Abstract
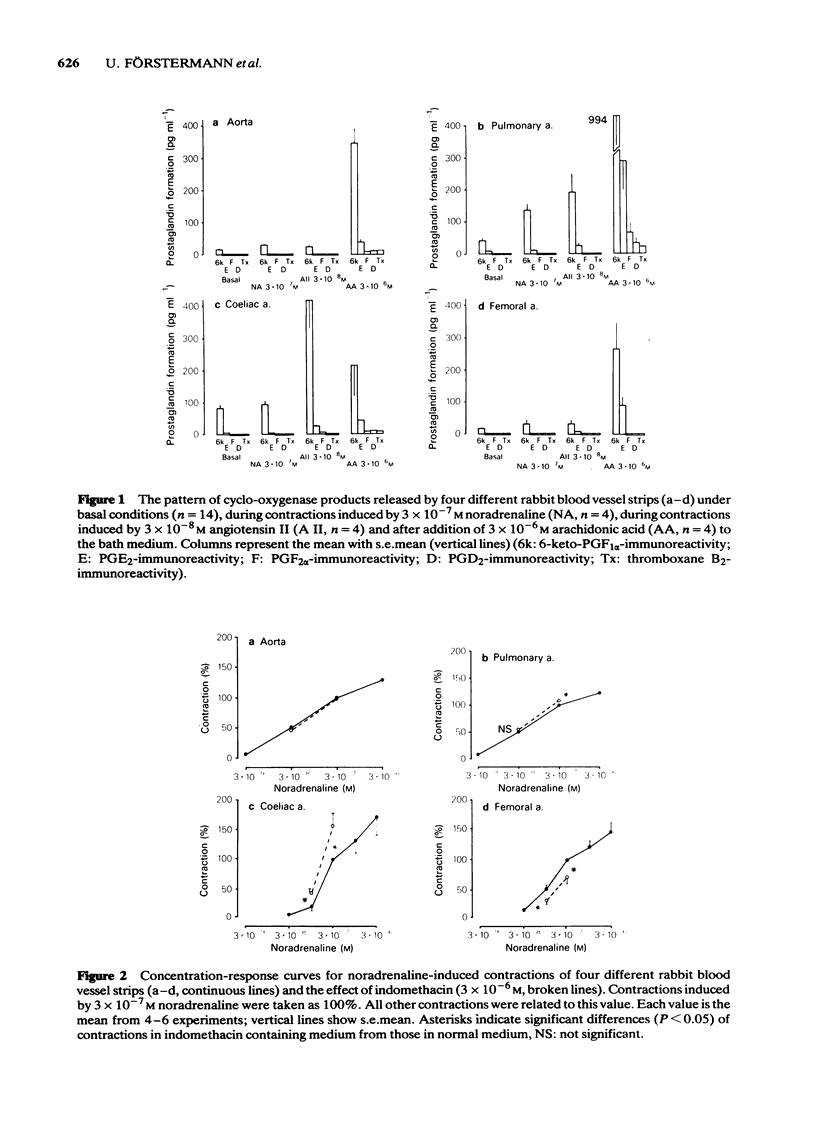
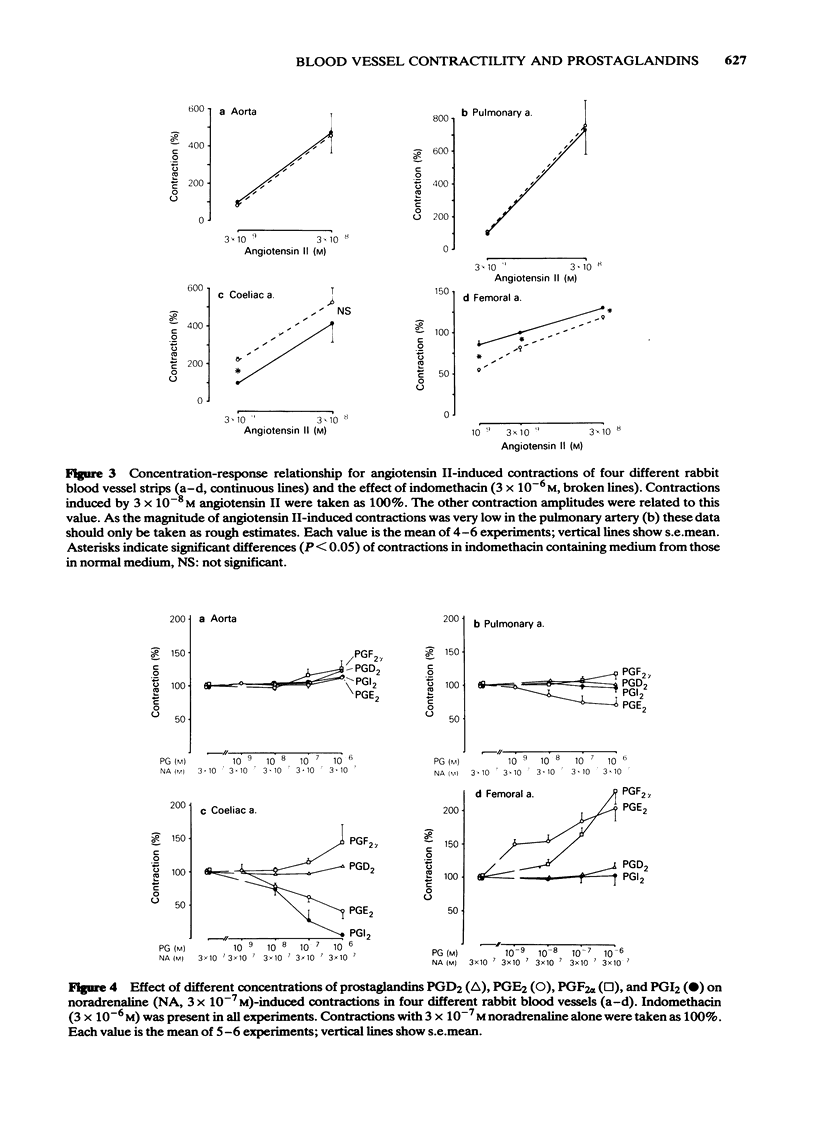
Helically cut strips of rabbit aorta, extrapulmonary artery, coeliac artery, and femoral artery were set up in organ baths. Contractions of the strips by noradrenaline and angiotensin II were recorded isotonically. The release of prostaglandins 6-keto-PGF1 alpha, E2, F2 alpha, D2 and thromboxane B2 from the strips was measured by means of sensitive and specific radioimmunoassays. All blood vessels released a characteristic pattern of cyclo-oxygenase products. Prostacyclin (PGI2, measured as 6-keto-PGF1 alpha) was the major compound formed, followed by smaller amounts of PGE2 and traces of PGF2 alpha, PGD2 and thromboxane A2 (measured as thromboxane B2). The pulmonary and the femoral artery had comparatively high abilities to synthesize PGE2. Contractions induced by noradrenaline increased prostaglandin release from the pulmonary artery but not from the other blood vessels. Angiotensin II-induced contractions were accompanied by a marked prostaglandin release from the coeliac artery. After angiotensin II, prostaglandin release was also enhanced in the pulmonary artery, but remained essentially unchanged in the aorta and femoral artery. Arachidonic acid markedly increased the levels of all prostaglandin formed. Indomethacin inhibited the formation of all prostaglandins below the detection limits of the respective radioimmunoassays. Indomethacin treatment induced a qualitatively similar shifting of the concentration-response curves of noradrenaline and angiotensin II in some vessels: the concentration-response curves remained unchanged for the aorta, were slightly shifted to the left of the pulmonary artery, were markedly shifted to the left for the coeliac artery, and were shifted to the right for the femoral artery.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Altura B. T. Vascular smooth muscle and prostaglandins. Fed Proc. 1976 Oct;35(12):2360–2366. [PubMed] [Google Scholar]
- Anhut H., Bernauer W., Peskar B. A. Radioimmunological determination of thromboxane release in cardiac anaphylaxis. Eur J Pharmacol. 1977 Jul 1;44(1):85–88. doi: 10.1016/0014-2999(77)90120-0. [DOI] [PubMed] [Google Scholar]
- Anhut H., Peskar B. A., Wachter W., Gräbling B., Peskar B. M. Radioimmunological determination of prostaglandin D2 synthesis in human thrombocytes. Experientia. 1978 Nov 15;34(11):1494–1496. doi: 10.1007/BF01932373. [DOI] [PubMed] [Google Scholar]
- Borda E. S., Sterin-Borda L., Gimeno M. F., Lazzari M. A., Gimeno A. L. The stimulatory effect of prostacyclin (PGI2) on isolated rabbit and rat aorta is probably associated to the generation of a thromboxane A2 (TXA2) "like-material". Arch Int Pharmacodyn Ther. 1983 Jan;261(1):79–89. [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Dusting G. J., Moncada S., Vane J. R. Prostacyclin (PGI2) is a weak contractor of coronary arteries of the pig. Eur J Pharmacol. 1977 Oct 1;45(3):301–304. doi: 10.1016/0014-2999(77)90014-0. [DOI] [PubMed] [Google Scholar]
- Fink G. D., Chapnick B. M., Goldberg M. R., Paustian P. W., Kadowitz P. J. Influence of prostaglandin E2, indomethacin, and reserpine on renal vascular responses to nerve stimulation, pressor and depressor hormones. Circ Res. 1977 Aug;41(2):172–178. doi: 10.1161/01.res.41.2.172. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Hertting G. The importance of Ca2+-mediated phospholipase A2 activation for stimulus-evoked PGE2-release from rabbit splenic capsular strips. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;307(3):243–249. doi: 10.1007/BF00505940. [DOI] [PubMed] [Google Scholar]
- Jobke A., Peskar B. A., Peskar B. M. On the specificity of antisera against prostaglandins A2 and E2. FEBS Lett. 1973 Dec 1;37(2):192–196. doi: 10.1016/0014-5793(73)80456-9. [DOI] [PubMed] [Google Scholar]
- Levy J. V. Contractile responses to prostacyclin (PGI2) of isolated human saphenous and rat venous tissue. Prostaglandins. 1978 Jul;16(1):93–97. doi: 10.1016/0090-6980(78)90205-8. [DOI] [PubMed] [Google Scholar]
- Machleidt C., Förstermann U., Anhut H., Hertting G. Formation and elimination of prostacyclin metabolites in the cat in vivo as determined by radioimmunoassay of unextracted plasma. Eur J Pharmacol. 1981 Aug 27;74(1):19–26. doi: 10.1016/0014-2999(81)90318-6. [DOI] [PubMed] [Google Scholar]
- Malik K. U. Prostaglandins--modulation of adrenergic nervous system. Fed Proc. 1978 Feb;37(2):203–207. [PubMed] [Google Scholar]
- Malik K. U., Ryan P., McGiff J. C. Modification by prostaglandins E1 and E2, indomethacin, and arachidonic acid of the vasoconstrictor responses of the isolated perfused rabbit and rat mesenteric arteries to adrenergic stimuli. Circ Res. 1976 Aug;39(2):163–168. doi: 10.1161/01.res.39.2.163. [DOI] [PubMed] [Google Scholar]
- Messina E. J., Weiner R., Kaley G. Prostaglandins and local circulatory control. Fed Proc. 1976 Oct;35(12):2367–2375. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Omini C., Moncada S., Vane J. R. The effects of prostacyclin (PGI2) on tissues which detect prostaglandins (PG'S). Prostaglandins. 1977 Oct;14(4):625–632. doi: 10.1016/0090-6980(77)90189-7. [DOI] [PubMed] [Google Scholar]
- Peskar B., Hertting G. Release of prostaglandins from isolated cat spleen by angiotensin and vasopressin. Naunyn Schmiedebergs Arch Pharmacol. 1973;279(3):227–234. doi: 10.1007/BF00500602. [DOI] [PubMed] [Google Scholar]
- Salzman P. M., Salmon J. A., Moncada S. Prostacyclin and thromboxane A2 synthesis by rabbit pulmonary artery. J Pharmacol Exp Ther. 1980 Oct;215(1):240–247. [PubMed] [Google Scholar]
- Sametz W., Juan H. Release of different prostaglandins from vascular tissue by different stimulators. Prostaglandins Leukot Med. 1982 Dec;9(6):593–602. doi: 10.1016/0262-1746(82)90017-8. [DOI] [PubMed] [Google Scholar]
- Simmet T., Förstermann U., Peskar B. A. Effect of exogenous and endogenous prostacyclin on the contractility of rabbit splenic capsular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1980 Jul;312(3):245–253. doi: 10.1007/BF00499154. [DOI] [PubMed] [Google Scholar]
- Simmet T., Hertting G. On the relation between contraction and prostaglandin release in rabbit mesenteric blood vessels. Eur J Pharmacol. 1980 Aug 8;65(4):325–331. doi: 10.1016/0014-2999(80)90335-0. [DOI] [PubMed] [Google Scholar]
- Yabek S. M., Avner B. P. Effects of prostacyclin (PGI2) and indomethacin on neonatal lamb mesenteric and renal artery responses to electrical stimulation and norepinephrine. Prostaglandins. 1980 Jan;19(1):23–29. doi: 10.1016/0090-6980(80)90150-1. [DOI] [PubMed] [Google Scholar]


