Abstract
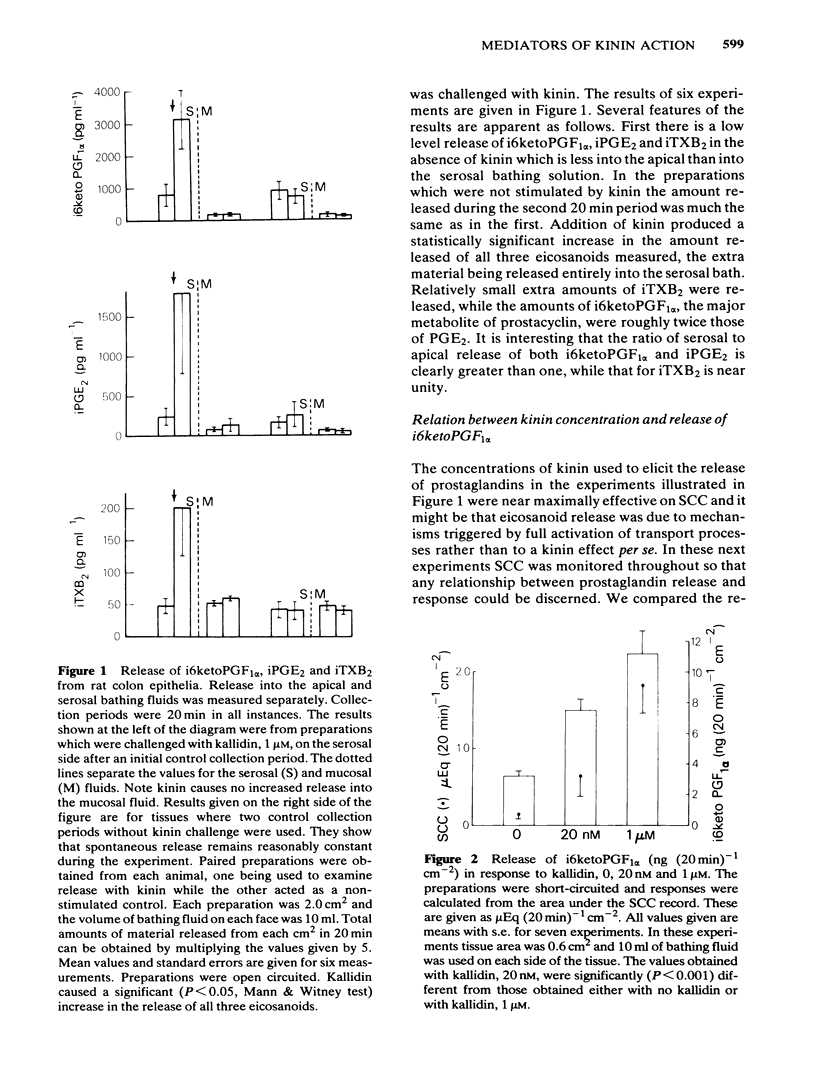
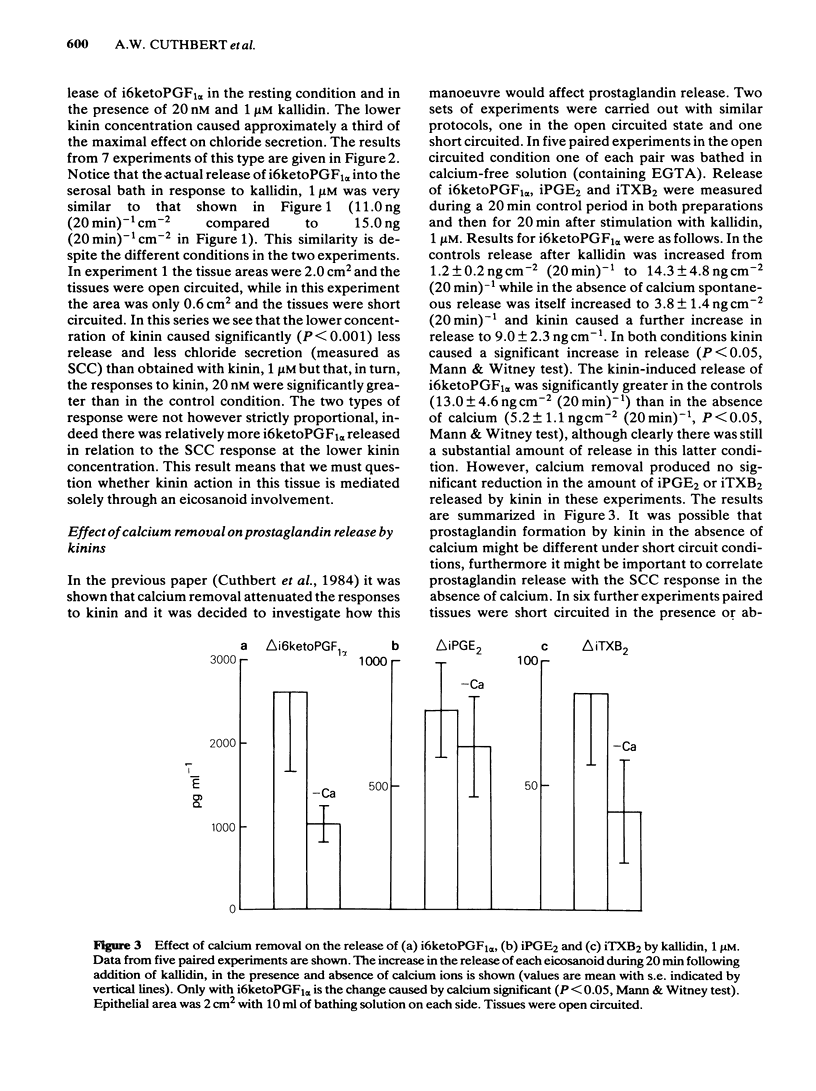
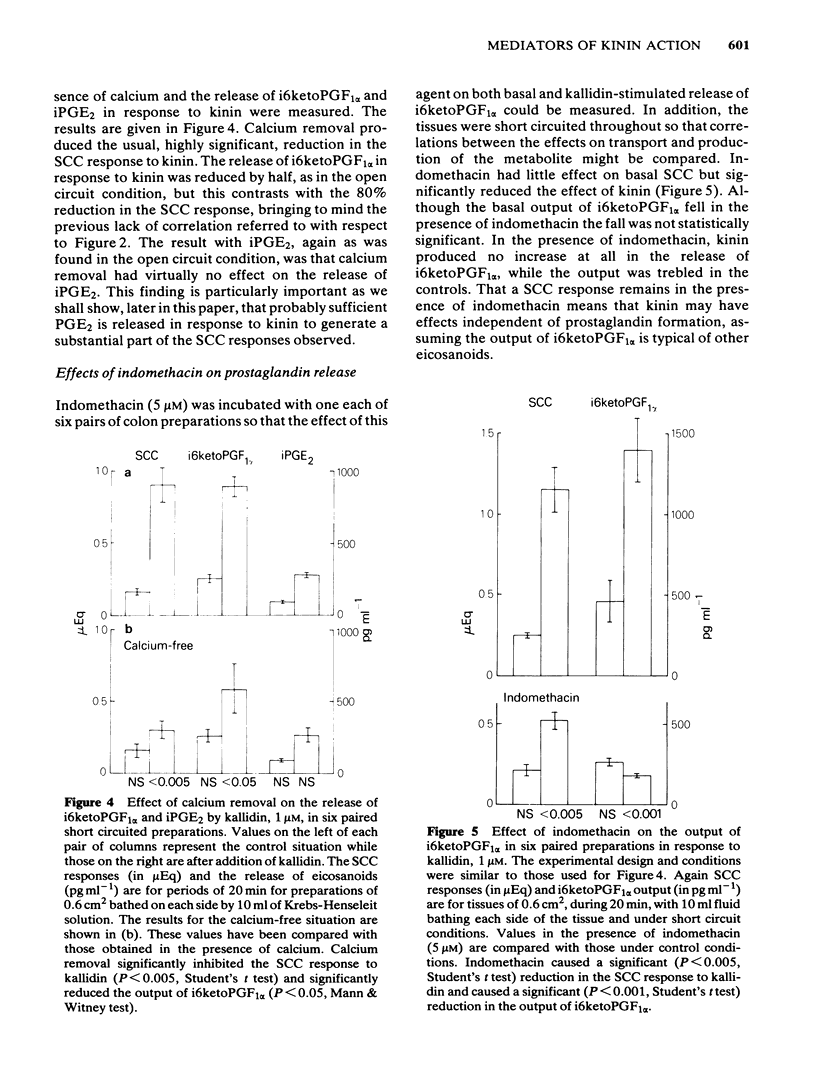
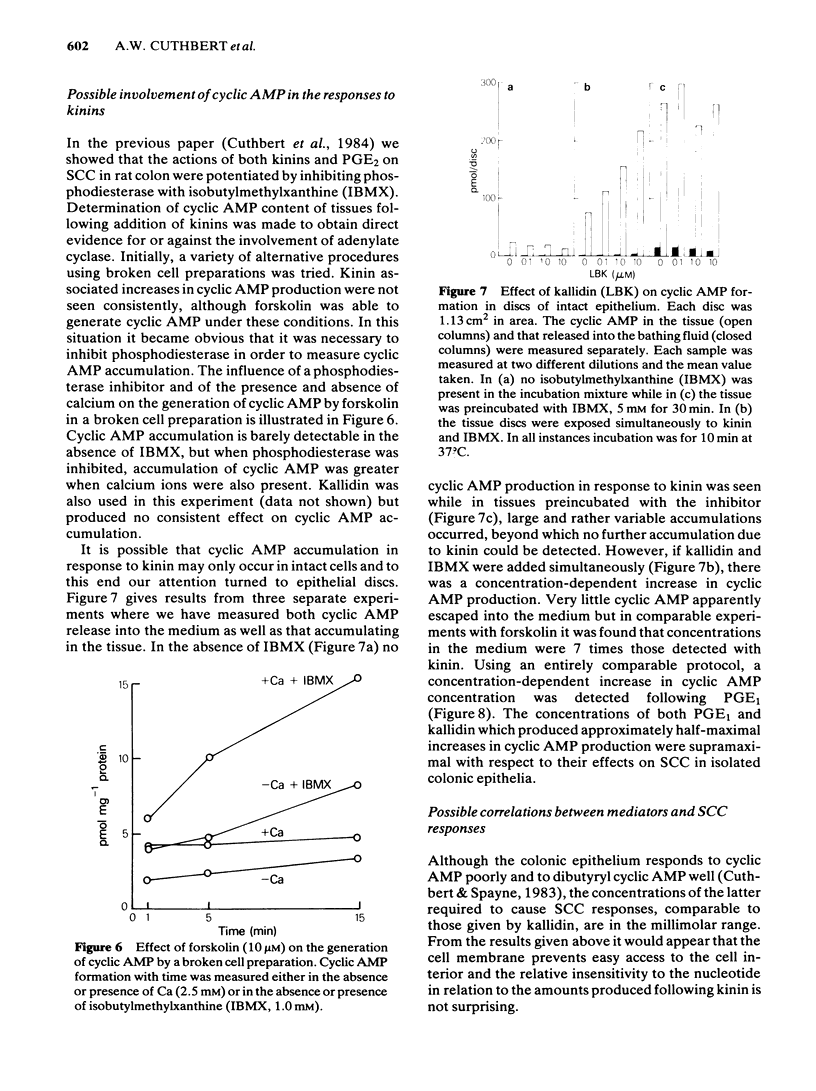
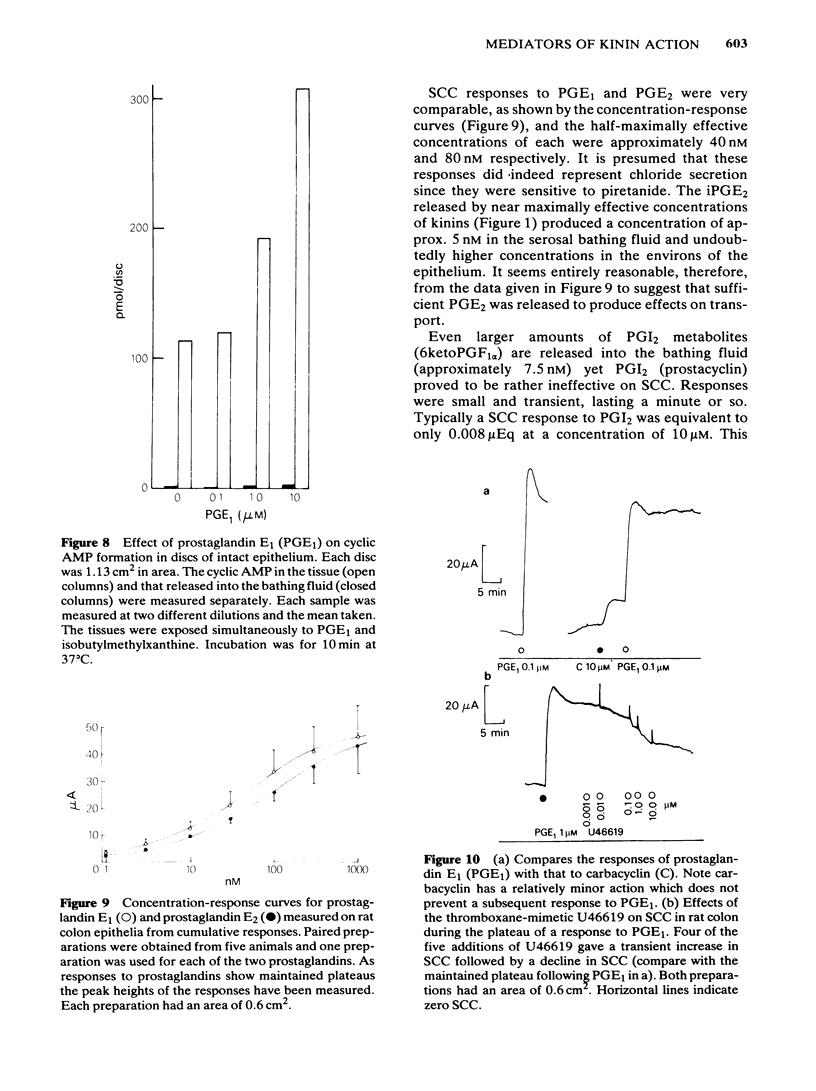
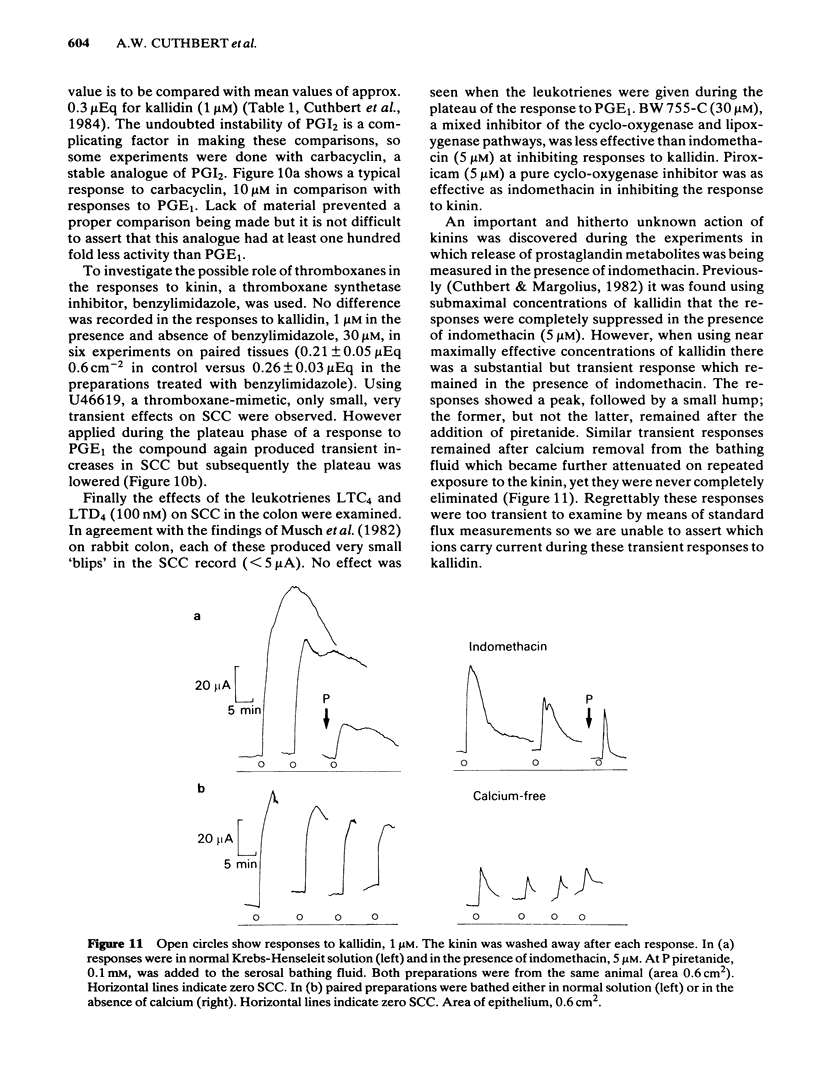
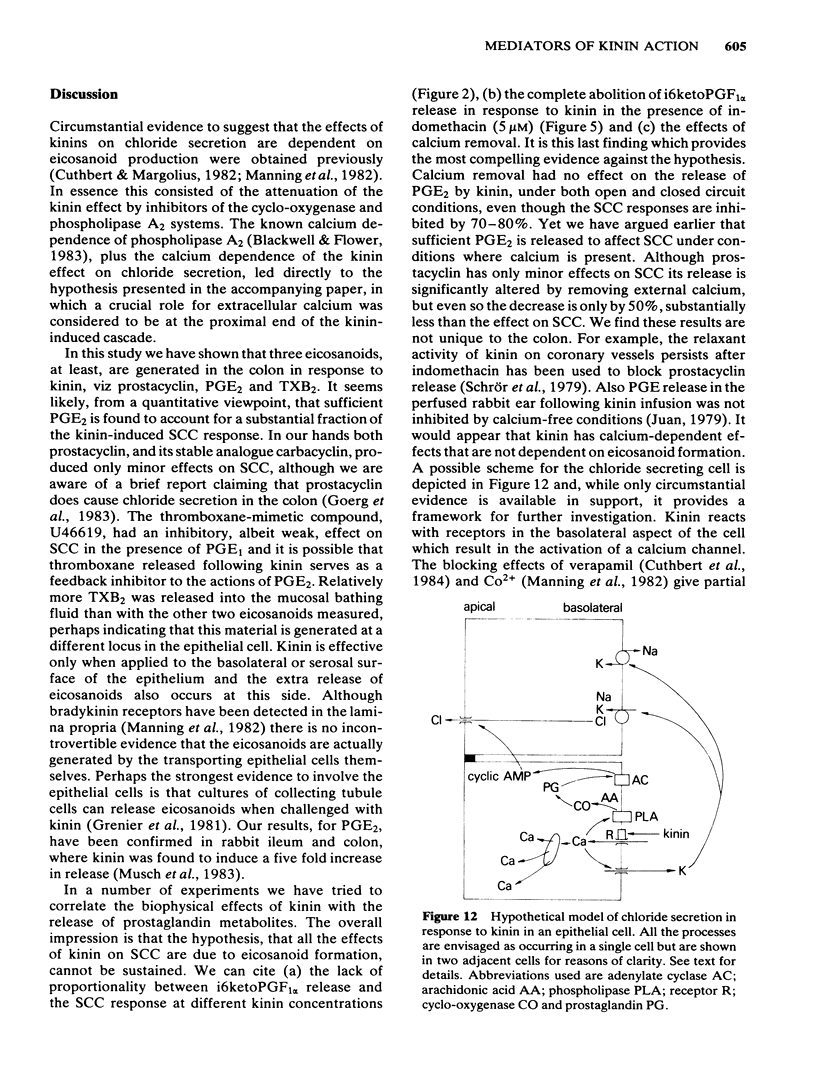
The output of immunoreactive (i) 6 keto prostaglandin F1 alpha (i6ketoPGF1 alpha), iPGE2 and ithromboxane B2 (iTXB2) from isolated colonic epithelium of the rat into the apical and basolateral bathing solution has been measured. In some instances tissues were also voltage clamped at 0 mV to measure short circuit current (SCC). Kallidin (lysylbradykinin) stimulated the output of all three eicosanoids, specifically from the basolateral face of the tissue. The output was similar whether or not the tissues were short circuited. Both the SCC response and eicosanoid output were dependent upon the concentration of kallidin, but not in a strictly proportional manner, there being relatively more eicosanoid output at submaximal kinin concentrations. Indomethacin, 5 microM, abolished the eicosanoid output, in response to kinin, while some part of the SCC response remained. Calcium removal from the basolateral bathing fluid severely attenuated the SCC response, reduced the output of i6ketoPGF1 alpha to half, but left the output of iPGE2 unchanged. In the presence or absence of calcium it is probable that sufficient PGE material is released to cause part of the SCC changes seen with kinin. Kinin and PGE1 increased the cyclic AMP content of intact epithelia, provided a phosphodiesterase inhibitor was added at the same time. It is proposed that kinin causes an increase in calcium influx at the basolateral pole of the tissue. This calcium is necessary for the production of some eicosanoids and the subsequent generation of cyclic AMP, which then increases apical chloride permeability. In addition, calcium may facilitate entry of chloride through the basolateral face of the cells by activating a cotransport mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bareis D. L., Manganiello V. C., Hirata F., Vaughan M., Axelrod J. Bradykinin stimulates phospholipid methylation, calcium influx, prostaglandin formation, and cAMP accumulation in human fibroblasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2514–2518. doi: 10.1073/pnas.80.9.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J. Inhibition of phospholipase. Br Med Bull. 1983 Jul;39(3):260–264. doi: 10.1093/oxfordjournals.bmb.a071830. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Knapp D. R., Halushka P. V. Vasopressin stimulates thromboxane synthesis in the toad urinary bladder: effects of imidazole. J Pharmacol Exp Ther. 1979 Sep;210(3):344–348. [PubMed] [Google Scholar]
- COLLIER H. O., SHORLEY P. G. Analgesic antipyretic drugs as antagonists of bradykinin. Br J Pharmacol Chemother. 1960 Dec;15:601–610. doi: 10.1111/j.1476-5381.1960.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield A. R. An effect of chloride on (Na+K) co-transport in human red blood cells. Nature. 1980 Jul 17;286(5770):281–282. doi: 10.1038/286281a0. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Halushka P. V., Margolius H. S., Spayne J. A. Role of calcium ions in kinin-induced chloride secretion. Br J Pharmacol. 1984 Jul;82(3):587–595. doi: 10.1111/j.1476-5381.1984.tb10797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Margolius H. S. Kinins stimulate net chloride secretion by the rat colon. Br J Pharmacol. 1982 Apr;75(4):587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Spayne J. A. Conversion of sodium channels to a form sensitive to cyclic AMP by component(s) from red cells. Br J Pharmacol. 1983 Jul;79(3):783–797. doi: 10.1111/j.1476-5381.1983.tb10017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Wilson S. A. Mechanisms for the effects of acetylcholine on sodium transport in frog skin. J Membr Biol. 1981 Mar 15;59(1):65–75. doi: 10.1007/BF01870822. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Grenier F. C., Rollins T. E., Smith W. L. Kinin-induced prostaglandin synthesis by renal papillary collecting tubule cells in culture. Am J Physiol. 1981 Jul;241(1):F94–104. doi: 10.1152/ajprenal.1981.241.1.F94. [DOI] [PubMed] [Google Scholar]
- Juan H. Role of calcium in prostaglandin E release induced by bradykinin and the ionophore A 23187. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jun;307(2):177–183. doi: 10.1007/BF00498461. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manning D. C., Snyder S. H., Kachur J. F., Miller R. J., Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982 Sep 16;299(5880):256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Kachur J. F., Miller R. J., Field M., Stoff J. S. Bradykinin-stimulated electrolyte secretion in rabbit and guinea pig intestine. Involvement of arachidonic acid metabolites. J Clin Invest. 1983 May;71(5):1073–1083. doi: 10.1172/JCI110857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch M. W., Miller R. J., Field M., Siegel M. I. Stimulation of colonic secretion by lipoxygenase metabolites of arachidonic acid. Science. 1982 Sep 24;217(4566):1255–1256. doi: 10.1126/science.6810465. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Orellana S. A., Kimberg L. S., Field M., Halm D. R., Krasny E. J., Jr, Frizzell R. A. Na+-K+-Cl- co-transport in the intestine of a marine teleost. Nature. 1982 Nov 25;300(5890):351–353. doi: 10.1038/300351a0. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Schrör K., Metz U., Krebs R. The bradykinin-induced coronary vasodilation. Evidence for an additional prostacyclin-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;307(3):213–221. doi: 10.1007/BF00505936. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Welsh M. J., Stoff J. S., Frizzell R. A. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol. 1982;70(3):217–226. doi: 10.1007/BF01870564. [DOI] [PubMed] [Google Scholar]
- Spraggs C. F., Margolius H. S., Cuthbert A. W. Selective activity of cyclokinins. J Pharm Pharmacol. 1983 Apr;35(4):266–268. doi: 10.1111/j.2042-7158.1983.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Stoner J., Manganiello V. C., Vaughan M. Effects of bradykinin and indomethacin on cyclic GMP and cyclic AMP in lung slices. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3830–3833. doi: 10.1073/pnas.70.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J. G., Saelens D. A., Halushka P. V. Biosynthesis of prostaglandin E by rat superior cervical ganglia. J Neurochem. 1978 Jul;31(1):13–19. doi: 10.1111/j.1471-4159.1978.tb12427.x. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Intracellular chloride activities in canine tracheal epithelium. Direct evidence for sodium-coupled intracellular chloride accumulation in a chloride-secreting epithelium. J Clin Invest. 1983 May;71(5):1392–1401. doi: 10.1172/JCI110892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Wise W. C., Cook J. A., Eller T., Halushka P. V. Ibuprofen improves survival from endotoxic shock in the rat. J Pharmacol Exp Ther. 1980 Oct;215(1):160–164. [PubMed] [Google Scholar]


