Abstract
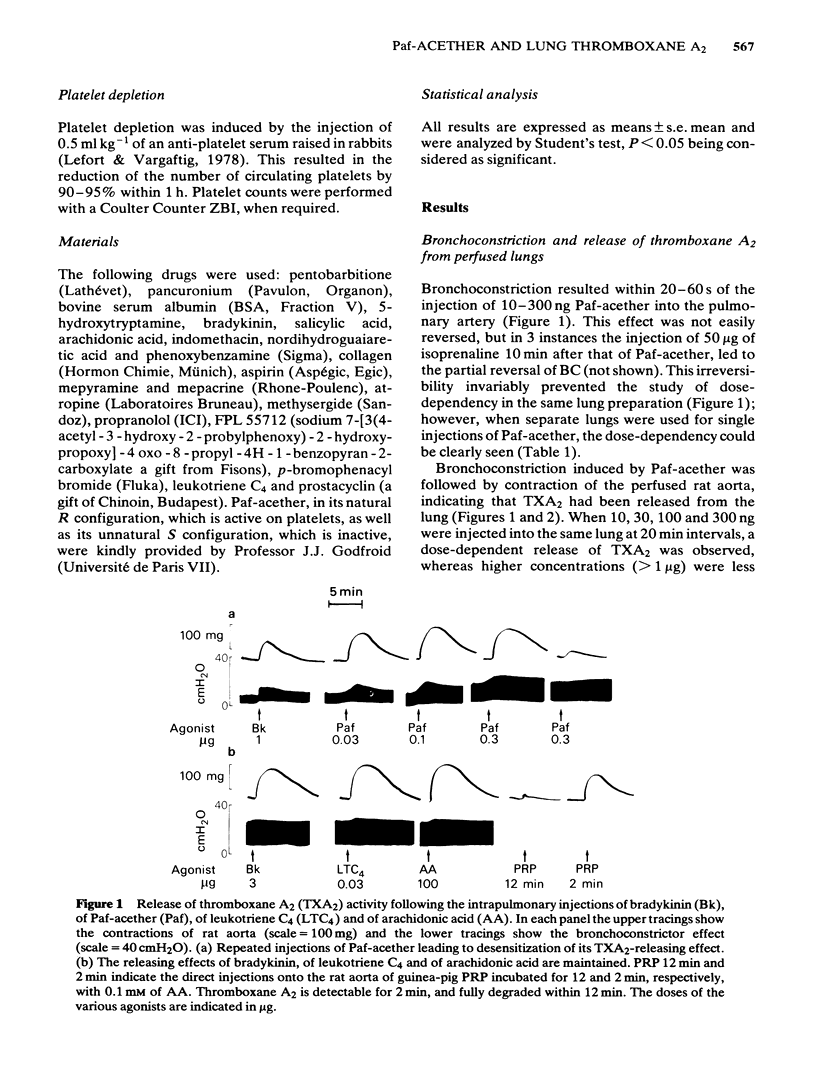
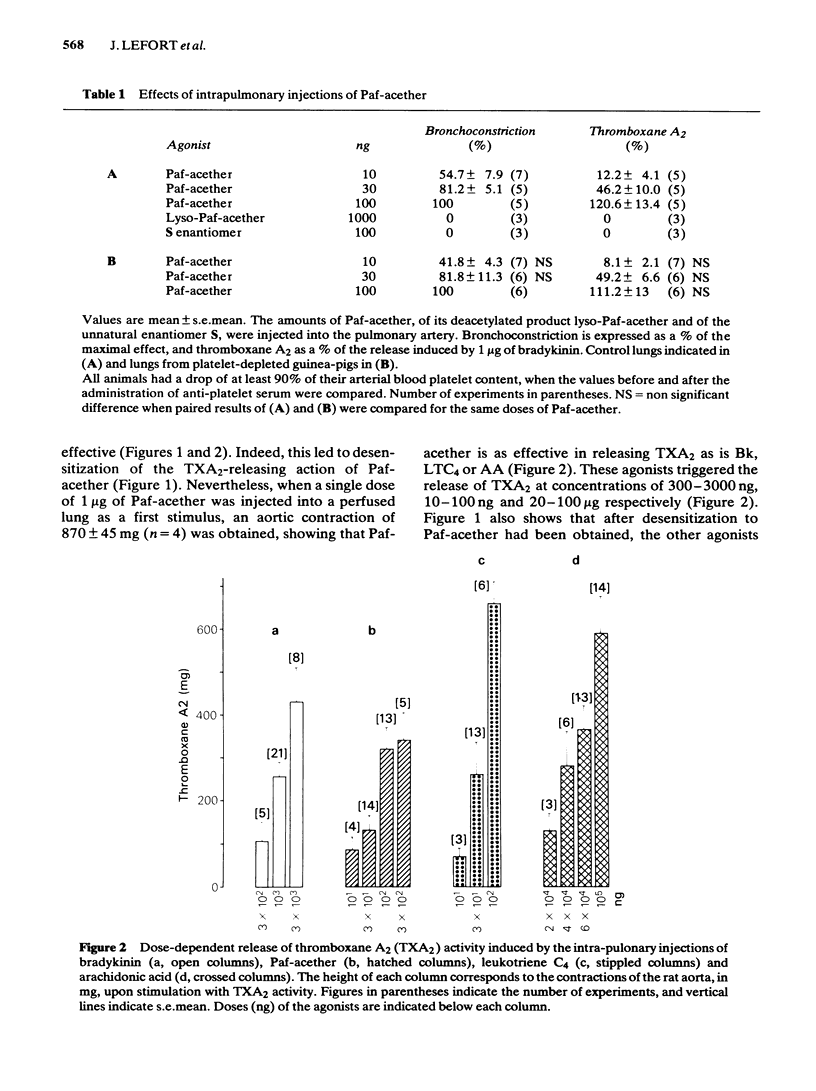
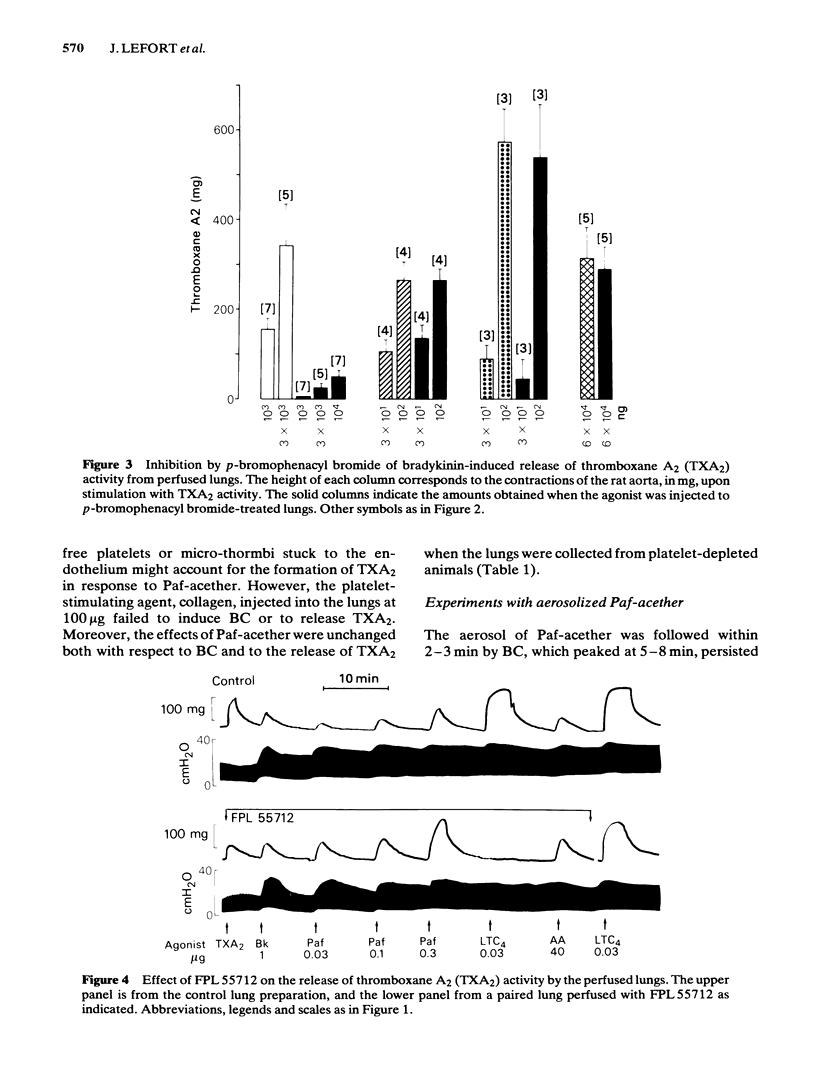
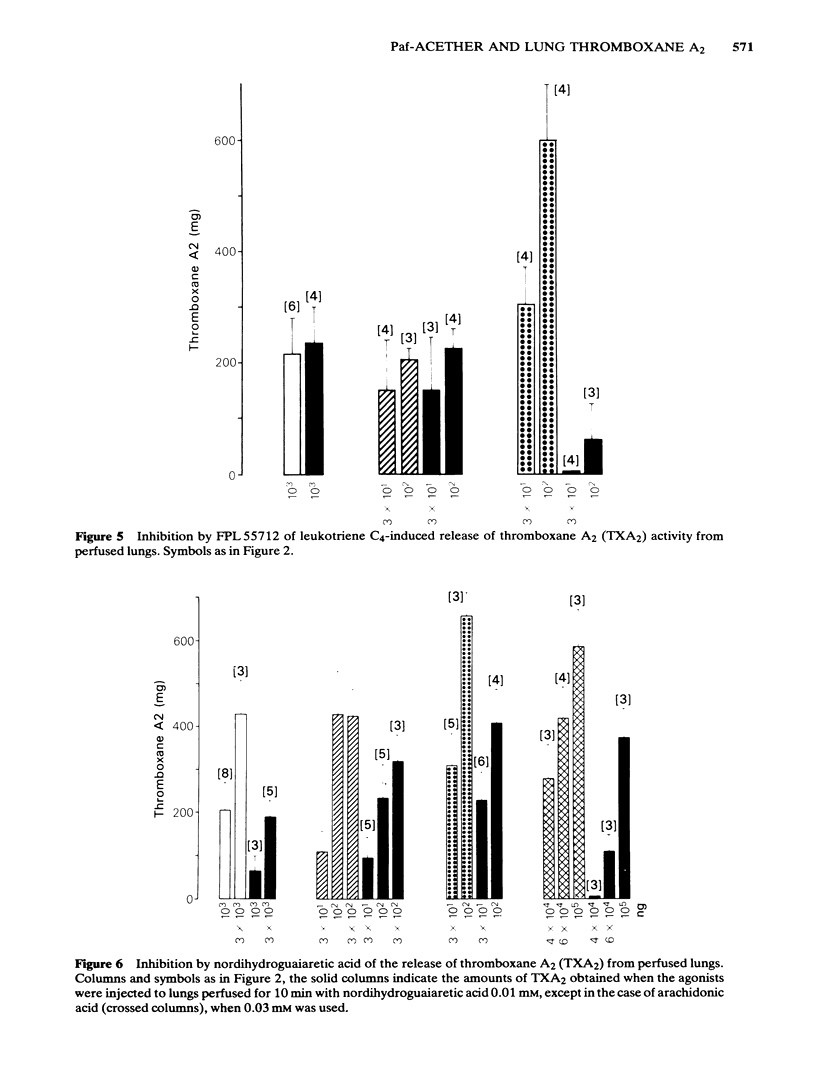
Intra-arterial injections of platelet-activating factor (Paf-acether, 10-300 ng) to the perfused guinea-pig lung induced a dose-related bronchoconstriction, followed by contraction of the rat aorta superfused with the lung effluent, indicating the release of thromboxane A2 (TXA2) activity. These effects were matched with injections of bradykinin (Bk) at 100-1000 ng, leukotriene C4(LTC4) at 10-300 ng or arachidonic acid (AA) at 30-300 micrograms. Repeated doses of Paf-acether led to a specific desensitization of the release of TXA2, under conditions where Bk, LTC4 and arachidonic acid retained their ability to release TXA2. Bronchoconstriction and the release of TXA2 induced by Paf-acether were suppressed when the lungs were perfused with acetylsalicylic acid, but not with salicylic acid. The phospholipase A2 inhibitor, p-bromophenacyl bromide suppressed the release of TXA2 by Bk, but did not interfere with its formation from AA, nor with its release with Paf-acether and LTC4. The lipoxygenase inhibitor, nordihydroguaiaretic acid, inhibited to a similar extent the release of TXA2 by Bk, LTC4 and Paf-acether but also reduced directly the formation of TXA2 from arachidonic acid, invalidating its use as a specific antilipoxygenase agent. The leukotriene C4/D4 antagonist, FPL 55712, suppressed the TXA2 releasing effects of LTC4, and was completely inactive against Paf-acether, Bk or arachidonic acid. The aerosol of Paf-acether was tested in the anaesthetized guinea-pig and resulted in bronchoconstriction, unaccompanied by thrombocytopenia. Unlike bronchoconstriction induced by intravenous Paf-acether, which is refractory to cyclo-oxygenase inhibitors, the effects of the aerosol were suppressed by aspirin. Platelet depletion, which blocks the intravenous effects of Paf-acether, failed to interfere with those of the aerosol. Paf-acether induced a marked contraction of the superfused guinea-pig isolated parenchyma lung strip, which was followed by total and irreversible desensitization to itself. The contractile effect was not inhibited by aspirin or indomethacin, atropine, mepyramine, methysergide, phenoxybenzamine or propranolol, indicating that cyclo-oxygenase products, cholinergic stimuli, histamine, 5-hydroxytryptamine and catecholamine mechanisms are not involved. Our results indicate that Paf-acether interacts with pulmonary sites distinct from those for Bk, LTC4 or AA, since no cross-desensitization between Paf-acether and the other agonists was noted, p-bromophenacyl bromide inhibited Bk only and FPL 55712 inhibited only LTC4.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ubaidi F., Bakhle Y. S. Differences in biological activation of arachidonic acid in perfused lungs from guinea pig, rat and man. Eur J Pharmacol. 1980 Mar 7;62(1):89–96. doi: 10.1016/0014-2999(80)90484-7. [DOI] [PubMed] [Google Scholar]
- Al-Ubaidi F., Bakhle Y. S. The fate of exogenous arachidonic acid in guinea-pig isolated lung. J Physiol. 1979 Oct;295:445–455. doi: 10.1113/jphysiol.1979.sp012979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally A. I., Boucher R., Knowles M. R., Eling T. E. Metabolism of prostaglandin endoperoxide by microsomes from human lung parenchyma and comparison with metabolites produced by pig, bovine, rat, mouse and guinea-pig. Prostaglandins. 1982 Oct;24(4):575–584. doi: 10.1016/0090-6980(82)90015-6. [DOI] [PubMed] [Google Scholar]
- BERRY P. A., COLLIER H. O. BRONCHOCONSTRICTOR ACTION AND ANTAGONISM OF A SLOW-REACTING SUBSTANCE FROM ANAPHYLAXIS OF GUINEA-PIG ISOLATED LUNG. Br J Pharmacol Chemother. 1964 Aug;23:201–216. doi: 10.1111/j.1476-5381.1964.tb01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J., Nijkamp F. P., Vane J. R. Phospholipase A2 activity of guinea-pig isolated perfused lungs: stimulation, and inhibition by anti-inflammatory steroids. Br J Pharmacol. 1978 Jan;62(1):79–89. doi: 10.1111/j.1476-5381.1978.tb07009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Fruteau de Laclos B., Maclouf J. New concepts in the modulation of leukotriene synthesis. Biochem Pharmacol. 1983 Feb 1;32(3):381–387. doi: 10.1016/0006-2952(83)90515-4. [DOI] [PubMed] [Google Scholar]
- COLLIER H. O., SHORLEY P. G. Antagonism by mefenamic and flufenamic acids of the bronchoconstrictor action of kinins in the guinea-pig. Br J Pharmacol Chemother. 1963 Apr;20:345–351. doi: 10.1111/j.1476-5381.1963.tb01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Benveniste J., Mustard J. F. Aggregation of rabbit platelets by platelet-activating factor is independent of the release reaction and the arachidonate pathway and inhibited by membrane-active drugs. Lab Invest. 1979 Sep;41(3):275–285. [PubMed] [Google Scholar]
- Crutchley D. J., Ryan J. W., Ryan U. S., Fisher G. H. Bradykinin-induced release of prostacyclin and thromboxanes from bovine pulmonary artery endothelial cells. Studies with lower homologs and calcium antagonists. Biochim Biophys Acta. 1983 Mar 22;751(1):99–107. doi: 10.1016/0005-2760(83)90261-8. [DOI] [PubMed] [Google Scholar]
- Damas J., Bourdon V. Libération d'acide arachidonique par la bradykinine. C R Seances Soc Biol Fil. 1974;168(10-11-12):1445–1448. [PubMed] [Google Scholar]
- Feinmark S. J., Bailey J. M. Lipid metabolism in cultured cells. Activators of endogenous thromboxane A2 synthesis in cultured lung fibroblasts. J Biol Chem. 1982 Mar 25;257(6):2816–2821. [PubMed] [Google Scholar]
- Franson R., Beckerdite S., Wang P., Waite M., Elsbach P. Some properties of phospholipases of alveolar macrophages. Biochim Biophys Acta. 1973 Feb 14;296(2):365–373. doi: 10.1016/0005-2760(73)90094-5. [DOI] [PubMed] [Google Scholar]
- Hopkins N. K., Sun F. F., Gorman R. R. Thromboxane A2 biosynthesis in human lung fibroblasts WI-38. Biochem Biophys Res Commun. 1978 Nov 29;85(2):827–836. doi: 10.1016/0006-291x(78)91237-8. [DOI] [PubMed] [Google Scholar]
- Lefort J., Vargaftig B. B. Role of platelets in aspirin-sensitive bronchoconstriction in the guinea-pig; interactions with salicylic acid. Br J Pharmacol. 1978 May;63(1):35–42. doi: 10.1111/j.1476-5381.1978.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H. W., Denborough M. A. The metabolism of arachidonic acid in the isolated tracheal and lung strip preparations of guinea-pigs. Lung. 1980;158(3):121–129. doi: 10.1007/BF02713714. [DOI] [PubMed] [Google Scholar]
- Omini C., Folco G. C., Viganò T., Rossoni G., Brunelli G., Berti F. Leukotriene-C4 induces generation of PGI2 and TXA2 in guinea-pig in vivo. Pharmacol Res Commun. 1981 Jul;13(7):633–640. doi: 10.1016/s0031-6989(81)80051-3. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R. Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature. 1969 Jul 5;223(5201):29–35. doi: 10.1038/223029a0. [DOI] [PubMed] [Google Scholar]
- Rankin J. A., Hitchcock M., Merrill W., Bach M. K., Brashler J. R., Askenase P. W. IgE-dependent release of leukotriene C4 from alveolar macrophages. Nature. 1982 May 27;297(5864):329–331. doi: 10.1038/297329a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg F. J., Gimber-Phillips P. E., Groblewski G. E., Davison C., Phillips D. K., Goralnick S. J., Cahill E. D. Acetylsalicylic acid: inhibition of platelet aggregation in the rabbit. J Pharmacol Exp Ther. 1971 Nov;179(2):410–418. [PubMed] [Google Scholar]
- Sahu S., Lynn W. S. Characterization of phospholipase A from pulmonary secretions of patients with alveolar proteinosis. Biochim Biophys Acta. 1977 Nov 24;489(2):307–317. doi: 10.1016/0005-2760(77)90150-3. [DOI] [PubMed] [Google Scholar]
- TAPPEL A. L., LUNDBERG W. O., BOYER P. D. Effect of temperature and antioxidants upon the lipoxidase-catalyzed oxidation of sodium linoleate. Arch Biochem Biophys. 1953 Feb;42(2):293–304. doi: 10.1016/0003-9861(53)90359-2. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B. Carrageenan and thrombin trigger prostaglandin synthetase-independent aggregation of rabbit platelets: inhibition by phospholipase A2 inhibitors. J Pharm Pharmacol. 1977 Apr;29(4):222–228. doi: 10.1111/j.2042-7158.1977.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Dao N. Release of vasoactive substances from guinea-pig lungs by slow-reacting substance c and arachidonic acid. Its blockade by nonsteroid anti-inflammatory agents. Pharmacology. 1971;6(2):99–108. doi: 10.1159/000136231. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Ferreira S. H. Blockade of the inflammatory effects of platelet-activating factor by cyclo-oxygenase inhibitors. Braz J Med Biol Res. 1981 Jul;14(2-3):187–189. [PubMed] [Google Scholar]
- Vargaftig B. B., Hai N. D. Selective inhibition by mepacrine of the release of "rabbit aorta contracting substance" evoked by the administration of bradykinin. J Pharm Pharmacol. 1972 Feb;24(2):159–161. doi: 10.1111/j.2042-7158.1972.tb08953.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Murphy R. C. Inhibition by aspirin of bronchoconstriction due to leukotrienes C4 and D4 in the guinea pig. Eur J Pharmacol. 1981 Jul 10;72(4):417–418. doi: 10.1016/0014-2999(81)90589-6. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Wal F., Chignard M., Medeiros M. C. Non-steroidal anti-inflammatory drugs if combined with anti-histamine and anti-serotonin agents interfere with the bronchial and platelet effects of "platelet-activating factor" (PAF-acether). Eur J Pharmacol. 1982 Aug 27;82(3-4):121–130. doi: 10.1016/0014-2999(82)90500-3. [DOI] [PubMed] [Google Scholar]
- Vensel L. A., Kantrowitz E. R. An essential arginine residue in porcine phospholipiase A2. J Biol Chem. 1980 Aug 10;255(15):7306–7310. [PubMed] [Google Scholar]
- Voelkel N. F., Worthen S., Reeves J. T., Henson P. M., Murphy R. C. Nonimmunological production of leukotrienes induced by platelet-activating factor. Science. 1982 Oct 15;218(4569):286–289. doi: 10.1126/science.7123233. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- Yoneda K. Ultrastructural localization of phospholipases in the Clara cell of the rat bronchiole. Am J Pathol. 1978 Dec;93(3):745–752. [PMC free article] [PubMed] [Google Scholar]


