Abstract
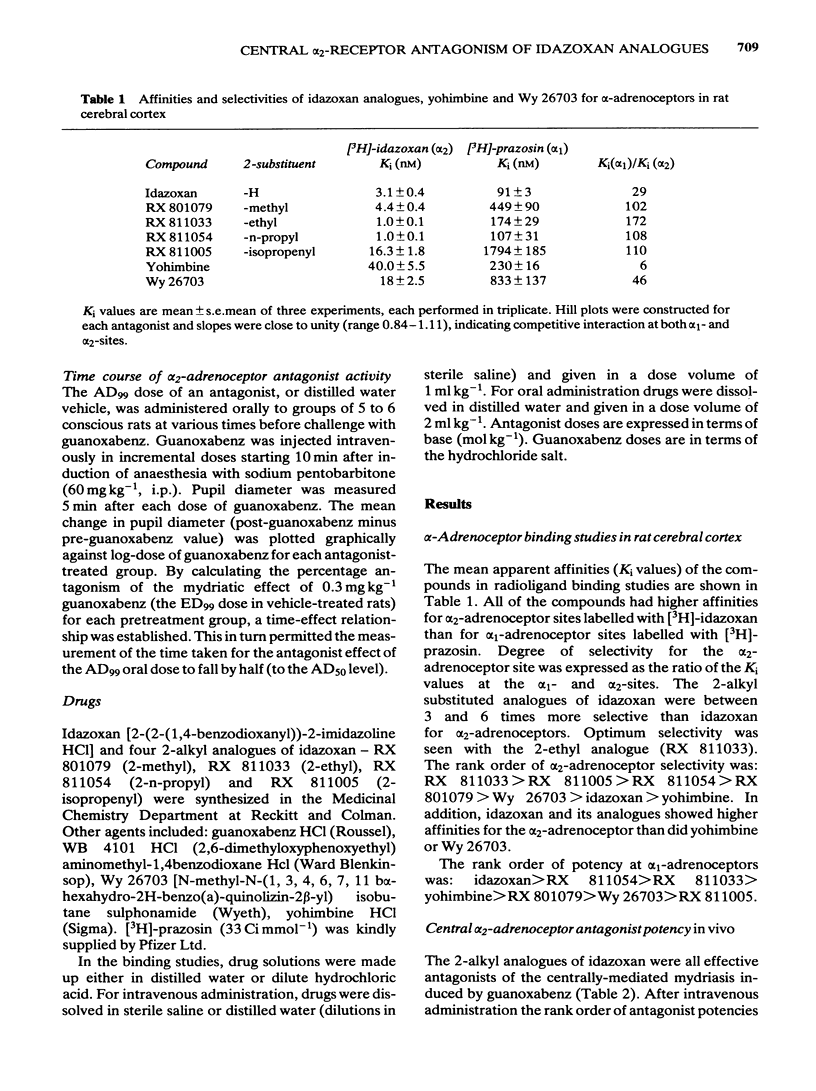
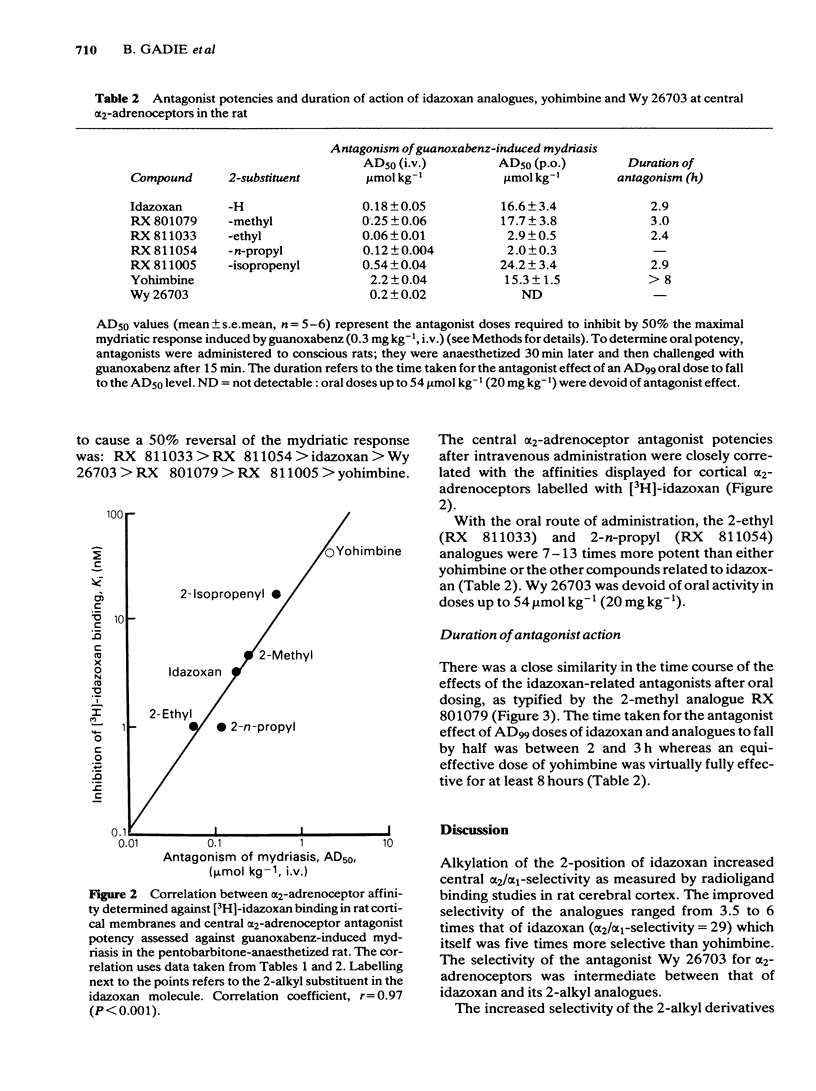
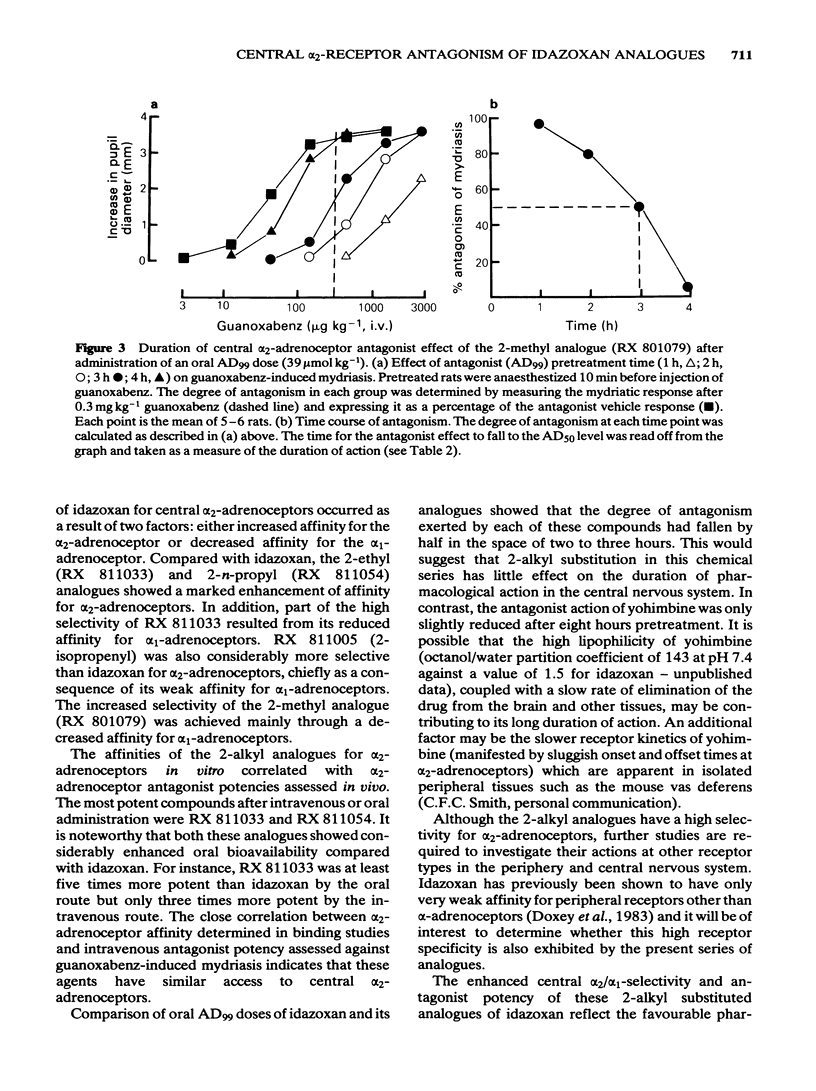
Four 2-alkyl (methyl, ethyl, n-propyl and isopropenyl) analogues of idazoxan (RX 781094) have been synthesized and assessed in terms of their central alpha 2/alpha 1-adrenoceptor selectivity and alpha 2-adrenoceptor antagonist potency using both in vitro and in vivo tests in the rat. In cortical binding assays using [3H]-idazoxan and [3H]-prazosin, idazoxan had a 5 times greater alpha 2/alpha 1-selectivity than yohimbine. The 2-alkyl substituted analogues all showed improved selectivity, being between 17 and 29 times more selective than yohimbine for [3H]-idazoxan binding sites. In terms of central antagonist potency in vivo, the most favourable substitutions were 2-ethyl (RX 811033) and 2-n-propyl (RX 811054). Compared with yohimbine, these analogues were, respectively, 36 and 18 times more potent intravenously and 5 and 7.5 times more potent orally in their antagonism of guanoxabenz-induced mydriasis in the pentobarbitone-anaesthetized rat. All the analogues had a duration of action similar to that of idazoxan, which was significantly shorter than that of yohimbine. The results indicate that introduction of alkyl groups in the 2-position of idazoxan greatly increases the alpha 2/alpha 1-adrenoceptor selectivity as measured in binding studies. Improved alpha 2-adrenoceptor affinity and antagonist potency were particularly associated with the 2-ethyl and 2-n-propyl analogues.
Full text
PDF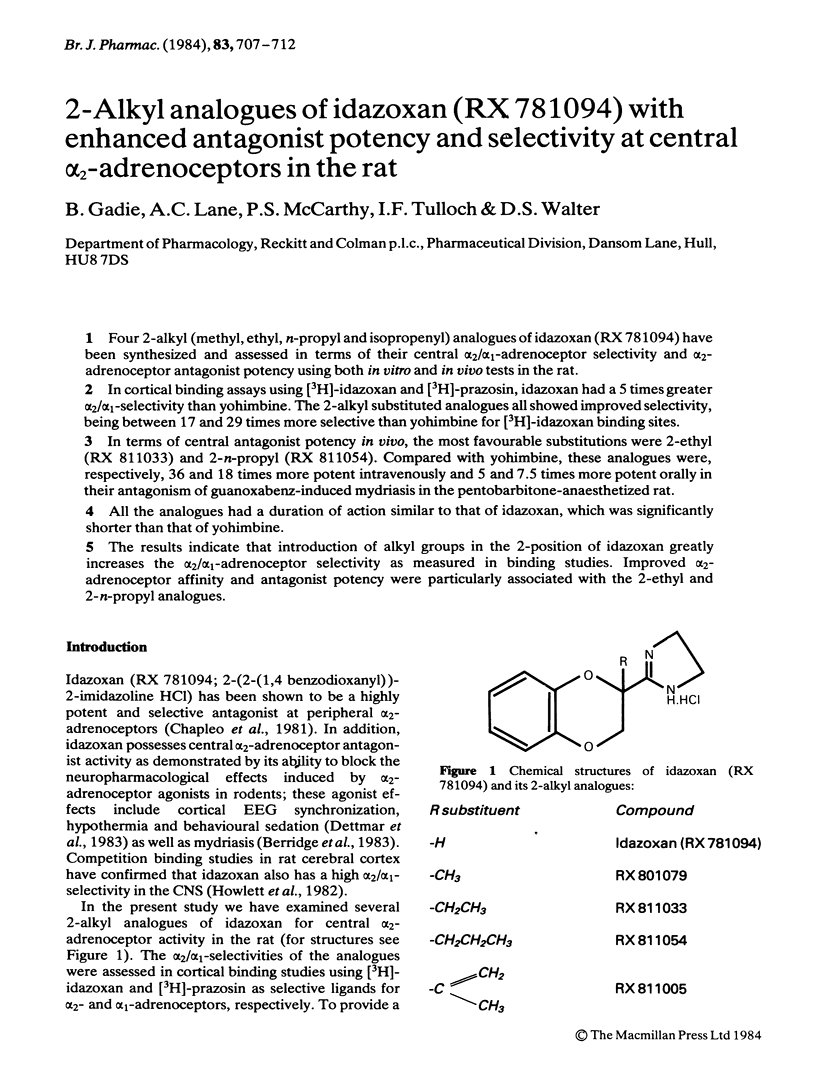


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge T. L., Gadie B., Roach A. G., Tulloch I. F. alpha 2-Adrenoceptor agonists induced mydriasis in the rat by an action within the central nervous system. Br J Pharmacol. 1983 Mar;78(3):507–515. doi: 10.1111/j.1476-5381.1983.tb08810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dettmar P. W., Lynn A. G., Tulloch I. F. Neuropharmacological studies in rodents on the action of RX 781094, a new selective alpha 2-adrenoceptor antagonist. Neuropharmacology. 1983 Jun;22(6):729–737. doi: 10.1016/0028-3908(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Doxey J. C., Gadie B., Lane A. C., Tulloch I. F. Evidence for pharmacological similarity between alpha 2-adrenoceptors in the vas deferens and central nervous system of the rat. Br J Pharmacol. 1983 Sep;80(1):155–161. doi: 10.1111/j.1476-5381.1983.tb11061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Smith C. F. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983 Mar;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Strachan D. A., Virdee N. K. Selectivity and potency of 2-alkyl analogues of the alpha 2-adrenoceptor antagonist idazoxan (RX 781094) in peripheral systems. Br J Pharmacol. 1984 Nov;83(3):713–722. doi: 10.1111/j.1476-5381.1984.tb16225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A. C., Howlett D. R., Walter D. S. The effects of metal ions on the binding of a new alpha 2-adrenoceptor antagonist radioligand (3H)-RX 781094 in rat cerebral cortex. Biochem Pharmacol. 1983 Oct 15;32(20):3122–3125. doi: 10.1016/0006-2952(83)90261-7. [DOI] [PubMed] [Google Scholar]


