Abstract
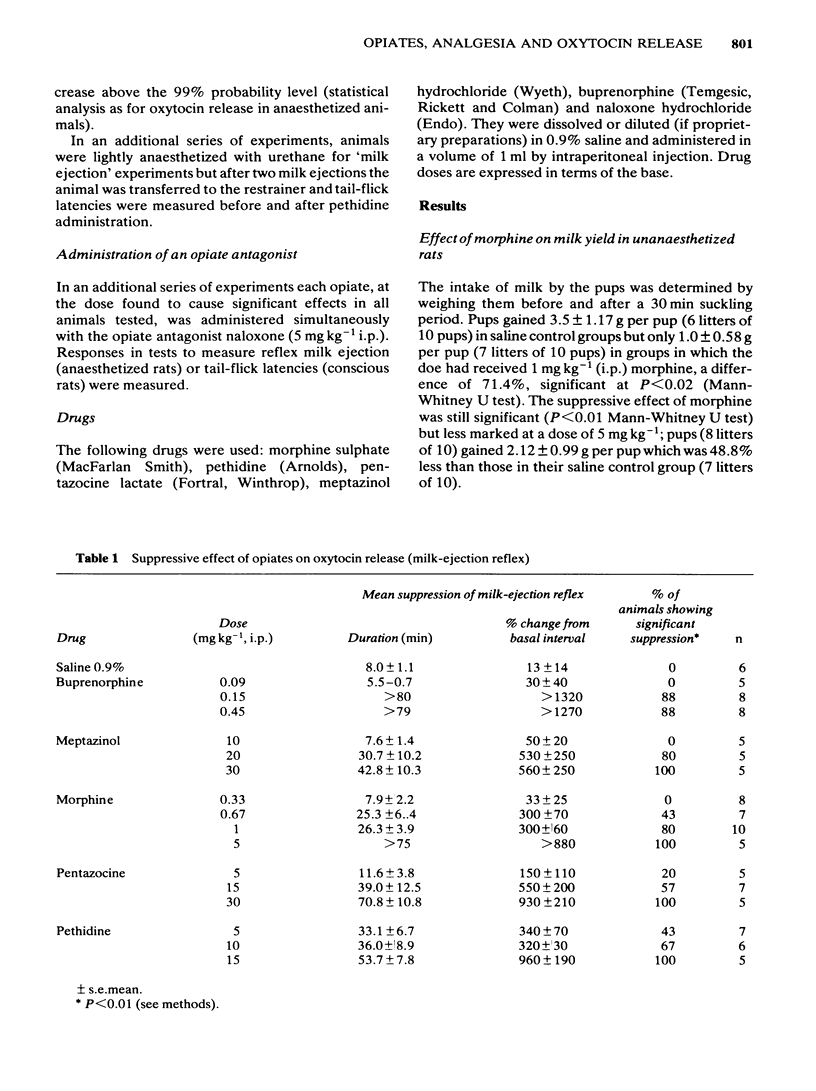
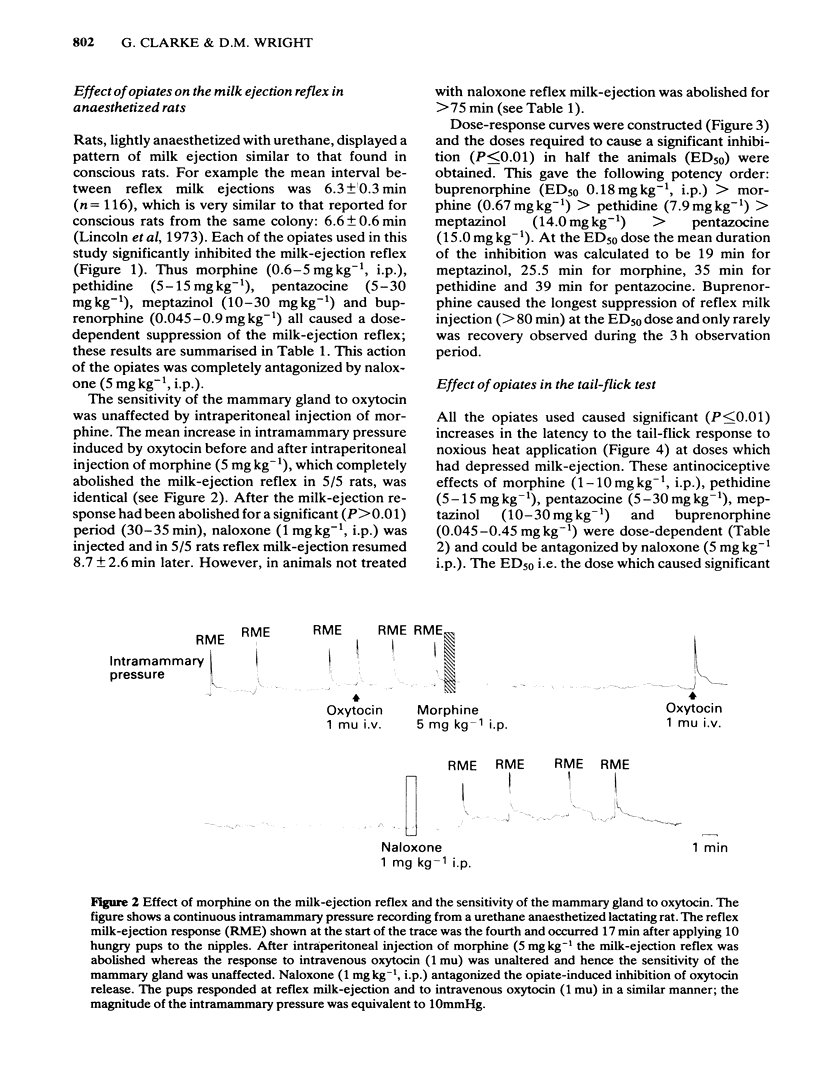
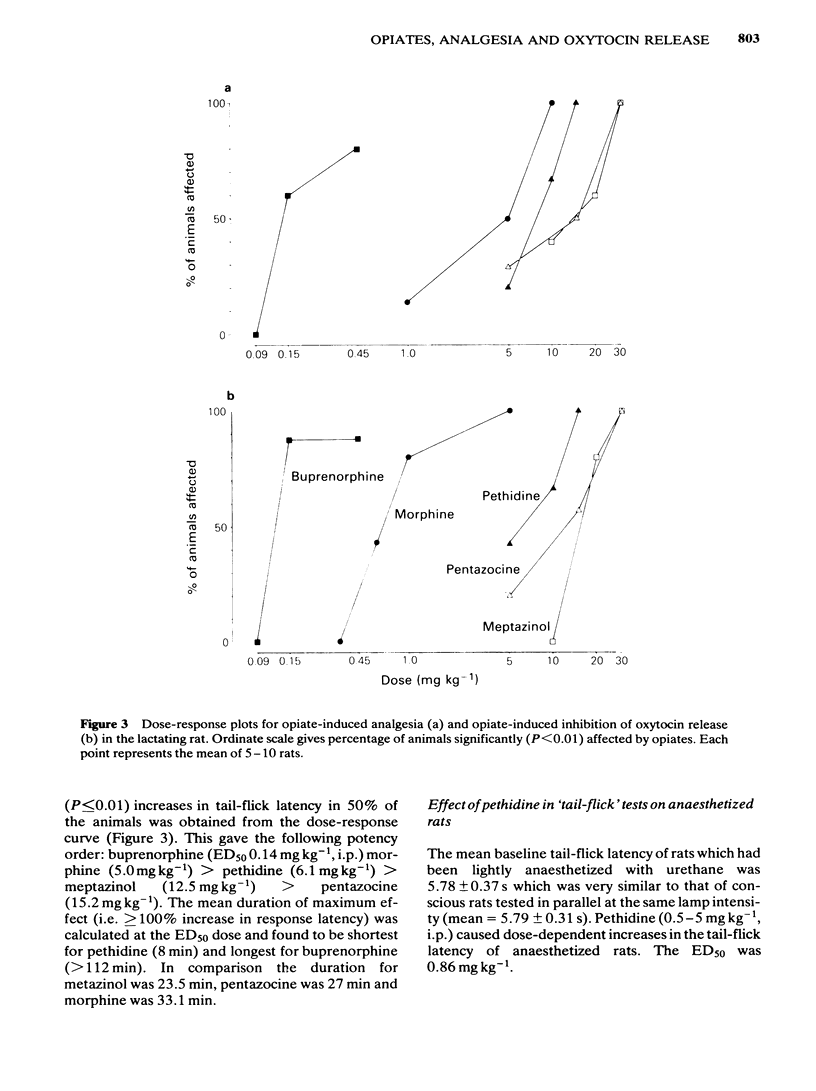
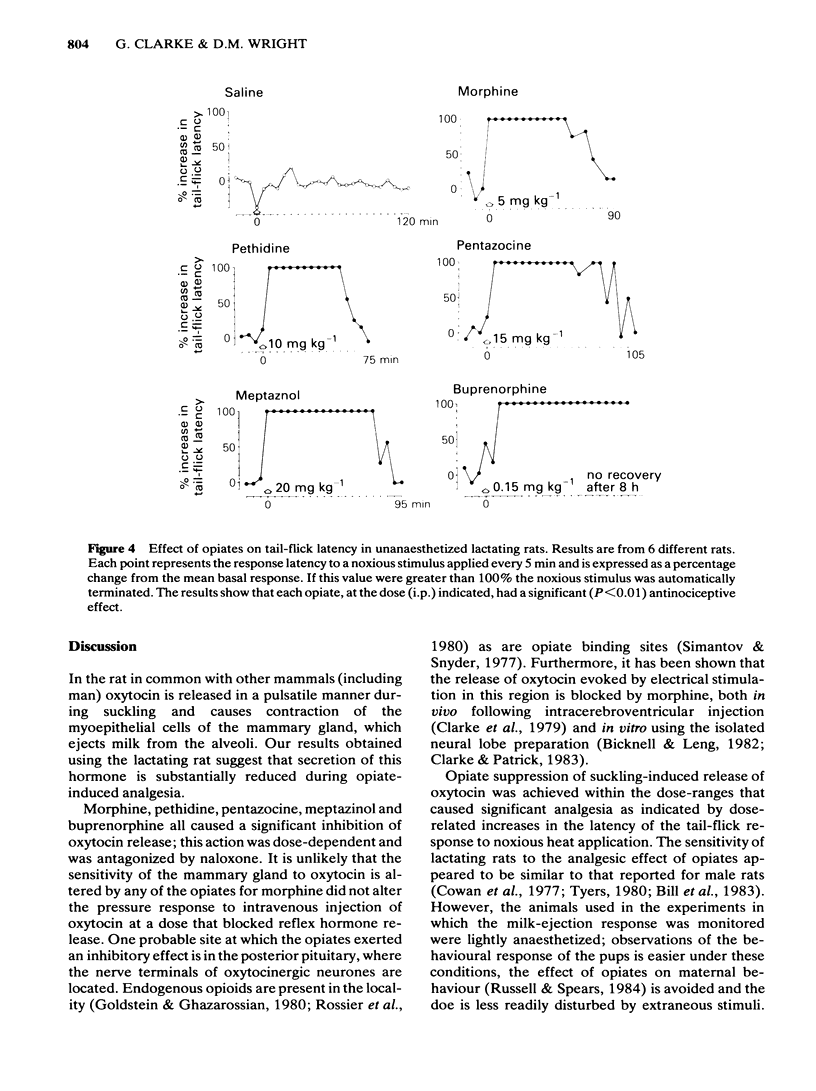
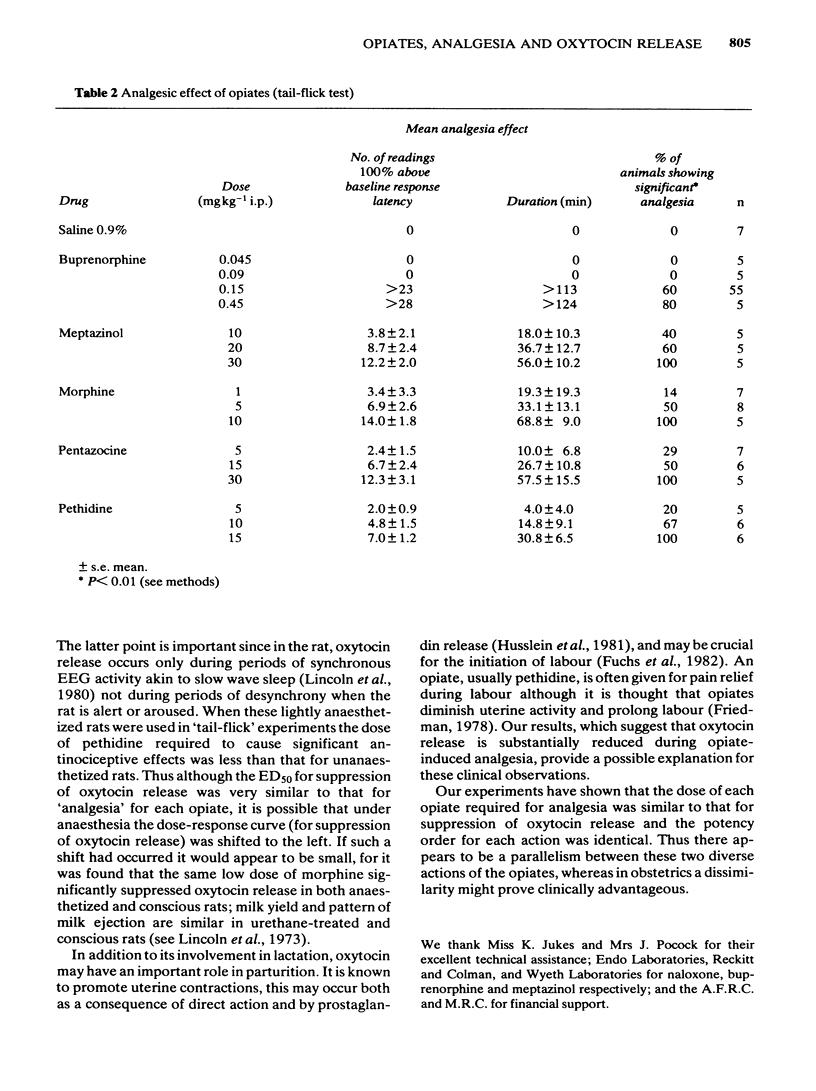
The potency of opiates for suppressing oxytocin release relative to their potency as analgesics was tested in lactating rats. Oxytocin release was evoked by the sucking of the young in urethane-anaesthetized and unanaesthetized rats, and was detected by the characteristic behaviour of the young and milk yield respectively. The tail-flick test, using noxious radiant heat, was used to assess analgesia. Intraperitoneal injection of morphine (1 mg kg-1 and 5 mg kg-1) significantly reduced milk yield in unanaesthetized rats. Urethane-anaesthetized rats displayed a pattern of reflex milk-ejection responses similar to that found in conscious rats. This reflex was significantly inhibited in a dose-related, naloxone-reversible manner by buprenorphine (ED50 0.18 mg kg-1), meptazinol (ED50: 14.0 mg kg-1), morphine (ED50: 0.67 mg kg-1), pentazocine (ED50: 15.0 mg kg-1) and pethidine (ED50: 7.9 mg kg-1). Although intraperitoneal injection of morphine (5 mg kg-1) abolished the increase in intramammary pressure occurring at reflex milk-ejection, that evoked by intravenous oxytocin (0.5-1 mu) was unaffected. Each opiate also caused significant, dose-related, naloxone-reversible increases in tail-flick latency. The ED50 doses were buprenorphine (ED50: 0.14 mg kg-1), meptazinol (ED50: 12.5 mg kg-1), morphine (ED50: 5.0 mg kg-1), pentazocine (ED50: 12.5 mg kg-1) and pethidine (ED50: 6.1 mg kg-1). The order of potency for analgesia and for suppression of oxytocin release were identical, namely: buprenorphine greater than morphine greater than pethidine greater than meptazinol greater than pentazocine. The results obtained with lactating rats suggest that secretion of the hormone oxytocin is substantially reduced during opiate-induced analgesia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atweh S. F., Kuhar M. J. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull. 1983 Jan;39(1):47–52. doi: 10.1093/oxfordjournals.bmb.a071789. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G. Endogenous opiates regulate oxytocin but not vasopressin secretion from the neurohypophysis. Nature. 1982 Jul 8;298(5870):161–162. doi: 10.1038/298161a0. [DOI] [PubMed] [Google Scholar]
- Bill D. J., Hartley J. E., Stephens R. J., Thompson A. M. The antinociceptive activity of meptazinol depends on both opiate and cholinergic mechanisms. Br J Pharmacol. 1983 May;79(1):191–199. doi: 10.1111/j.1476-5381.1983.tb10512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Fall C. H., Lincoln D. W., Merrick L. P. Effects of cholinoceptor antagonists on the suckling-induced and experimentally evoked release of oxytocin. Br J Pharmacol. 1978 Jul;63(3):519–527. doi: 10.1111/j.1476-5381.1978.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Patrick G. Differential inhibitory action by morphine on the release of oxytocin and vasopressin from the isolated neural lobe. Neurosci Lett. 1983 Aug 29;39(2):175–180. doi: 10.1016/0304-3940(83)90073-3. [DOI] [PubMed] [Google Scholar]
- Clarke G., Wood P., Merrick L., Lincoln D. W. Opiate inhibition of peptide release from the neurohumoral terminals of hypothalamic neurones. Nature. 1979 Dec 13;282(5740):746–748. doi: 10.1038/282746a0. [DOI] [PubMed] [Google Scholar]
- Cowan A., Lewis J. W., Macfarlane I. R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977 Aug;60(4):537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A. R., Fuchs F., Husslein P., Soloff M. S., Fernström M. J. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982 Mar 12;215(4538):1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Ghazarossian V. E. Immunoreactive dynorphin in pituitary and brain. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6207–6210. doi: 10.1073/pnas.77.10.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husslein P., Fuchs A. R., Fuchs F. Oxytocin and the initiation of human parturition. I. Prostaglandin release during induction of labor by oxytocin. Am J Obstet Gynecol. 1981 Nov 15;141(6):688–693. doi: 10.1016/s0002-9378(15)33312-3. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hentzen K., Hin T., van der Schoot P., Clarke G., Summerlee A. J. Sleep: a prerequisite for reflex milk ejection in the rat. Exp Brain Res. 1980 Jan;38(2):151–162. doi: 10.1007/BF00236736. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hill A., Wakerley J. B. The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol. 1973 Jun;57(3):459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- Rossier J., Pittman Q., Bloom F., Guillemin R. Distribution of opioid peptides in the pituitary: a new hypothalamic-pars nervosa enkephalinergic pathway. Fed Proc. 1980 Jun;39(8):2555–2560. [PubMed] [Google Scholar]
- Simantov R., Snyder S. H. Opiate receptor binding in the pituitary gland. Brain Res. 1977 Mar 18;124(1):178–184. doi: 10.1016/0006-8993(77)90877-0. [DOI] [PubMed] [Google Scholar]
- Tyers M. B. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980 Jul;69(3):503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. Intermittent release of oxytocin during suckling in the rat. Nat New Biol. 1971 Oct 6;233(40):180–181. doi: 10.1038/newbio233180a0. [DOI] [PubMed] [Google Scholar]
- Wright D. M., Pill C. E., Clarke G. Effect of ACTH on opiate inhibition of oxytocin release. Life Sci. 1983;33 (Suppl 1):495–498. doi: 10.1016/0024-3205(83)90549-0. [DOI] [PubMed] [Google Scholar]


