Abstract
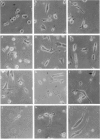
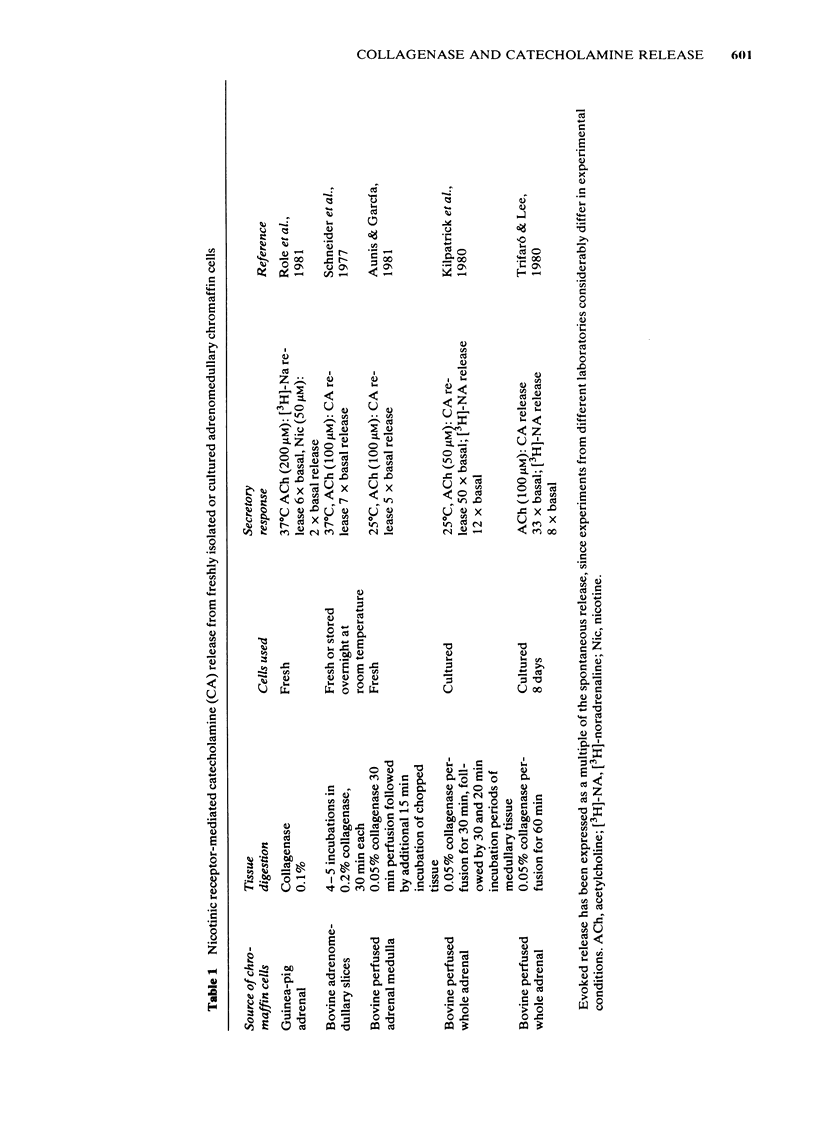
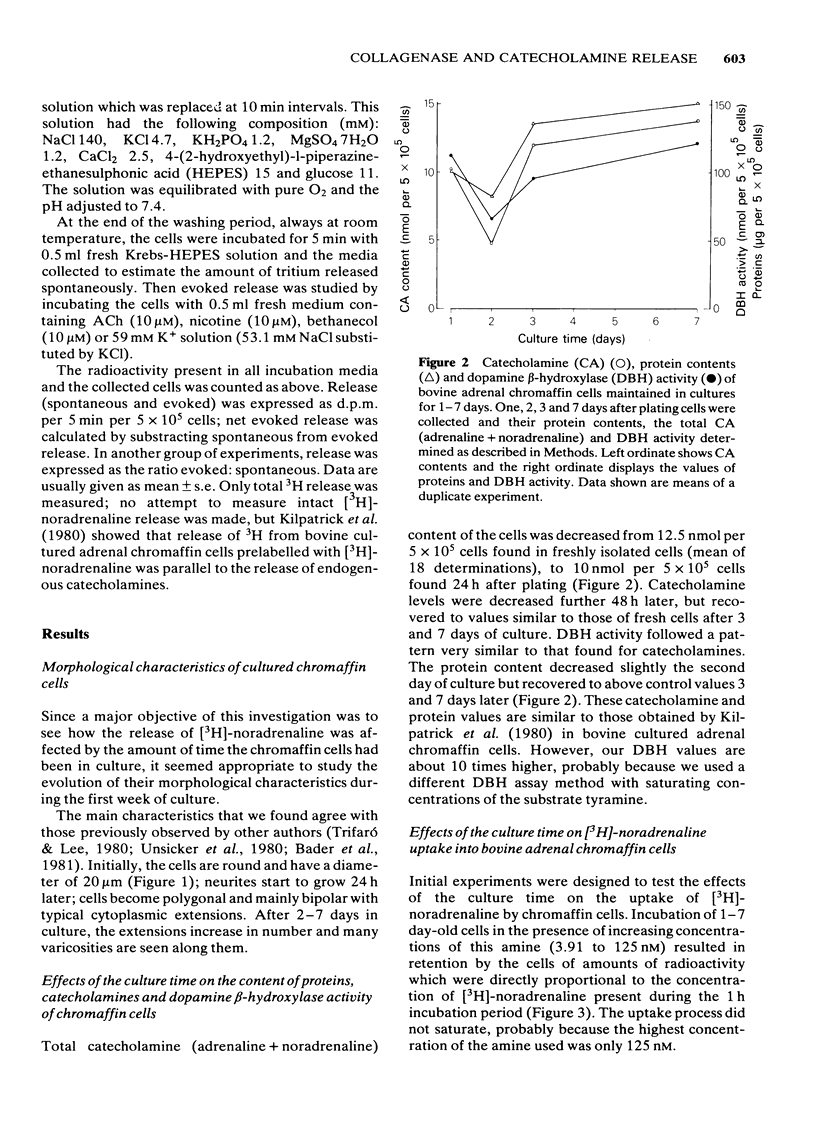
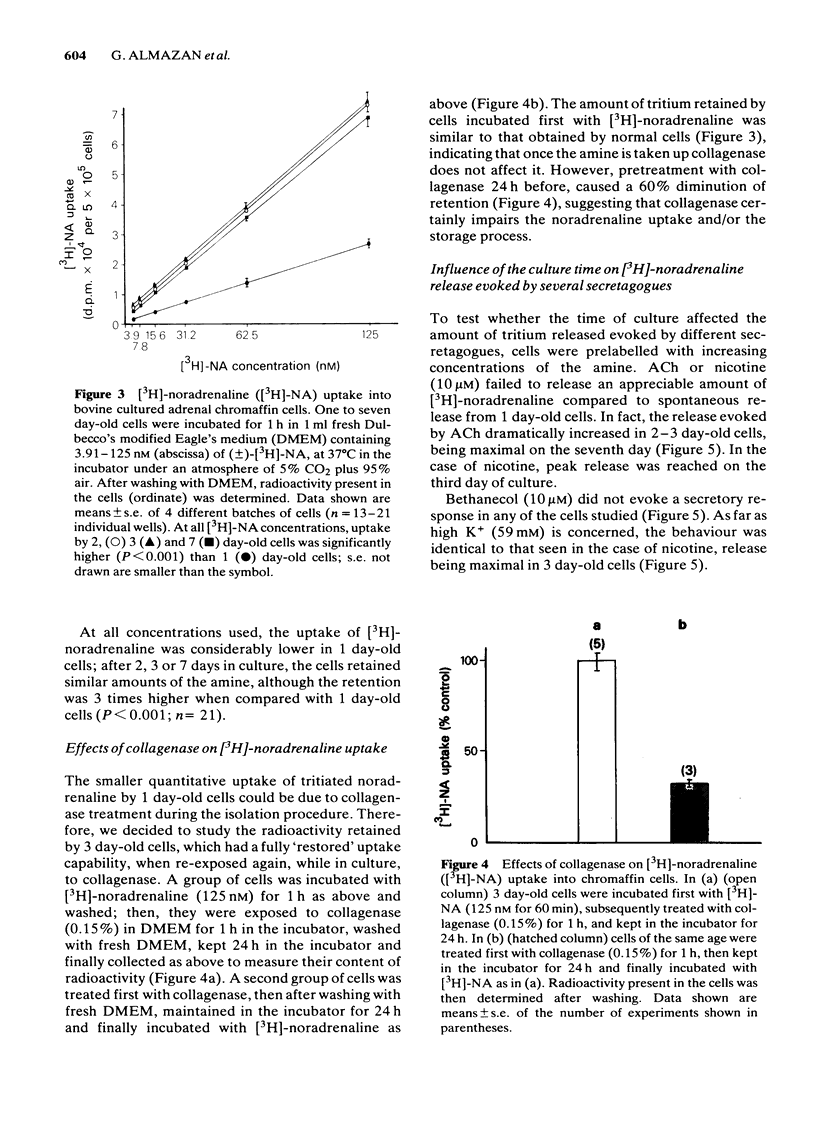
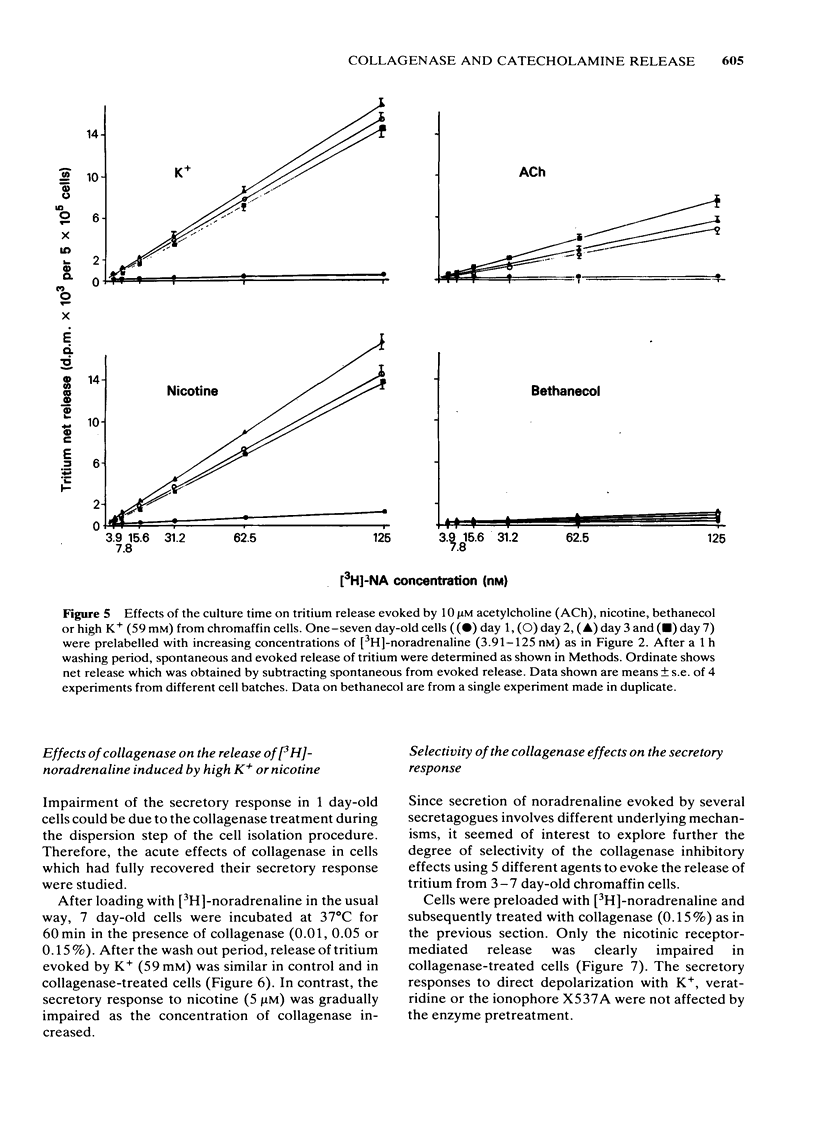
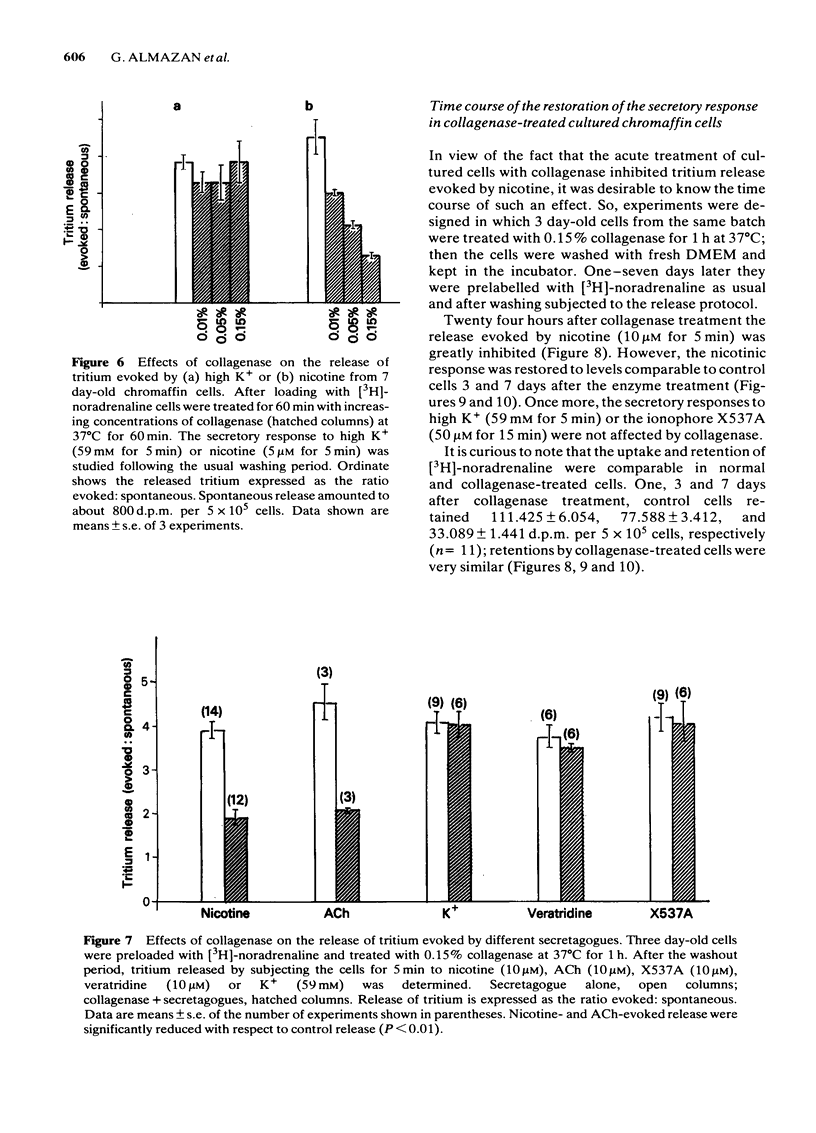
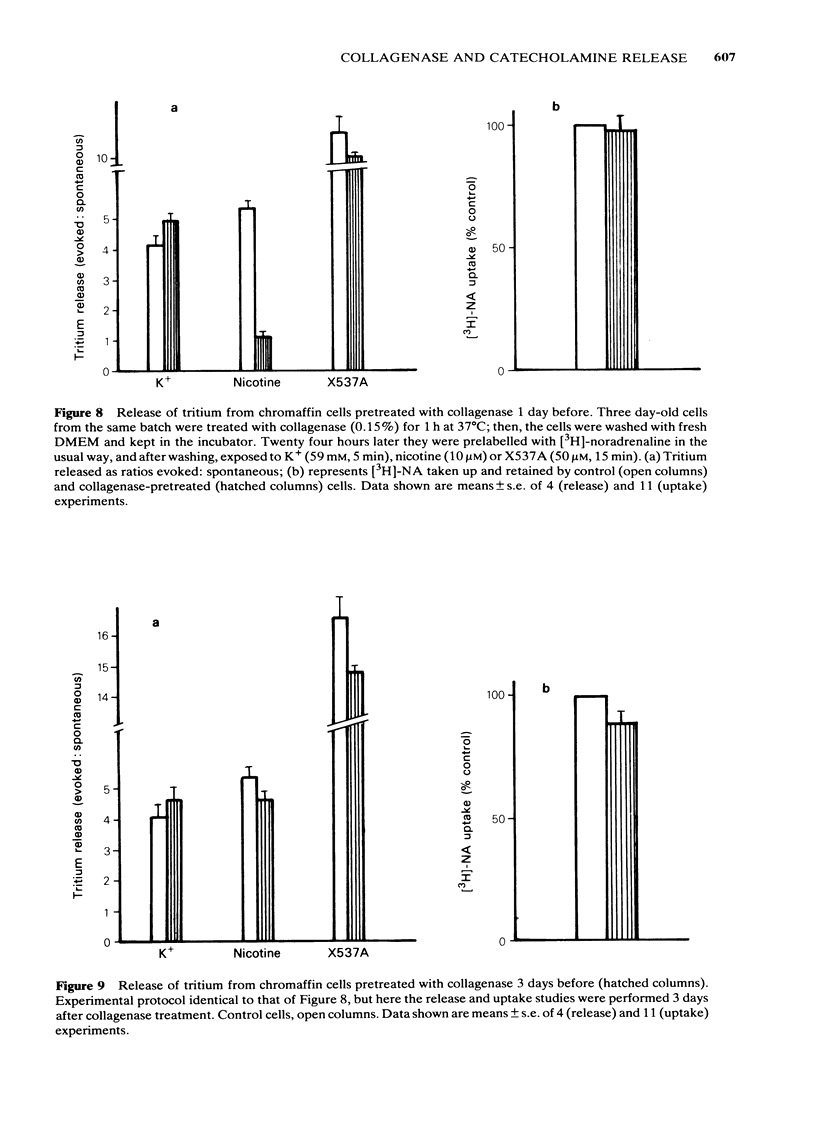
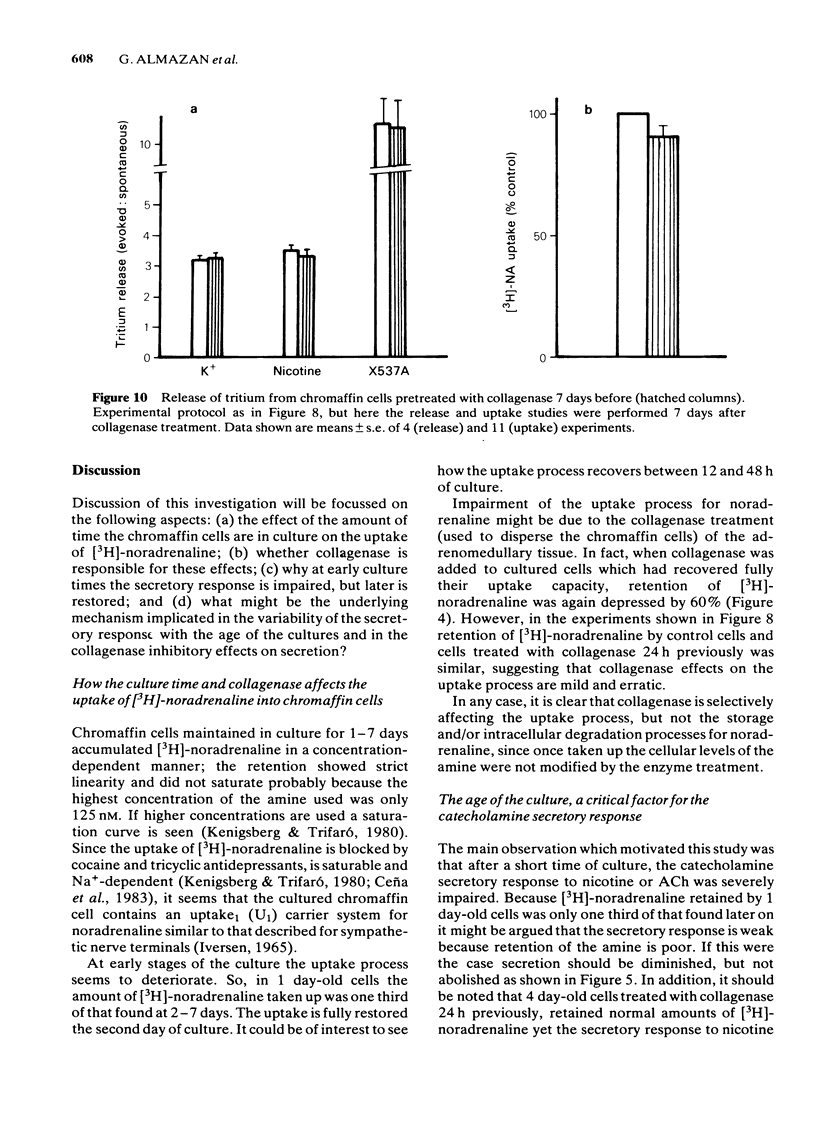
Bovine isolated adrenal chromaffin cells maintained in culture at 37 degrees C for 1-7 days become polygonal and bipolar, with typical varicosity-like extensions. Catecholamine levels and dopamine beta-hydroxylase activity decreased after 24-48 h of culture, but recovered to normal levels 3-7 days later. Incubation of 1-7 day-old cells in the presence of increasing concentrations of [3H]-noradrenaline (3.91 to 125 nM) resulted in the retention by the cells of amounts of radioactivity directly proportional to the amine present in the media. One day-old cells took up and retained only one third of the radioactivity found in 2-7 day-old cells. The addition of collagenase to cultured cells caused a decrease in the uptake of tritium. However, the enzyme treatment did not affect the amine taken up by the cell before collagenase treatment. Release of tritium from cultured cells evoked by nicotine, acetylcholine (ACh) or 59 mM K+ was very poor in 24 h-old cells; the secretory response to nicotine, ACh or K+ was dramatically increased after 2-7 days of culture. Bethanecol did not cause any secretory response. When treated with collagenase, cultured cells which had recovered fully their secretory response, lost again the ability to release tritium evoked by ACh or nicotine. However, the responses to high K+, veratridine or ionophore X537A were not affected. The nicotinic response was recovered two days after collagenase treatment. The data suggest that the use of collagenase to disperse the adrenomedullary tissue during the isolation procedure might be responsible for the lost secretory response of young cultured chromaffin cells. Since collagenase specifically impairs the nicotinic cholinoceptor-mediated catecholamine release, it seems likely that the enzyme is exerting its action on the ACh receptor complex. It is unlikely that either voltage-sensitive Na+ or Ca2+ channels are affected by collagenase as the responses induced by high K+ or veratridine were unaffected by this enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aunis D., García A. G. Correlation between catecholamine secretion from bovine isolated chromaffin cells and [3H]-ouabain binding to plasma membranes. Br J Pharmacol. 1981 Jan;72(1):31–40. doi: 10.1111/j.1476-5381.1981.tb09101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M. F., Ciesielski-Treska J., Thierse D., Hesketh J. E., Aunis D. Immunocytochemical study of microtubules in chromaffin cells in culture and evidence that tubulin is not an integral protein of the chromaffin granule membrane. J Neurochem. 1981 Oct;37(4):917–933. doi: 10.1111/j.1471-4159.1981.tb04479.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Brooks J. C. The isolated bovine adrenomedullary chromaffin cell: a model of neuronal excitation-secretion. Endocrinology. 1977 Nov;101(5):1369–1378. doi: 10.1210/endo-101-5-1369. [DOI] [PubMed] [Google Scholar]
- Carbone E. Removal of Na+ channels in squid giant axons by perfusion with trypsin. Biochim Biophys Acta. 1982 Dec 8;693(1):188–194. doi: 10.1016/0005-2736(82)90486-2. [DOI] [PubMed] [Google Scholar]
- Ceña V., García A. G., Montiel C., Sánchez-García P. Uptake of [3H]-nicotine and [3H]-noradrenaline by cultured chromaffin cells. Br J Pharmacol. 1984 Jan;81(1):119–123. doi: 10.1111/j.1476-5381.1984.tb10751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Fajdiga P. B., Howe N. B., Livett B. G. Functional and morphological characterization of isolated bovine adrenal medullary cells. J Cell Biol. 1978 Jan;76(1):12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. P., Miledi R., Perez de la Mora M., Vincent A. Acetylcholine receptors. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):551–559. doi: 10.1098/rstb.1975.0031. [DOI] [PubMed] [Google Scholar]
- Hochman J., Perlman R. L. Catecholamine secretion by isolated adrenal cells. Biochim Biophys Acta. 1976 Jan 14;421(1):168–175. doi: 10.1016/0304-4165(76)90180-x. [DOI] [PubMed] [Google Scholar]
- Kenigsberg R. L., Trifaró J. M. Presence of a high affinity uptake system for catecholamines in cultured bovine adrenal chromaffin cells. Neuroscience. 1980;5(9):1547–1556. doi: 10.1016/0306-4522(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Ledbetter F. H., Carson K. A., Kirshner A. G., Slepetis R., Kirshner N. Stability of bovine adrenal medulla cells in culture. J Neurochem. 1980 Sep;35(3):679–692. doi: 10.1111/j.1471-4159.1980.tb03707.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagatsu T., Udenfriend S. Photometric assay of dopamine- -hydroxylase activity in human blood. Clin Chem. 1972 Sep;18(9):980–983. [PubMed] [Google Scholar]
- Role L. W., Leeman S. E., Perlman R. L. Somatostatin and substance P inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience. 1981;6(9):1813–1821. doi: 10.1016/0306-4522(81)90215-3. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Herz R., Rosenheck K. Stimulus-secretion coupling in chromaffin cells isolated from bovine adrenal medulla. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5036–5040. doi: 10.1073/pnas.74.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaragli G. P., Sen I., Baba A., Schulz R. A., Cooper J. R. The mechanism of actions of collagenase on the inhibition of release of acetylcholine from synaptosomal preparations. Brain Res. 1977 Sep 23;134(1):113–123. doi: 10.1016/0006-8993(77)90929-5. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Lee R. W. Morphological characteristics and stimulus-secretion coupling in bovine adcrenal chromaffin cell cultures. Neuroscience. 1980;5(9):1533–1546. doi: 10.1016/0306-4522(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Griesser G. H., Lindmar R., Löffelholz K., Wolf U. Establishment, characterization and fibre outgrowth of isolated bovine adrenal medullary cells in long-term cultures. Neuroscience. 1980;5(8):1445–1460. doi: 10.1016/0306-4522(80)90006-8. [DOI] [PubMed] [Google Scholar]