Abstract
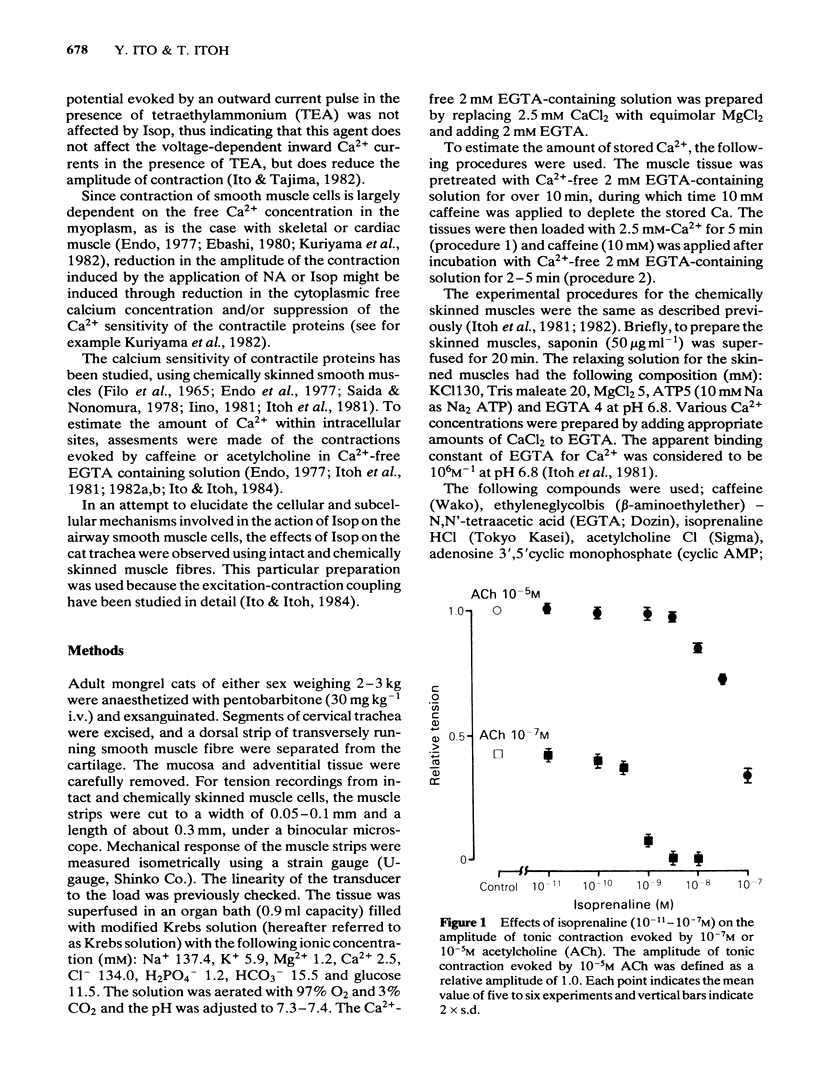
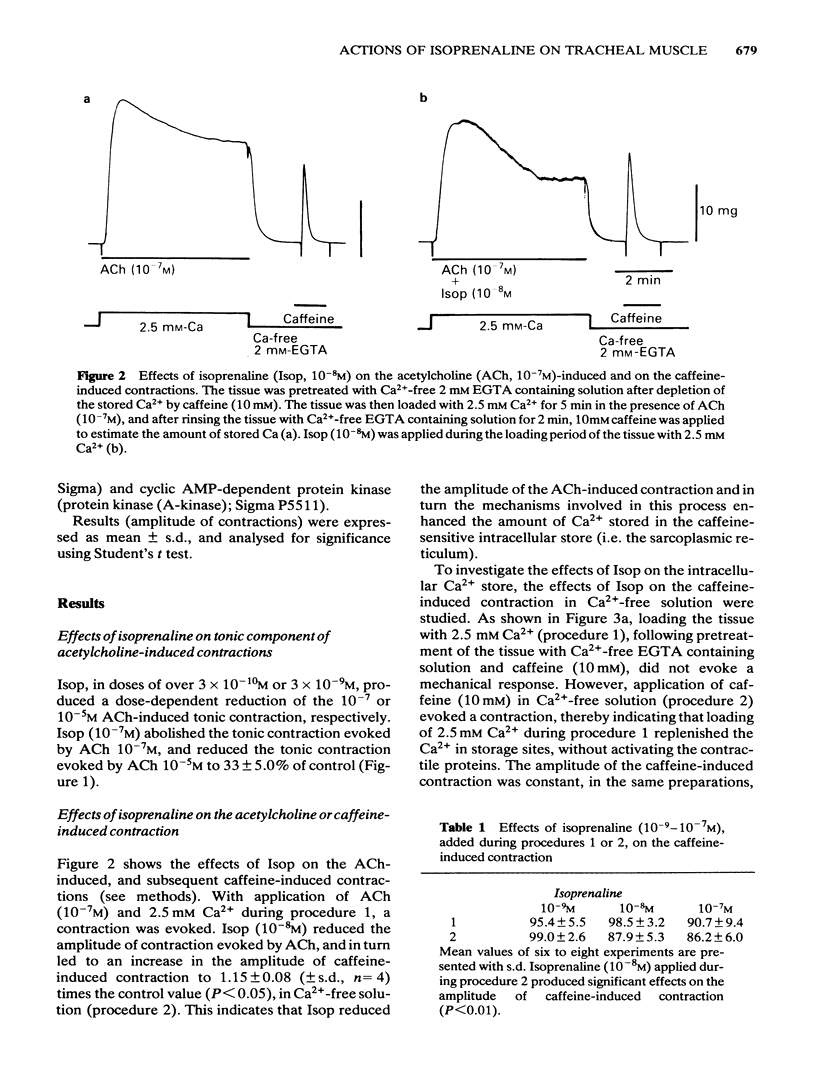
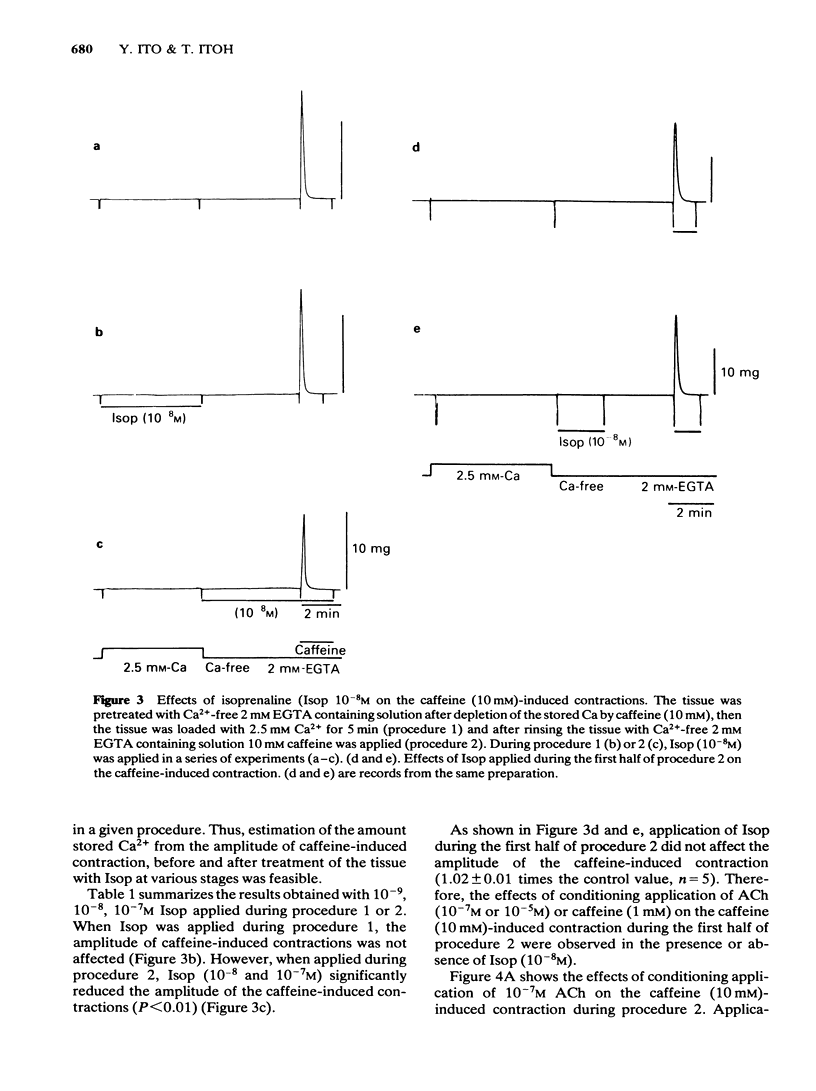
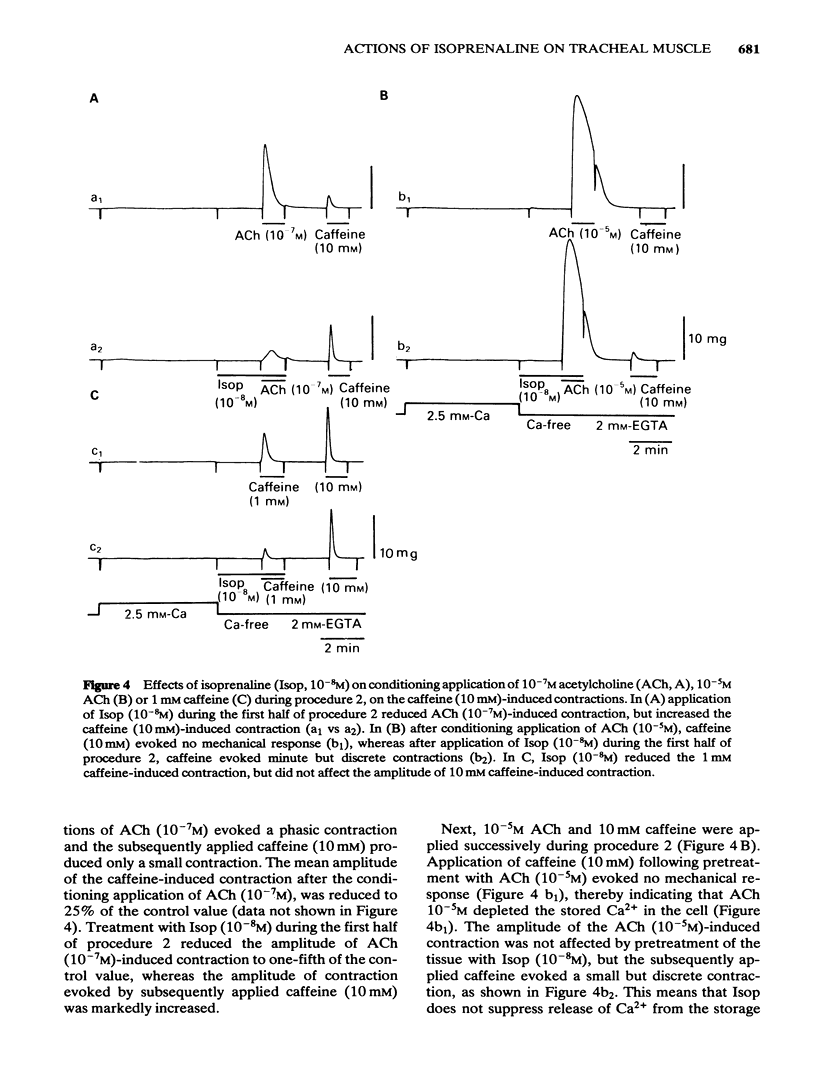
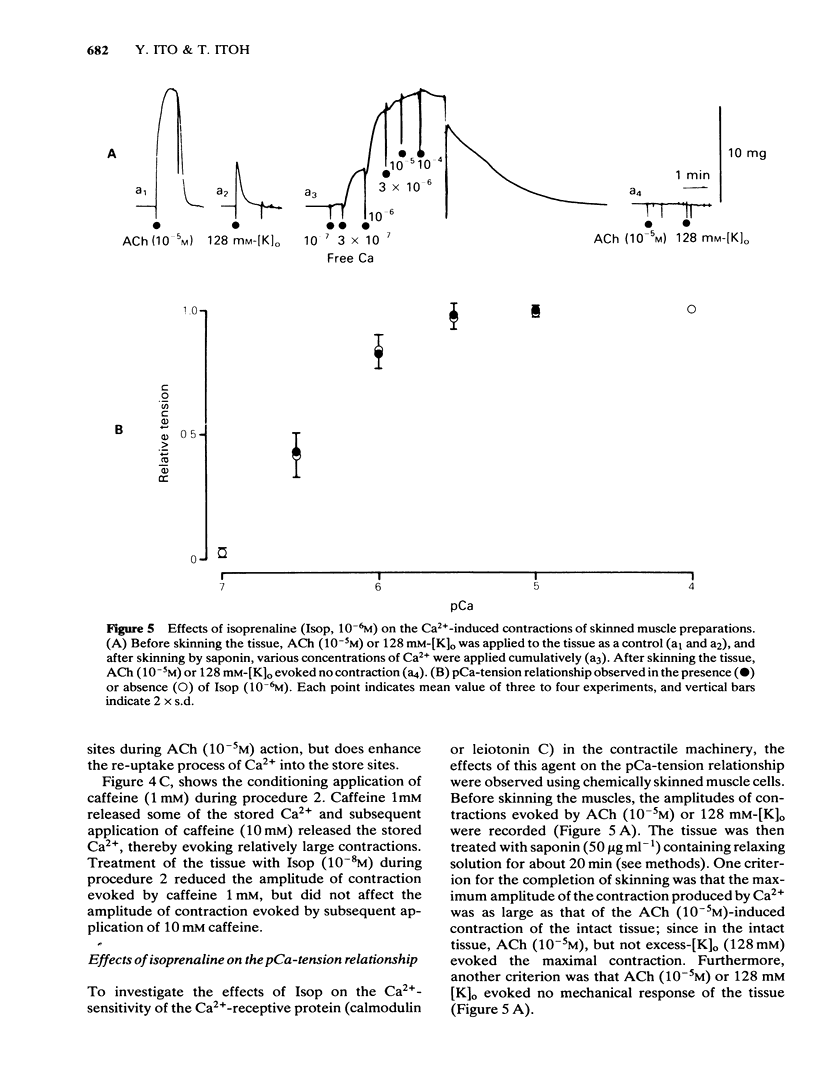
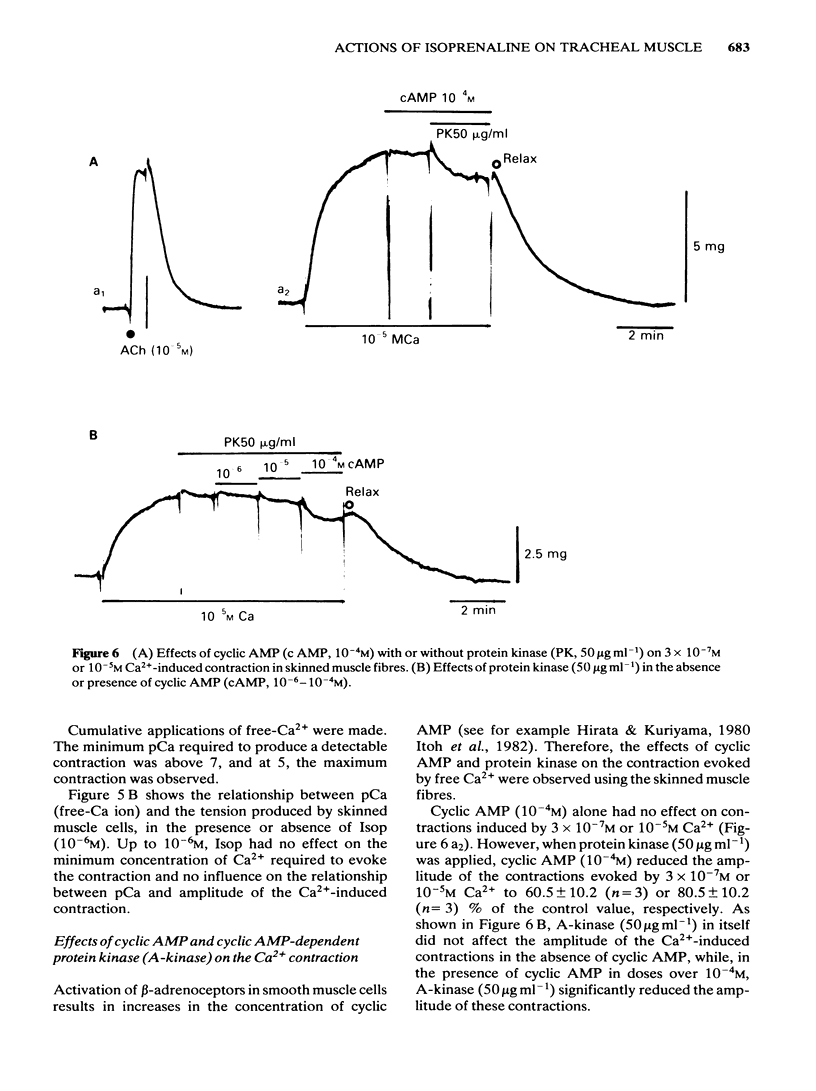
Effects of isoprenaline (Isop) on the contractile properties of the smooth muscle cells of cat trachea were investigated using intact and chemically skinned muscle preparations and an isometric tension recording method. In the intact muscle preparations, Isop 3 X 10(-10) or 3 X 10(-9) M significantly suppressed the amplitude of tonic contractions evoked by acetylcholine (ACh) 10(-7) M or 10(-5) M, respectively. Following treatment of the tissue with Ca2+-free 2 mM EGTA-containing solution after depletion of stored Ca2+ with caffeine, 2.5 mM Ca2+ was applied for 5 min (procedure 1), and subsequently 10 mM caffeine was applied in Ca2+-free 2 mM EGTA containing solution. The object was to estimate the amount of stored Ca2+ during procedure 1 from the amplitude of the caffeine (10 mM)-induced contraction (procedure 2). Isop, applied during procedure 1, did not affect the amplitude of the caffeine-induced contraction; however, when applied during procedure 2, this agent (10(-8)M) significantly suppressed the amplitude of the caffeine-induced contraction to about 90% of the control value. ACh (10(-5)M), applied during procedure 1, evoked phasic and tonic contractions. Isop (10(-8)M), applied simultaneously with ACh (10(-5)M), suppressed the amplitude of the ACh-induced contraction yet increased the amplitude of contraction evoked by the subsequent application of caffeine 10 mM (procedure 2). Effects of conditioning application of ACh (10(-7) or 10(-5)M) on the caffeine-induced contraction were observed in the presence or absence of Isop during procedure 2. When ACh 10(-5)M was used, subsequent application of caffeine 10 mM evoked no mechanical response, in control conditions. However, after the pretreatment of the tissue with Isop during procedure 2, the amplitude of the ACh (10(-5)M)-induced contraction was not affected, yet the subsequent application of caffeine (10 mM) evoked minute but discrete contractions, indicating that Isop did enhance the sequestration of free Ca2+ into the storage sites. In the saponin-treated skinned muscles, the minimum concentration of Ca2+ required to produce contraction was 1 X 10(-7)M, and the maximum contraction was obtained with 1 X 10(-5)M Ca2+. Isop (10(-6)M) had no effect on the relationship between free-Ca2+ and the amplitude of the contraction. However, simultaneous application of high concentrations of cyclic AMP (10(-4)M) and cyclic AMP-dependent protein kinase (50 micrograms ml-1) significantly suppressed contractions evoked by 3 X 10(-7) or 10(-5)M Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A., Hathaway D. R., Klee C. B. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3': 5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Dec 10;253(23):8347–8350. [PubMed] [Google Scholar]
- Bülbring E., den Hertog A. The action of isoprenaline on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1980 Jul;304:277–296. doi: 10.1113/jphysiol.1980.sp013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas G. A., Graf P. D., Nadel J. A. Sympathetic versus parasympathetic nervous regulation of airways in dogs. J Appl Physiol. 1971 Nov;31(5):651–655. doi: 10.1152/jappl.1971.31.5.651. [DOI] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G., Shepherd J. T. Isoproterenol-induced relaxation of venous smooth muscle contracted by agents which mobilize different calcium pools. J Pharmacol Exp Ther. 1979 Jun;209(3):359–365. [PubMed] [Google Scholar]
- Ebashi S. The Croonian lecture, 1979: Regulation of muscle contraction. Proc R Soc Lond B Biol Sci. 1980 Mar 21;207(1168):259–286. doi: 10.1098/rspb.1980.0024. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- FILO R. S., BOHR D. F., RUEGG J. C. GLYCERINATED SKELETAL AND SMOOTH MUSCLE: CALCIUM AND MAGNESIUM DEPENDENCE. Science. 1965 Mar 26;147(3665):1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kuriyama H. Does activation of cyclic AMP dependent phosphorylation induced by beta-adrenergic agent control the tone of vascular muscle? J Physiol. 1980 Oct;307:143–161. doi: 10.1113/jphysiol.1980.sp013428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol. 1981 Nov;320:449–467. doi: 10.1113/jphysiol.1981.sp013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Itoh T. The roles of stored calcium in contractions of cat tracheal smooth muscle produced by electrical stimulation, acetylcholine and high K+. Br J Pharmacol. 1984 Nov;83(3):667–676. doi: 10.1111/j.1476-5381.1984.tb16220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Dual effects of catecholamines on pre- and post-junctional membranes in the dog trachea. Br J Pharmacol. 1982 Mar;75(3):433–440. doi: 10.1111/j.1476-5381.1982.tb09158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Izumi H., Kuriyama H. Mechanisms of relaxation induced by activation of beta-adrenoceptors in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1982 May;326:475–493. doi: 10.1113/jphysiol.1982.sp014207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., van Breemen C. Effects of beta-adrenergic stimulation on calcium movements in rabbit aortic smooth muscle: relationship with cyclic AMP. J Physiol. 1982 Oct;331:429–441. doi: 10.1113/jphysiol.1982.sp014380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. E., Sellick H., Widdicombe J. G. Activity of lung irritant receptors in pulmonary microembolism, anaphylaxis and drug-induced bronchoconstrictions. J Physiol. 1969 Aug;203(2):337–357. doi: 10.1113/jphysiol.1969.sp008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow M. P., Mrwa U., Hofmann F., Rüegg J. C. Calmodulin is essential for smooth muscle contraction. FEBS Lett. 1981 Mar 23;125(2):141–145. doi: 10.1016/0014-5793(81)80704-1. [DOI] [PubMed] [Google Scholar]


