Abstract
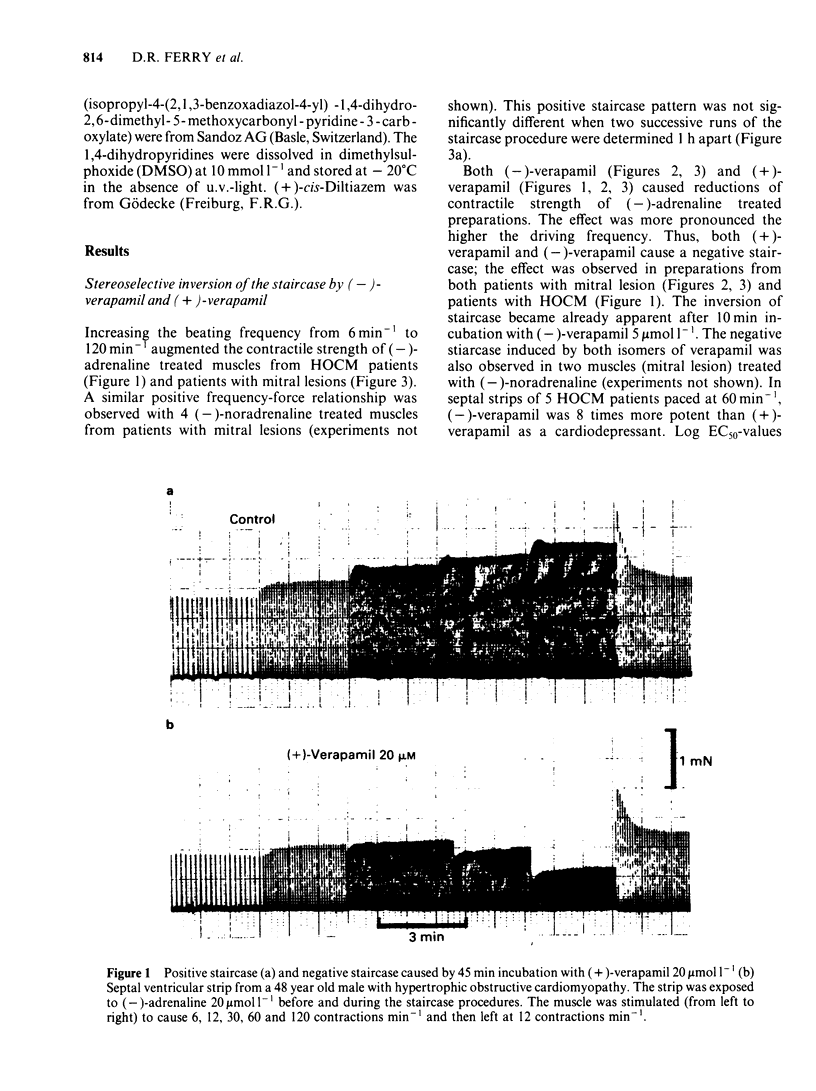
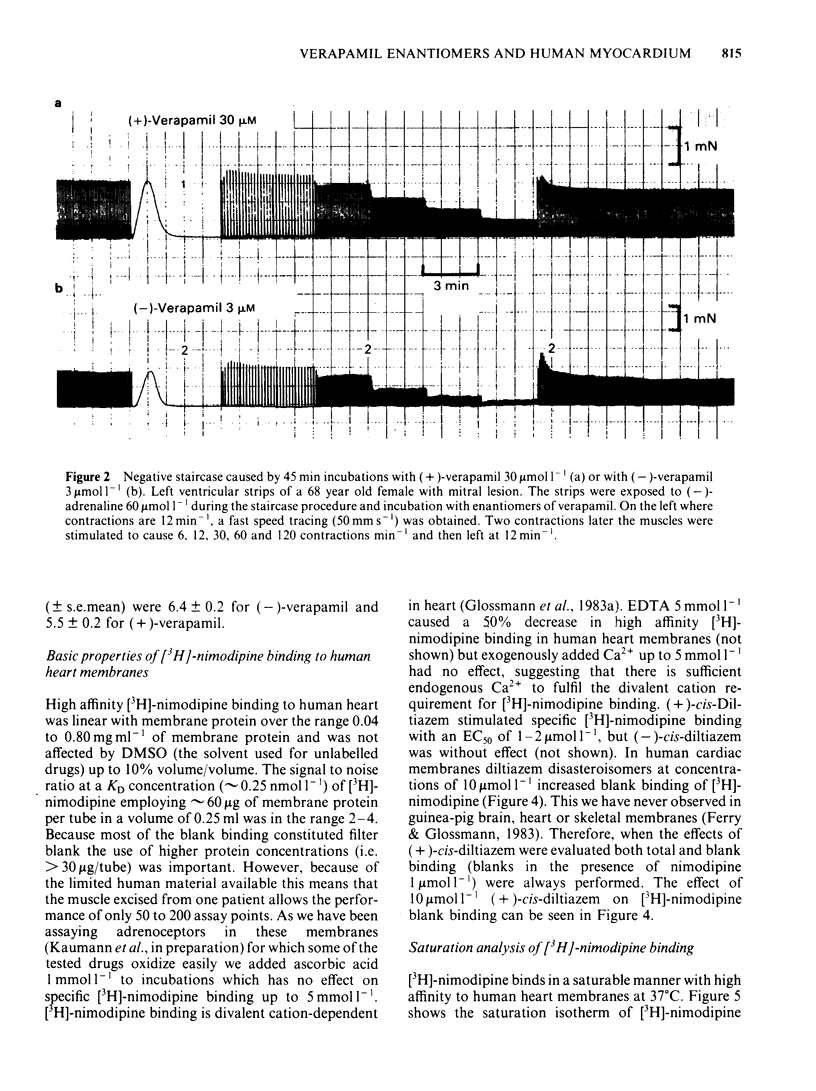
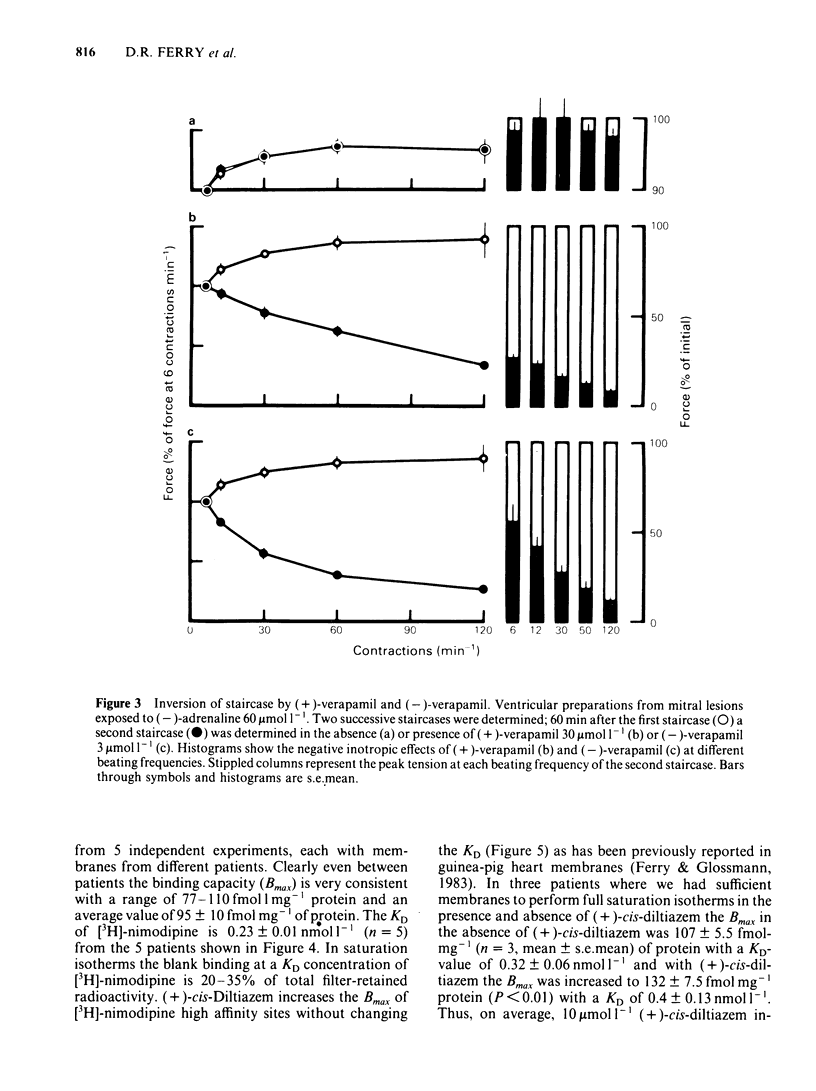
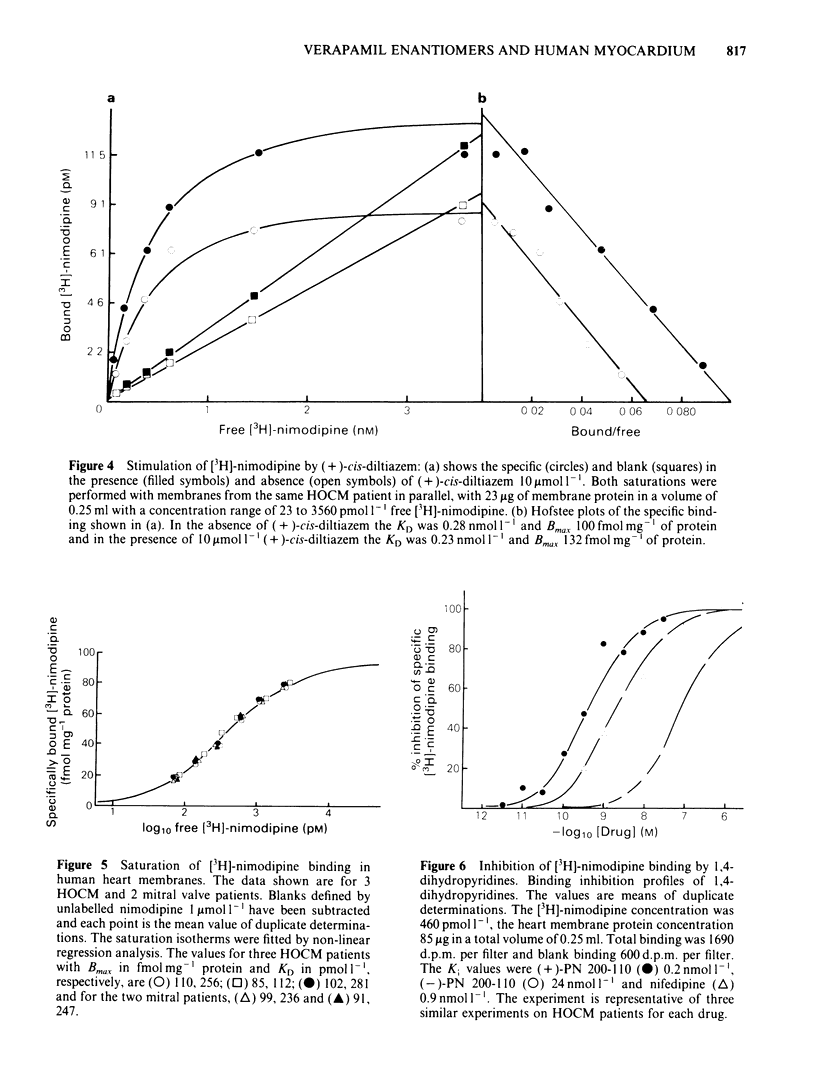
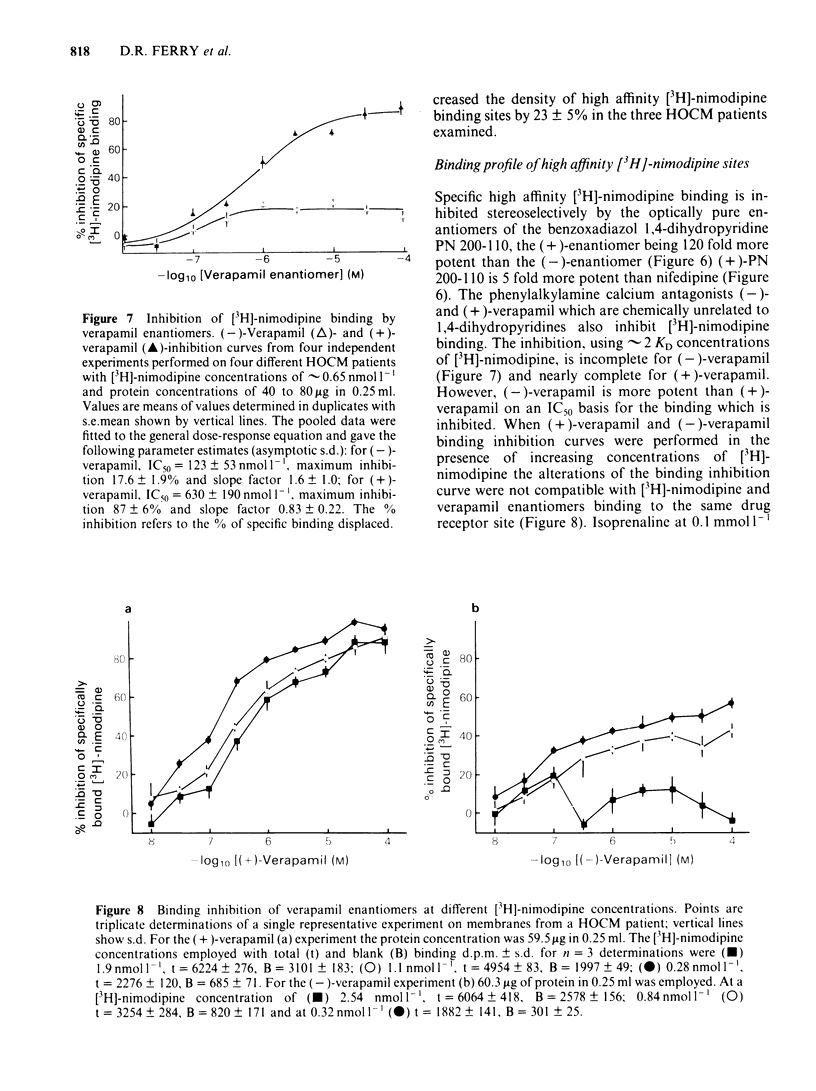
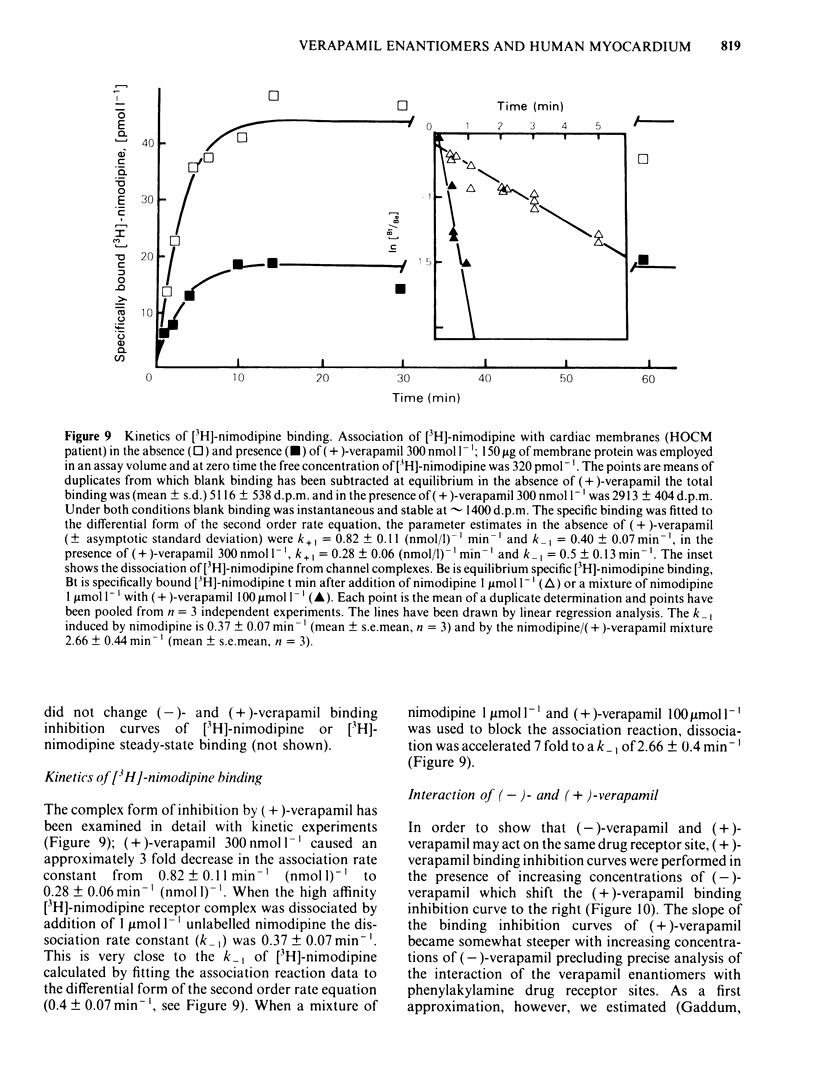
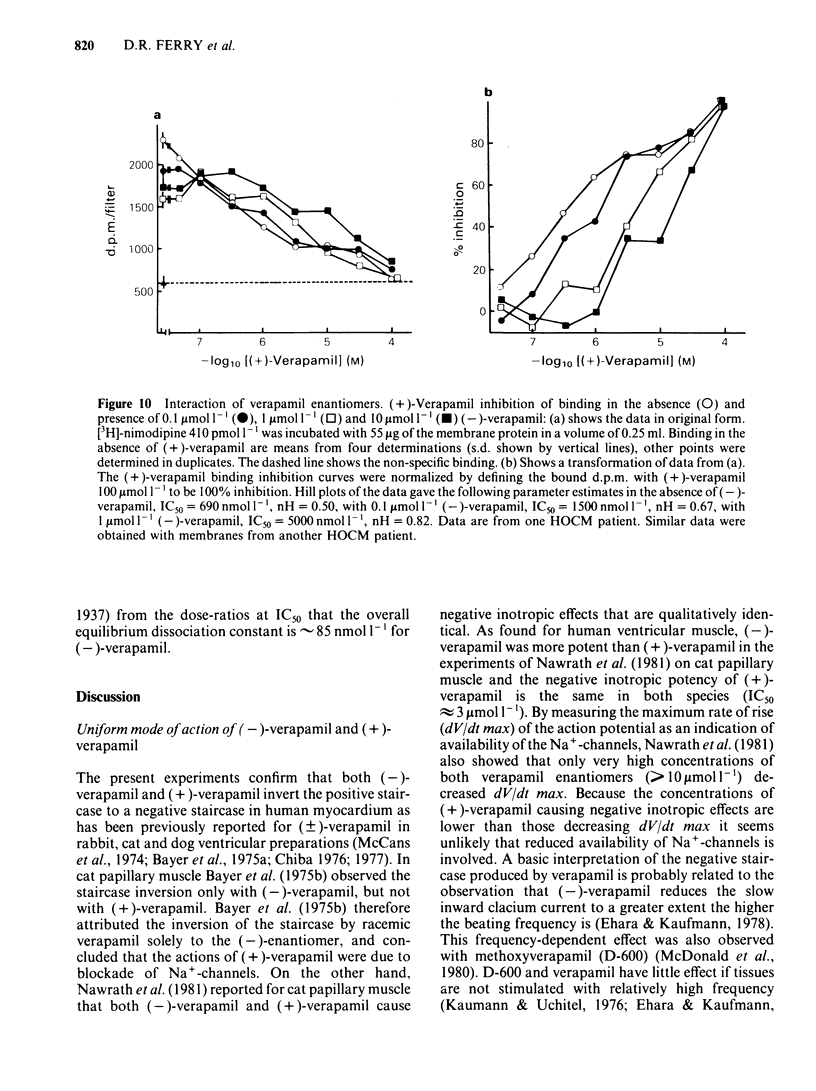
Ventricular preparations from patients with mitral disease and hypertrophic obstructive cardiomyopathy (HOCM) were set up to contract isometrically. Ventricular membrane particles were also prepared and putative calcium channels were labelled with [3H]-nimodipine. Positive staircase was induced by varying the rate of stimulation of isolated strips from 6 min-1 to 120 min-1 in the presence of 6-60 microM (-)-adrenaline or (-)-noradrenaline. (-)-Verapamil 3-5 microM or (+)-verapamil 20-30 microM reversed the force-frequency relationship (i.e. caused negative staircase) in preparations from patients with mitral disease or HOCM. In subendocardial strips of ventricular septum from 5 patients with HOCM paced at 60 min-1, both (-)-verapamil and (+)-verapamil caused cardiodepression. Half-maximal cardiodepression was observed with 0.4 microM (-)-verapamil and with 3 microM (+)-verapamil. [3H]-nimodipine bound to ventricular membrane particles in a saturable, reversible fashion to a high affinity site with an equilibrium dissociation constant of 0.23 nM. The density of these sites was 95 fmol mg-1 of membrane protein. Binding of the tritiated 1,4-dihydropyridine was stereoselectively inhibited by 1,4-dihydropyridine enantiomers and nifedipine. (-)-Verapamil and (+)-verapamil inhibited high affinity [3H]-nimodipine binding in a negative heterotropic allosteric manner with (-)-verapamil being 5 times more potent than (+)-verapamil on an IC50 basis. At a given [3H]-nimodipine concentration, (+)-verapamil inhibited a greater fraction of specific [3H]-nimodipine binding. The allosteric mode of (+)-verapamil inhibition of [3H]-nimodipine binding was confirmed by kinetic studies. (-)-Verapamil shifted (+)-verapamil-binding inhibition curves to the right in an apparently competitive fashion. The inversion of staircase caused by both verapamil enantiomers suggests that they cause a use-dependent channel blockade. The similar potency ratios for binding and for cardiodepression are indicative of a common locus of action for both verapamil enantiomers within the calcium channel.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer R., Hennekes R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. I. Pattern of inotropic effects of the racemic compounds. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):49–68. doi: 10.1007/BF00499989. [DOI] [PubMed] [Google Scholar]
- Bayer R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. II. Pattern of inotropic effects of the optical isomers. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):69–80. doi: 10.1007/BF00499990. [DOI] [PubMed] [Google Scholar]
- Blinks J. R. Convenient apparatus for recording contractions of isolated heart muscle. J Appl Physiol. 1965 Jul;20(4):755–757. doi: 10.1152/jappl.1965.20.4.755. [DOI] [PubMed] [Google Scholar]
- Bolger G. T., Gengo P., Klockowski R., Luchowski E., Siegel H., Janis R. A., Triggle A. M., Triggle D. J. Characterization of binding of the Ca++ channel antagonist, [3H]nitrendipine, to guinea-pig ileal smooth muscle. J Pharmacol Exp Ther. 1983 May;225(2):291–309. [PubMed] [Google Scholar]
- Chiba S. Effect of pentobarbital, verapamil and manganese on the frequency-force relationship of the isolated atrium and ventricle of the dog heart. Eur J Pharmacol. 1976 Dec;40(2):225–232. doi: 10.1016/0014-2999(76)90056-x. [DOI] [PubMed] [Google Scholar]
- Chiba S. Effect of verapamil of frequency-force relationship in isolated dog left ventricular muscle. Jpn J Pharmacol. 1977 Feb;27(1):175–177. doi: 10.1254/jjp.27.175. [DOI] [PubMed] [Google Scholar]
- DePover A., Grupp I. L., Grupp G., Schwartz A. Diltiazem potentiates the negative inotropic action of nimodipine in heart. Biochem Biophys Res Commun. 1983 Aug 12;114(3):922–929. doi: 10.1016/0006-291x(83)90648-4. [DOI] [PubMed] [Google Scholar]
- DePover A., Lee S. W., Matlib M. A., Whitmer K., Davis B. A., Powell T., Schwartz A. [3H]nimodipine specific binding to cardiac myocytes and subcellular fractions. Biochem Biophys Res Commun. 1983 May 31;113(1):185–191. doi: 10.1016/0006-291x(83)90449-7. [DOI] [PubMed] [Google Scholar]
- Ehara T., Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther. 1978 Oct;207(1):49–55. [PubMed] [Google Scholar]
- Eichelbaum M., Birkel P., Grube E., Gütgemann U., Somogyi A. Effects of verapamil on P-R-intervals in relation to verapamil plasma levels following single I.V. and oral administration and during chronic treatment. Klin Wochenschr. 1980 Sep 15;58(18):919–925. doi: 10.1007/BF01477049. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Mikus G., Vogelgesang B. Pharmacokinetics of (+)-, (-)- and (+/-)-verapamil after intravenous administration. Br J Clin Pharmacol. 1984 Apr;17(4):453–458. doi: 10.1111/j.1365-2125.1984.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Somogyi A., von Unruh G. E., Dengler H. J. Simultaneous determination of the intravenous and oral pharmacokinetic parameters of D,L-verapamil using stable isotope-labelled verapamil. Eur J Clin Pharmacol. 1981 Jan;19(2):133–137. doi: 10.1007/BF00568400. [DOI] [PubMed] [Google Scholar]
- Fairhurst A. S., Whittaker M. L., Ehlert F. J. Interactions of D600 (methoxyverapamil) and local anesthetics with rat brain alpha-adrenergic and muscarinic receptors. Biochem Pharmacol. 1980 Feb;29(2):155–162. doi: 10.1016/0006-2952(80)90323-8. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Glossmann H. Evidence of multiple receptor sites within the putative calcium channel. Naunyn Schmiedebergs Arch Pharmacol. 1982 Oct;321(1):80–83. doi: 10.1007/BF00586355. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Glossmann H. Identification of putative calcium channels in skeletal muscle microsomes. FEBS Lett. 1982 Nov 8;148(2):331–337. doi: 10.1016/0014-5793(82)80835-1. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Goll A., Gadow C., Glossmann H. (-)-3H-desmethoxyverapamil labelling of putative calcium channels in brain: autoradiographic distribution and allosteric coupling to 1,4-dihydropyridine and diltiazem binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1984 Sep;327(2):183–187. doi: 10.1007/BF00500915. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A., Kammermeier H., Döring H. J., Freund H. J. Zum Wirkungsmechanismus neuartiger Koronardilatatoren mit gleichzeitig Sauerstoff-einsparenden Myokard-Effekten, Prenylamin und Iproveratril. 1. Z Kreislaufforsch. 1967 Jul;56(7):716–744. [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Lazdunski M. [3H] verapamil binding sites in skeletal muscle transverse tubule membranes. Biochem Biophys Res Commun. 1984 Jan 13;118(1):239–245. doi: 10.1016/0006-291x(84)91092-1. [DOI] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Glossmann H. Target size analysis and molecular properties of Ca2+ channels labelled with [3H]verapamil. Eur J Biochem. 1984 May 15;141(1):177–186. doi: 10.1111/j.1432-1033.1984.tb08172.x. [DOI] [PubMed] [Google Scholar]
- Holck M., Thorens S., Haeusler G. Characterization of [3H]nifedipine binding sites in rabbit myocardium. Eur J Pharmacol. 1982 Dec 3;85(3-4):305–315. doi: 10.1016/0014-2999(82)90217-5. [DOI] [PubMed] [Google Scholar]
- Janis R. A., Maurer S. C., Sarmiento J. G., Bolger G. T., Triggle D. J. Binding of [3H]nimodipine to cardiac and smooth muscle membranes. Eur J Pharmacol. 1982 Aug 27;82(3-4):191–194. doi: 10.1016/0014-2999(82)90511-8. [DOI] [PubMed] [Google Scholar]
- Kaltenbach M., Hopf R., Keller M. Calciumantagonistische Therapie bei hypertroph-obstruktiver Kardiomyopathie. Dtsch Med Wochenschr. 1976 Aug 27;101(35):1284–1287. doi: 10.1055/s-0028-1104257. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J. Adrenergic receptors in heart muscle: relations among factors influencing the sensitivity of the cat papillary muscle to catecholamines. J Pharmacol Exp Ther. 1970 Jun;173(2):383–398. [PubMed] [Google Scholar]
- Kaumann A. J., Birnbaumer L. Studies on receptor-mediated activation of adenylyl cyclases. IV. Characteristics of the adrenergic receptor coupled to myocardial adenylyl cyclase: stereospecificity for ligands and determination of apparent affinity constants for beta-blockers. J Biol Chem. 1974 Dec 25;249(24):7874–7885. [PubMed] [Google Scholar]
- Kaumann A. J., Lemoine H., Morris T. H., Schwederski U. An initial characterization of human heart beta-adrenoceptors and their mediation of the positive inotropic effects of catecholamines. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):216–221. doi: 10.1007/BF00495868. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Serur J. R. Optical isomers of verapamil on canine heart. Prevention of ventricular fibrillation induced by coronary artery occlusion, impaired atrioventricular conductance and negative inotropic and chronotropic effects. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(4):347–358. doi: 10.1007/BF00501793. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Uchitel O. D. Reversible Inhibition of Potassium Contractures by optical isomers of verapamil and D 600 on slow muscle fibres of the frog. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(1):21–27. doi: 10.1007/BF00506485. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Mnich Z. Studies on the inhibitory effect of verapamil on the slow inward current in mammalian ventricular myocardium. J Mol Cell Cardiol. 1978 Nov;10(11):1037–1052. doi: 10.1016/0022-2828(78)90400-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCans J. L., Lindenmayer G. E., Munson R. G., Evans R. W., Schwartz A. A dissociation of positive staircase (Bowditch) from ouabain-induced positive inotropism. Circ Res. 1974 Sep;35(3):439–447. doi: 10.1161/01.res.35.3.439. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. On the mechanism of slow calcium channel block in heart. Pflugers Arch. 1980 May;385(2):175–179. doi: 10.1007/BF00588699. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Gould R. J., Largent B. L., Snyder S. H. A unitary mechanism of calcium antagonist drug action. Proc Natl Acad Sci U S A. 1983 Feb;80(3):860–864. doi: 10.1073/pnas.80.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Raschack M. Relationship of antiarrhythmic to inotropic activity and antiarrhythmic qualities of the optical isomers of verapamil. Naunyn Schmiedebergs Arch Pharmacol. 1976 Sep;294(3):285–291. doi: 10.1007/BF00508397. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing D. R., Kent K. M., Borer J. S., Seides S. F., Maron B. J., Epstein S. E. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. I. Hemodynamic effects. Circulation. 1979 Dec;60(6):1201–1207. doi: 10.1161/01.cir.60.6.1201. [DOI] [PubMed] [Google Scholar]
- Towart R., Wehinger E., Meyer H. Effects of unsymmetrical ester substituted 1,4-dihydropyridine derivatives and their optical isomers on contraction of smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):183–185. doi: 10.1007/BF00500079. [DOI] [PubMed] [Google Scholar]
- Wagner J., Schümann H. J., Knorr A., Rohm N., Reidemeister J. C. Stimulation by adrenaline and dopamine but not by noradrenaline of myocardial alpha-adrenoceptors mediating positive inotropic effects in human atrial preparations. Naunyn Schmiedebergs Arch Pharmacol. 1980 May;312(1):99–102. doi: 10.1007/BF00502581. [DOI] [PubMed] [Google Scholar]


