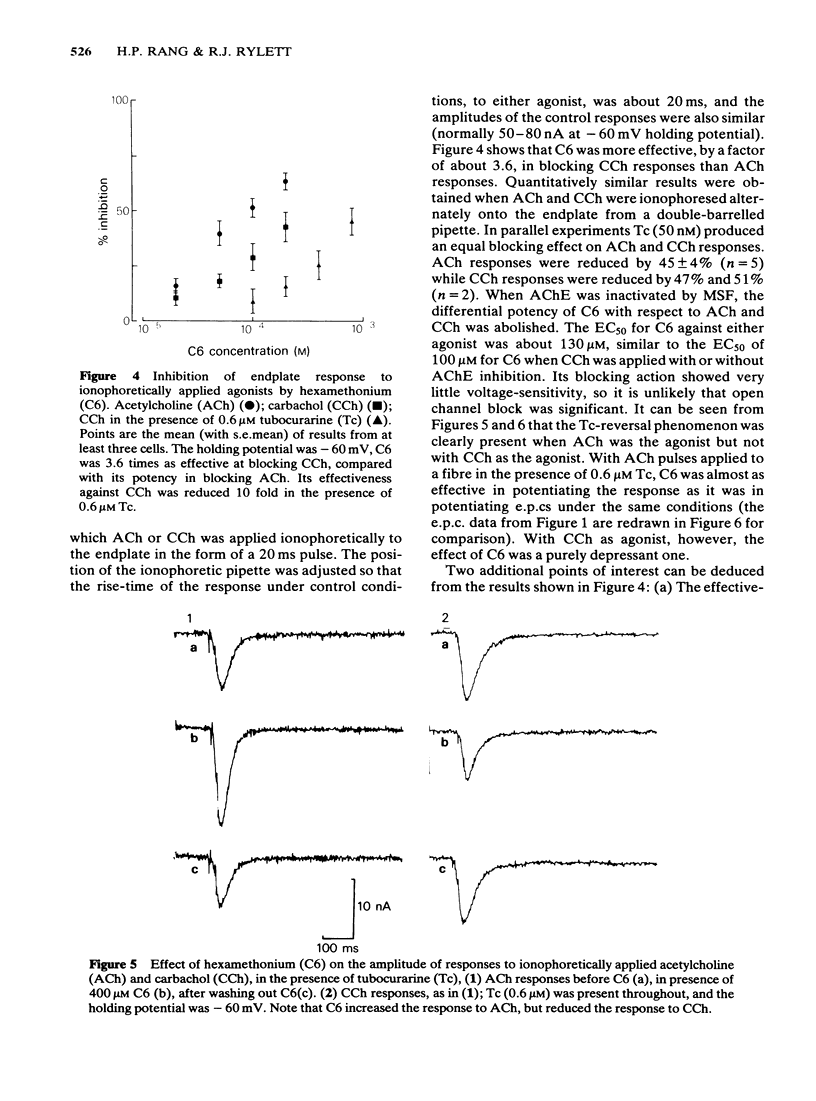
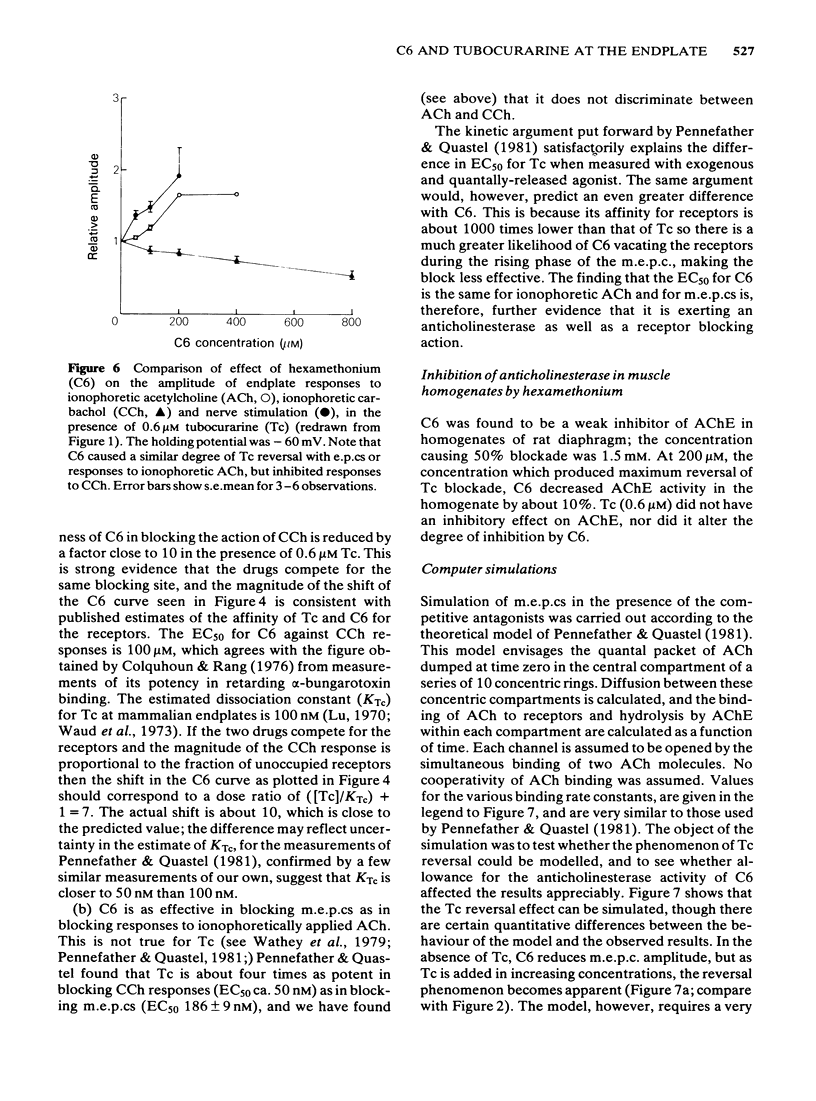
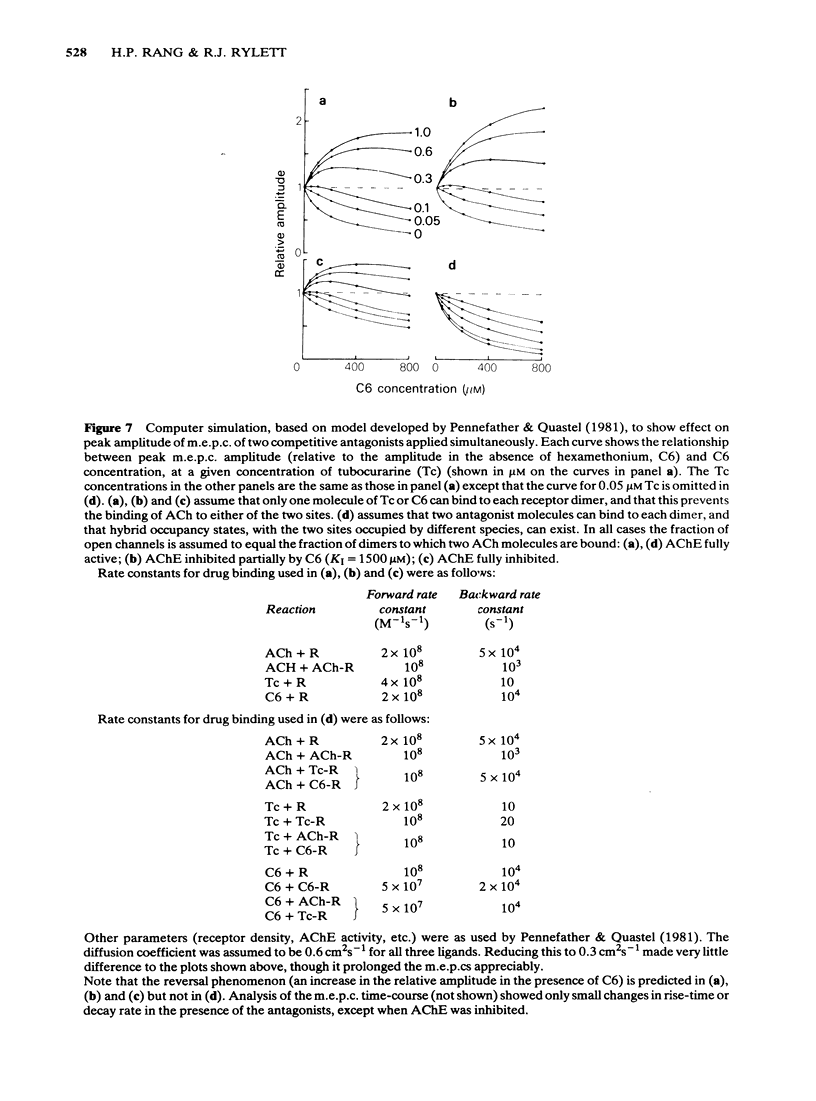
The ability of hexamethonium (C6) to reverse the neuromuscular blocking action of tubocurarine (Tc) has been reinvestigated at the voltage clamped endplate of the omohyoid muscle of rat. The possibility that a weak anticholinesterase action of C6 could contribute to the paradoxical potentiation of the peak amplitude of the endplate response has been examined. C6 (50-200 microM) caused an increase in the amplitude of nerve-evoked endplate currents (e.p.cs) recorded in the presence of 0.6 microM Tc. The effect decreased with hyperpolarization of the muscle fibre. Irreversible inhibition of acetylcholinesterase resulted in a loss of the anti-curare effect of C6. C6 did not cause an increase in e.p.c. amplitude when acetylcholine (ACh) receptors were blocked irreversibly by alpha-bungaratoxin. When transmission was blocked by increased Mg2+ concentration, C6 (50-400 microM) reduced the amplitude of e.p.cs without appreciably affecting their time course. C6 caused a decrease in the amplitude of miniature endplate currents (m.e.p.cs) the effect being slightly increased when the fibre was hyperpolarized. An e-fold increase in the effectiveness of C6 occurred with approximately 58 mV hyperpolarization. High concentrations (greater than 400 microM) affected the time course of m.e.p.cs in a manner suggestive of open channel block, but this was not evident at 200 microM, the concentration that was most effective in reversing Tc block. When tested against responses to short ionophoretic pulses of agonists, C6 was less effective against ACh (EC50ca. 300 microM) than against carbachol (CCh) (EC50 100 microM). When cholinesterase was irreversibly inhibited, C6 blocked responses to both agonists equally (EC50ca. 100 microM). The effectiveness of C6 in blocking the action of CCh was reduced 10 fold in the presence of 0.6 microM Tc, implying that the two antagonists compete for the same binding site. C6 (50-200 microM) in the presence of Tc (0.6 microM) increased the response to ionophoretically applied ACh but not that to CCh. C6 was equipotent in blocking m.e.p.cs and responses to ionophoretically applied ACh whereas Tc was more potent against the exogenously applied agonist. C6 was a weak inhibitor of acetylcholinesterase activity in rat muscle homogenates (EC50 1.5 mM). The results are discussed in terms of the kinetic hypothesis advanced by Ginsborg & Stephenson (1974) to account for the Tc reversal phenomenon.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Feltz A. End-plate channel opening and the kinetics of quinacrine (mepacrine) block. J Physiol. 1980 Sep;306:283–306. doi: 10.1113/jphysiol.1980.sp013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. Quinacrine (mepacrine) action at frog end-plate. J Physiol. 1980 Sep;306:261–281. doi: 10.1113/jphysiol.1980.sp013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Oliveira A. C., Albuquerque E. X., Mansour N. A., Eldefrawi A. T. Reaction of tetraethylammonium with the open and closed conformations of the acetylcholine receptor ionic channel complex. J Gen Physiol. 1979 Jul;74(1):129–152. doi: 10.1085/jgp.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Gauldie R. W., Milne R. J. Interaction of competitive antagonists: the anti-curare action of hexamethonium and other antagonists at the skeletal neuromuscular junction. Br J Pharmacol. 1975 May;54(1):91–100. doi: 10.1111/j.1476-5381.1975.tb07414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Mechanisms of drug action at the voluntary muscle endplate. Annu Rev Pharmacol. 1975;15:307–325. doi: 10.1146/annurev.pa.15.040175.001515. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Range H. P. Effects of inhibitors of the binding of iodinated alpha-bungarotoxin to acetylcholine receptors in rat muscle. Mol Pharmacol. 1976 Jul;12(4):519–535. [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The effect of tubocurarine competition on the kinetics of agonist action on the nicotinic receptor. Br J Pharmacol. 1982 Jan;75(1):77–86. doi: 10.1111/j.1476-5381.1982.tb08759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Farley J. M., Yeh J. Z., Watanabe S., Narahashi T. Endplate channel block by guanidine derivatives. J Gen Physiol. 1981 Mar;77(3):273–293. doi: 10.1085/jgp.77.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry C. B., Marshall A. R. An anti-curare effect of hexamethonium at the mammalian neuromuscular junction. Br J Pharmacol. 1973 Feb;47(2):353–362. doi: 10.1111/j.1476-5381.1973.tb08333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry C. B., Marshall A. R. An anticurare effect of hexamethonium at the mammalian neuromuscular junction. Br J Pharmacol. 1971 Feb;41(2):380P–381P. [PMC free article] [PubMed] [Google Scholar]
- Gingsborg B. L., Stephenson R. P. On the simultaneous action of two competitive antagonists. Br J Pharmacol. 1974 Jun;51(2):287–300. doi: 10.1111/j.1476-5381.1974.tb09659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Masukawa L. M., Albuquerque E. X. Voltage- and time-dependent action of histrionicotoxin on the endplate current of the frog muscle. J Gen Physiol. 1978 Sep;72(3):351–367. doi: 10.1085/jgp.72.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R. J., Byrne J. H. Effects of hexamethonium and decamethonium on end-plate current parameters. Mol Pharmacol. 1981 Mar;19(2):276–281. [PubMed] [Google Scholar]
- Pennefather P., Quastel D. M. Relation between subsynaptic receptor blockade and response to quantal transmitter at the mouse neuromuscular junction. J Gen Physiol. 1981 Sep;78(3):313–344. doi: 10.1085/jgp.78.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Colquhoun D., Rang H. P. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol. 1982 Jan;75(1):151–168. doi: 10.1111/j.1476-5381.1982.tb08768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L. Quantitative simulation of endplate currents at neuromuscular junctions based on the reaction of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys J. 1979 May;26(2):263–289. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R. P., Ginsborg B. L. Potentiation by an antagonist. Nature. 1969 May 24;222(5195):790–791. doi: 10.1038/222790a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI N. Some properties of conductance changes at the end-plate membrane during the action of acetylcholine. J Physiol. 1963 Jun;167:128–140. doi: 10.1113/jphysiol.1963.sp007136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Bakry N. M., Eldefrawi M. E., Eldefrawi A. T. Voltage- and time-dependent actions of piperocaine on the ion channel of the acetylcholine receptor. Mol Pharmacol. 1979 Nov;16(3):909–921. [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud B. E., Cheng M. C., Waud D. R. Comparison of drug-receptor dissociation constants at the mammalian neuromuscular junction in the presence and absence of halothane. J Pharmacol Exp Ther. 1973 Oct;187(1):40–46. [PubMed] [Google Scholar]


