Abstract
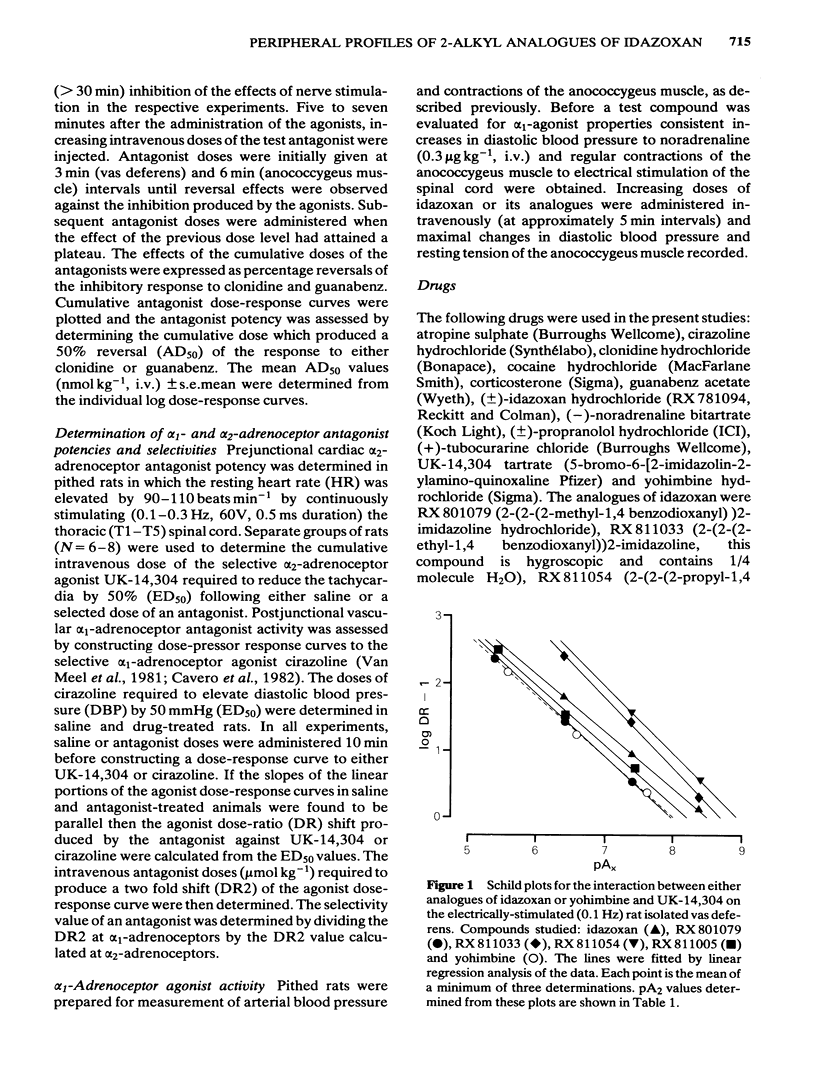
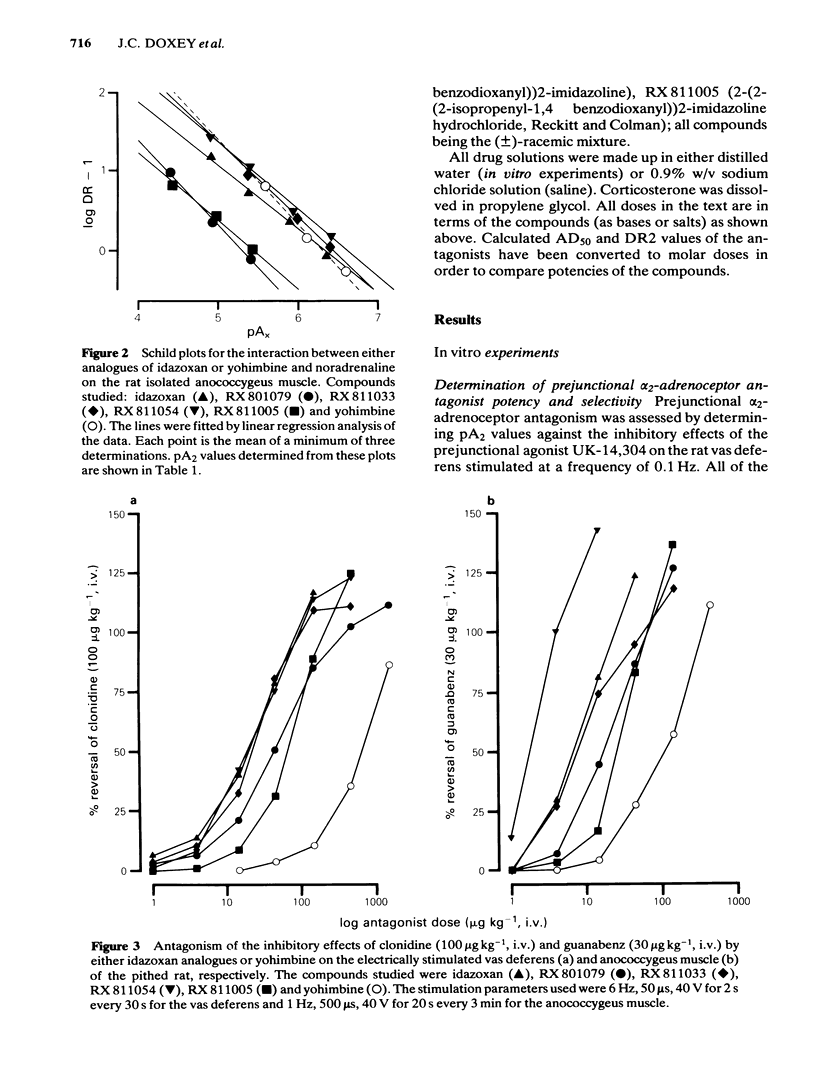
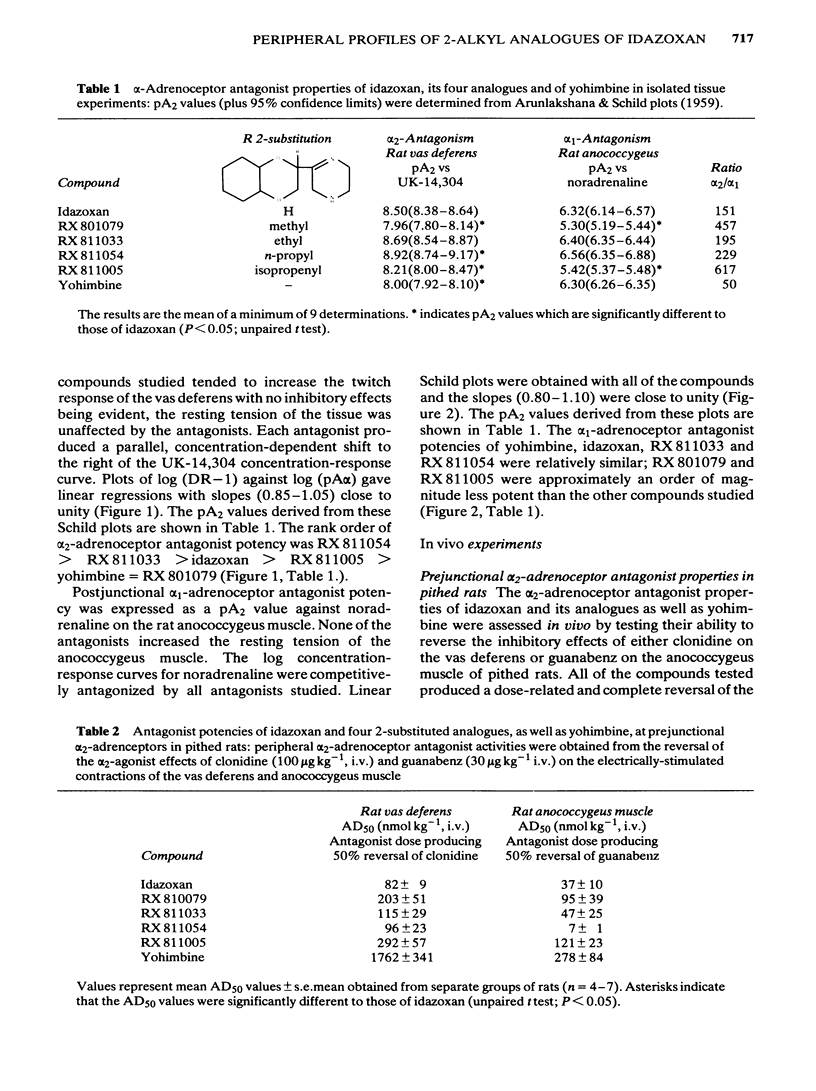
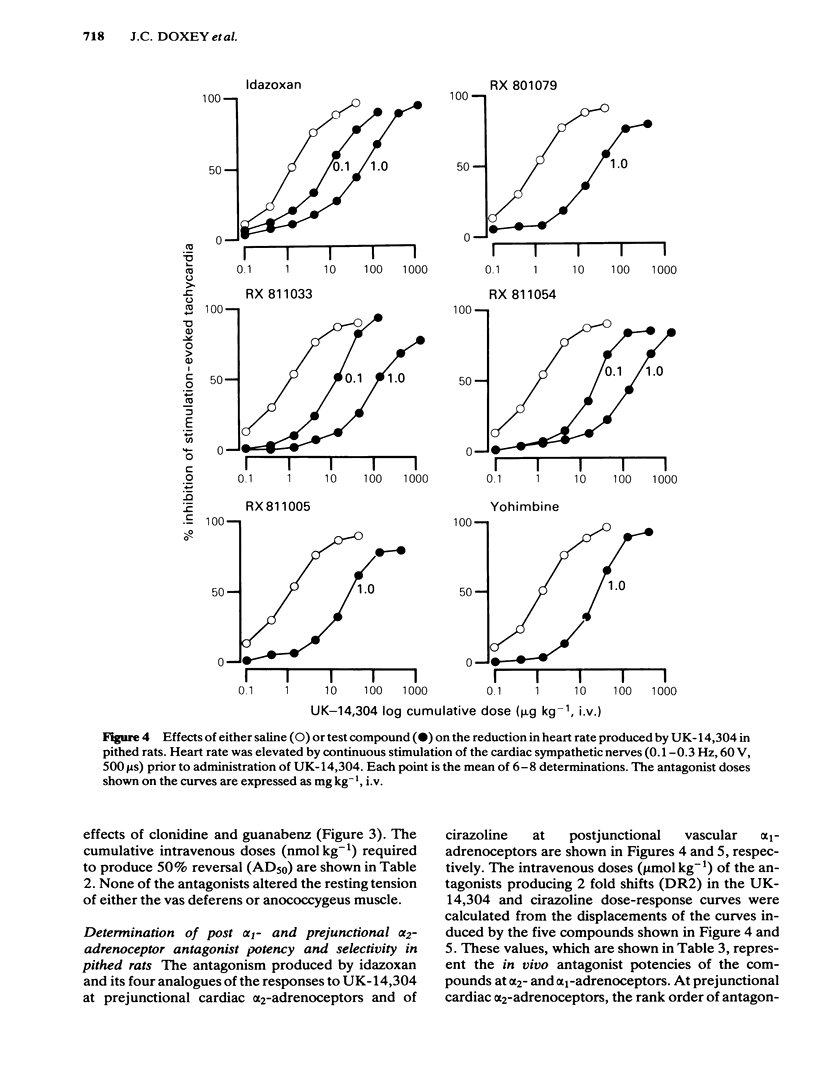
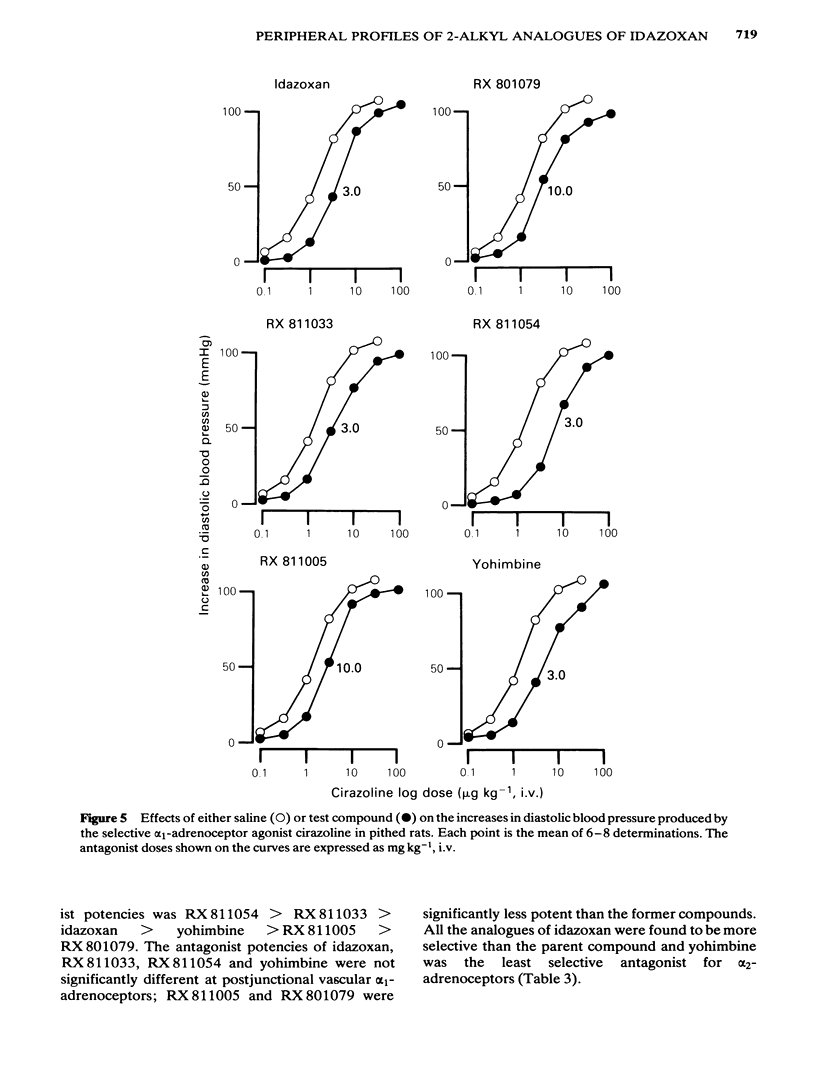
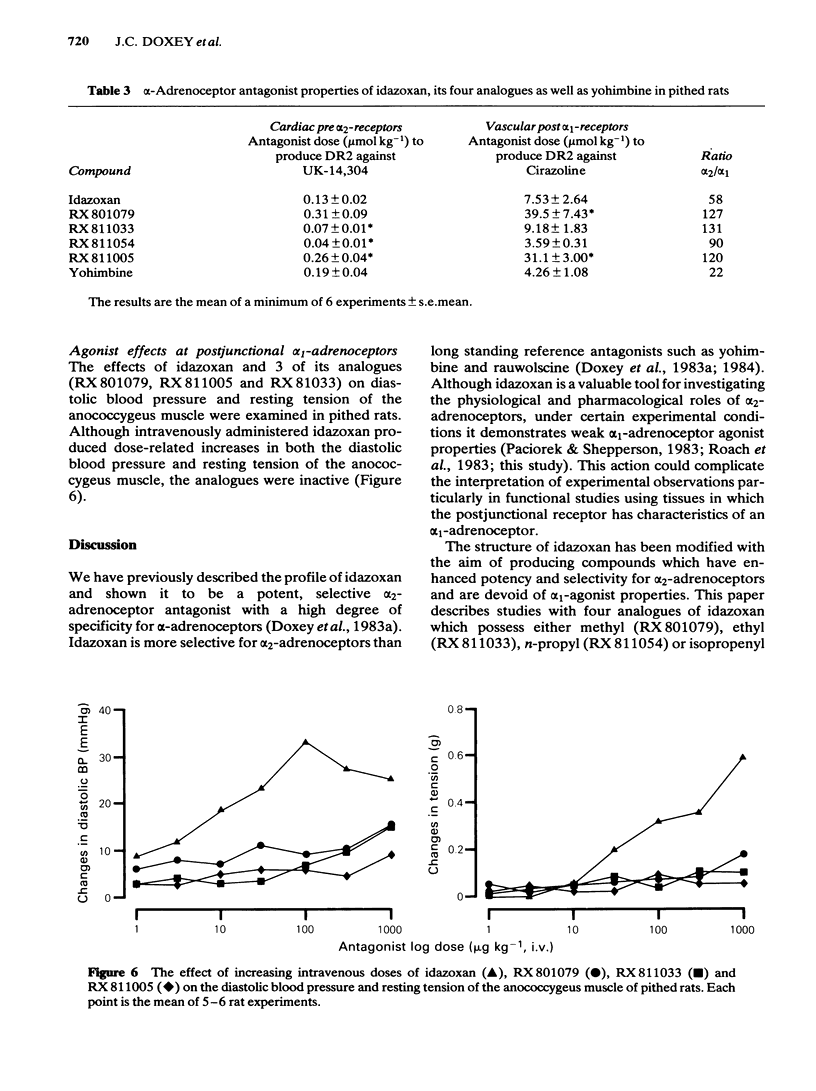
The profiles of four analogues of idazoxan have been examined at alpha-adrenoceptors and the results compared to those obtained with idazoxan and yohimbine. The compounds possessed either a methyl (RX 801079), ethyl (RX 811033), n-propyl (RX 811054) or isopropenyl (RX 811005) group at the two position of idazoxan. The rank order of antagonist potency against UK-14,304 at prejunctional alpha 2-adrenoceptors of the rat isolated vas deferens was RX 811054 greater than RX 811033 greater than idazoxan greater than RX 811005 greater than yohimbine = RX 801079. All compounds were competitive antagonists. The rank order of antagonist potency against noradrenaline at postjunctional alpha 1-adrenoceptors of the rat isolated anococcygeus muscle was RX 811054 = RX 811033 = idazoxan = yohimbine greater than RX 811005 = RX801079. All compounds were competitive antagonists. The rank order of alpha-adrenoceptor selectivity (alpha 2/alpha 1) was RX 811005 greater than RX 801079 greater than RX 811054 greater than RX 811033 greater than idazoxan greater than yohimbine. In pithed rats, intravenous administration of all compounds fully reversed the prejunctional alpha 2-adrenoceptor agonist effects of clonidine and guanabenz on electrically-induced contractions of the vas deferens and anococcygeus muscle respectively. In pithed rats the rank order of antagonist potency against UK-14,304 at cardiac prejunctional alpha 2-adrenoceptors was RX 811054 greater than RX 811033 greater than idazoxan greater than yohimbine greater than RX 811005 greater than RX 801079. In contrast, the rank order of antagonist potency against cirazoline pressor effects (vascular postjunctional alpha 1-adrenoceptors) was RX 811054 greater than RX 811033 greater than yohimbine greater than idazoxan greater than RX 811005 greater than RX 801079. The rank order of alpha 2-adrenoceptor selectivity was RX 811033 = RX 801079 = RX 801005 greater than RX 811054 greater than idazoxan greater than yohimbine. Although idazoxan produced contractions of the anococcygeus muscle and increased blood pressure in pithed rats, three of the analogues (RX 811005, RX 801079 and RX 811033) were inactive. In conclusion, alkyl substitution in the 2-position of idazoxan can enhance either alpha 2-adrenoceptor antagonist potency or selectivity or both and furthermore, the weak partial alpha 1-adrenoceptor agonist properties of idazoxan can be removed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge D. UK-14,304, a potent and selective alpha2-agonist for the characterisation of alpha-adrenoceptor subtypes. Eur J Pharmacol. 1981 Jul 10;72(4):413–415. doi: 10.1016/0014-2999(81)90588-4. [DOI] [PubMed] [Google Scholar]
- Cavero I., Lefèvre-Borg F., Roach A. G., Gomeni R., Scatton B. Functional and biochemical evidence for the lack of cardiac presynaptic alpha-2 adrenoceptor stimulant properties of cirazoline (LD 3098), a potent alpha-1 adrenoceptor agonist in dogs and rats. J Pharmacol Exp Ther. 1982 Oct;223(1):241–250. [PubMed] [Google Scholar]
- Dettmar P. W., Lynn A. G., Tulloch I. F. Neuropharmacological studies in rodents on the action of RX 781094, a new selective alpha 2-adrenoceptor antagonist. Neuropharmacology. 1983 Jun;22(6):729–737. doi: 10.1016/0028-3908(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Docherty J. R., McGrath J. C. A comparison of pre- and post-junctional potencies of several alpha-adrenoceptor agonists in the cardiovascular system and anococcygeus muscle of the rat. Evidence for two types of post-junctional alpha-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol. 1980 Jun;312(2):107–116. doi: 10.1007/BF00569718. [DOI] [PubMed] [Google Scholar]
- Doxey J. C., Everitt J. Inhibitory effects of clonidine on responses to sympathetic nerve stimulation in the pithed rat. Br J Pharmacol. 1977 Dec;61(4):559–566. doi: 10.1111/j.1476-5381.1977.tb07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Lane A. C., Roach A. G., Virdee N. K. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):136–144. doi: 10.1007/BF00506193. [DOI] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Smith C. F. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983 Mar;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadie B., Lane A. C., McCarthy P. S., Tulloch I. F., Walter D. S. 2-Alkyl analogue of idazoxan (RX 781094) with enhanced antagonist potency and selectivity at central alpha 2-adrenoceptors in the rat. Br J Pharmacol. 1984 Nov;83(3):707–712. doi: 10.1111/j.1476-5381.1984.tb16224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek P. M., Shepperson N. B. alpha 1-Adrenoceptor agonist activity of alpha 2-adrenoceptor antagonists in the pithed rat preparation. Br J Pharmacol. 1983 May;79(1):12–14. doi: 10.1111/j.1476-5381.1983.tb10488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel J. C., de Jonge A., Timmermans P. B., van Zwieten P. A. Selectivity of some alpha adrenoceptor agonists for peripheral alpha-1 and alpha-2 adrenoceptors in the normotensive rat. J Pharmacol Exp Ther. 1981 Dec;219(3):760–767. [PubMed] [Google Scholar]


