Abstract
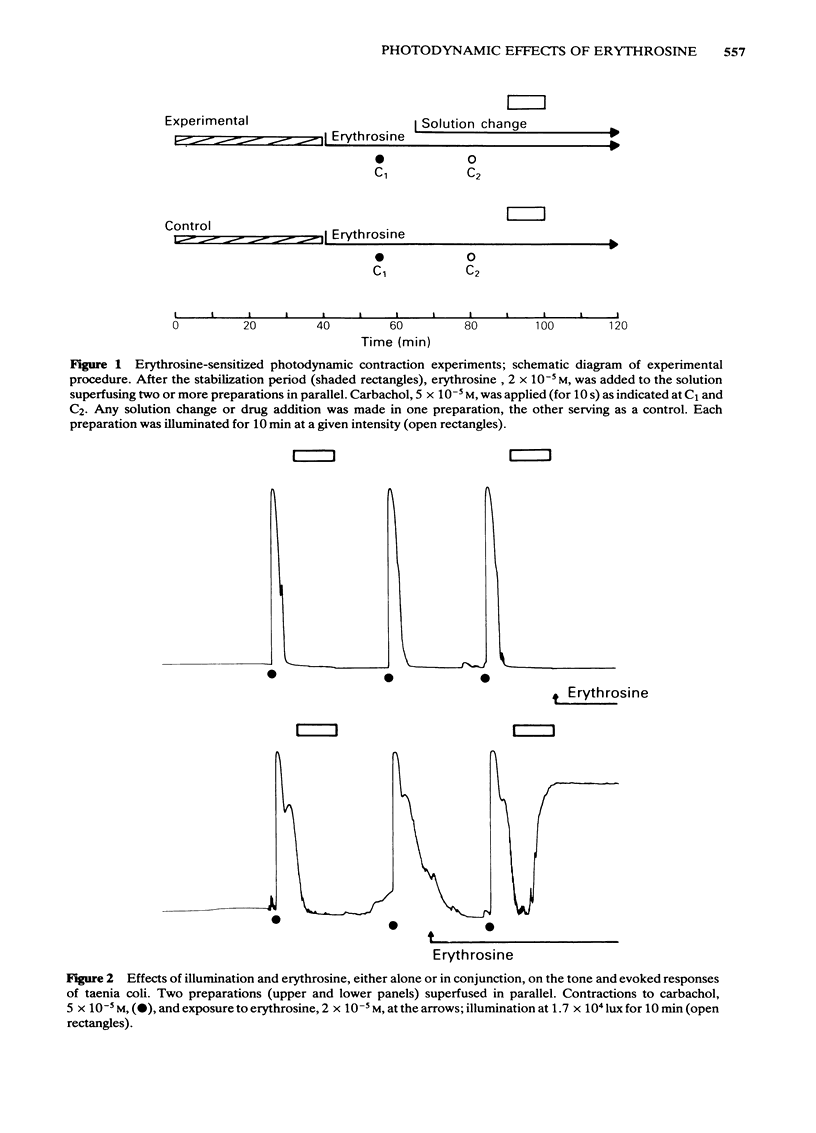
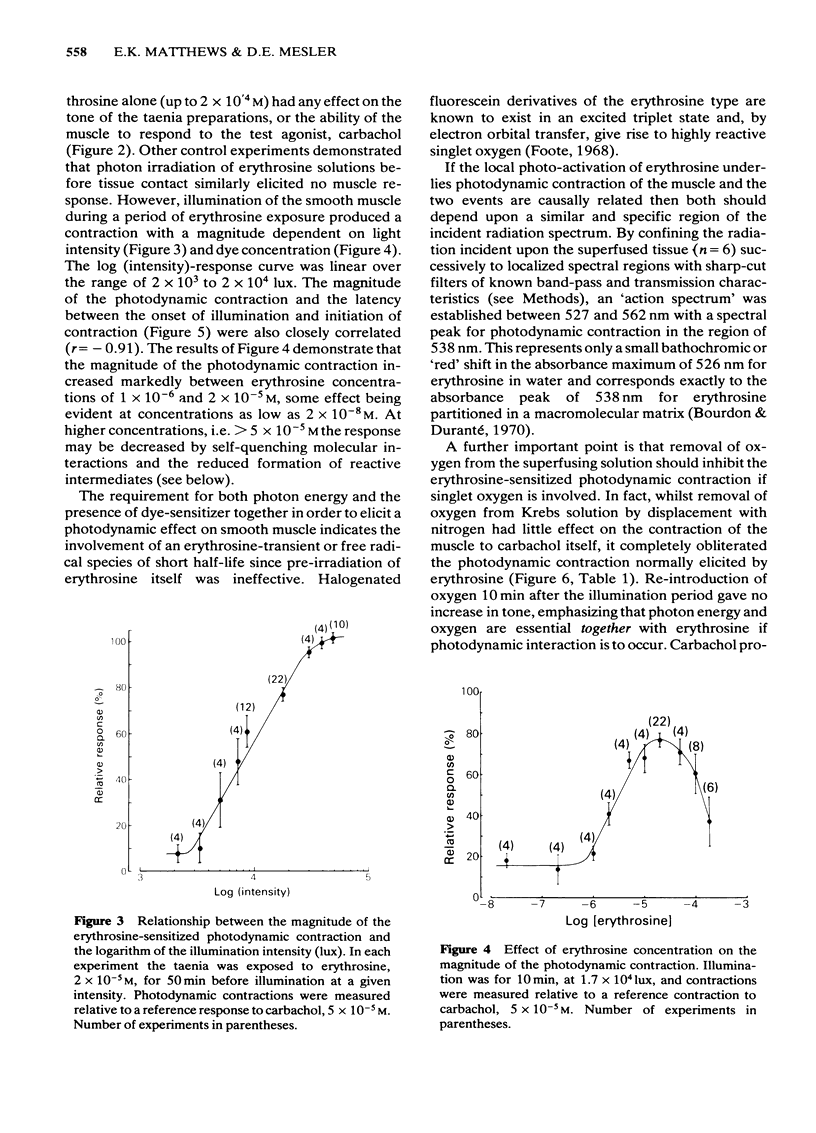
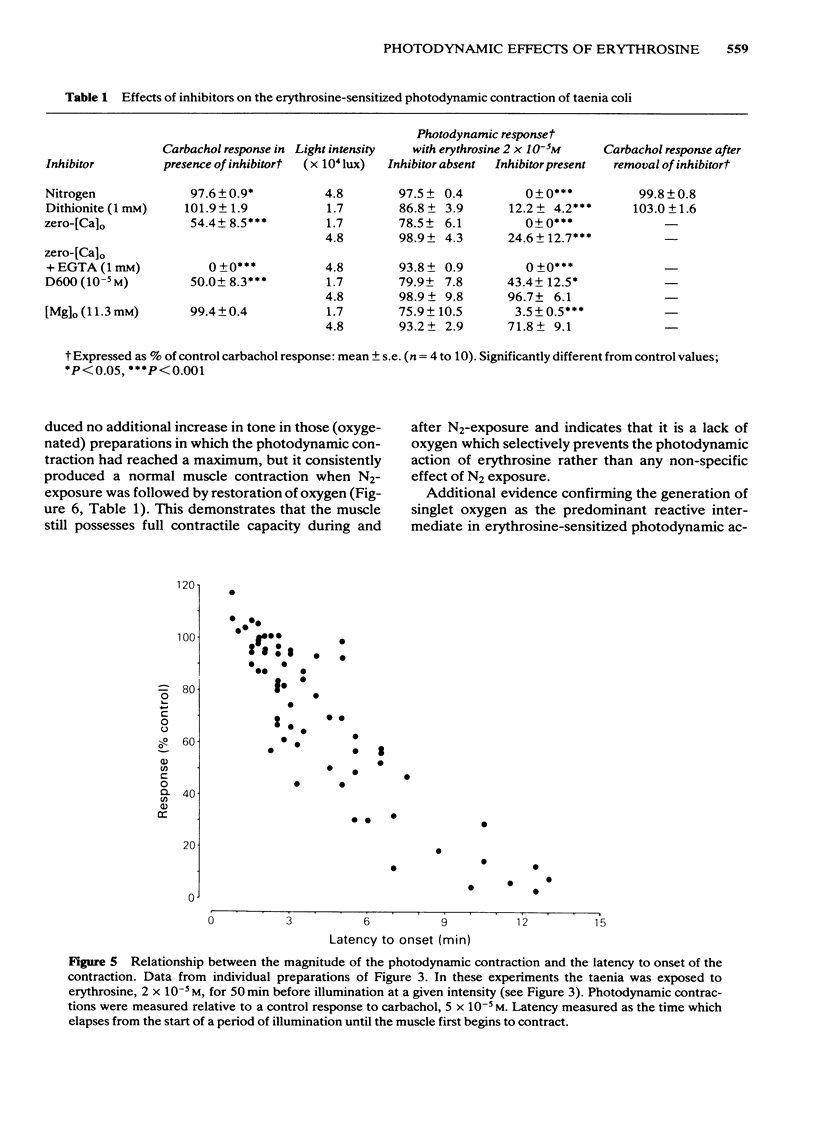
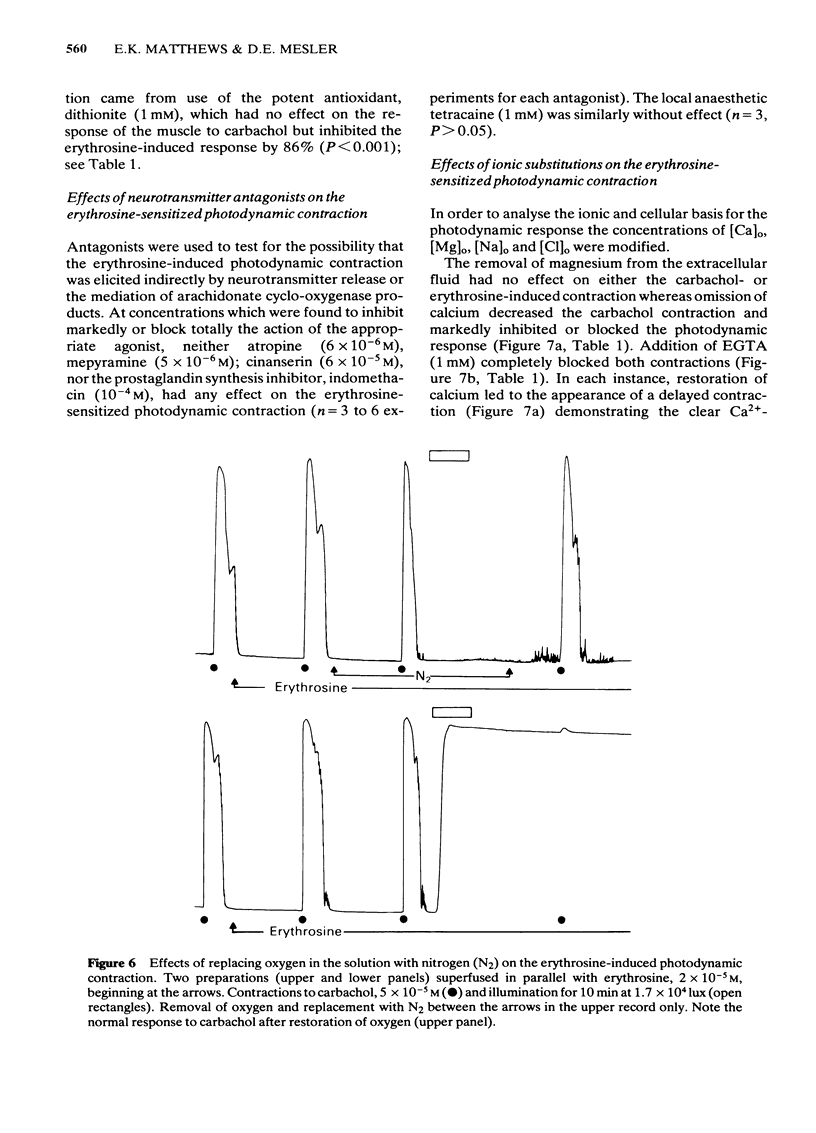
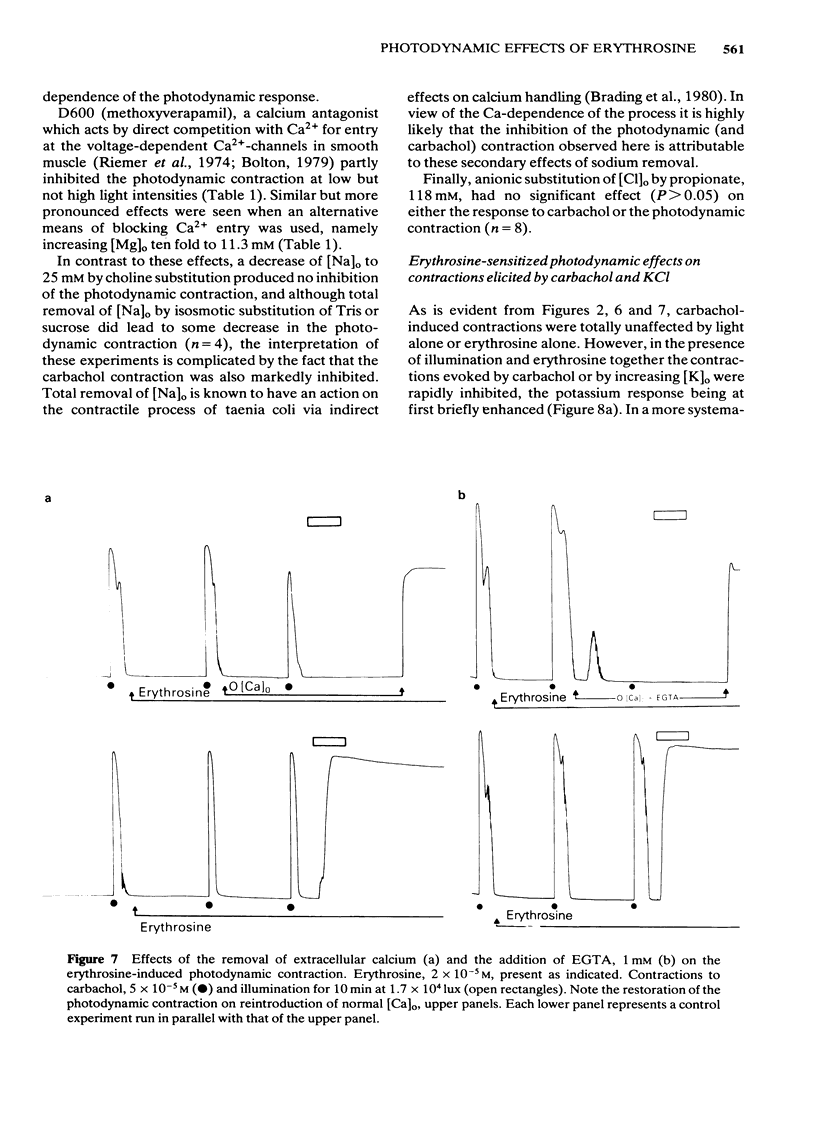
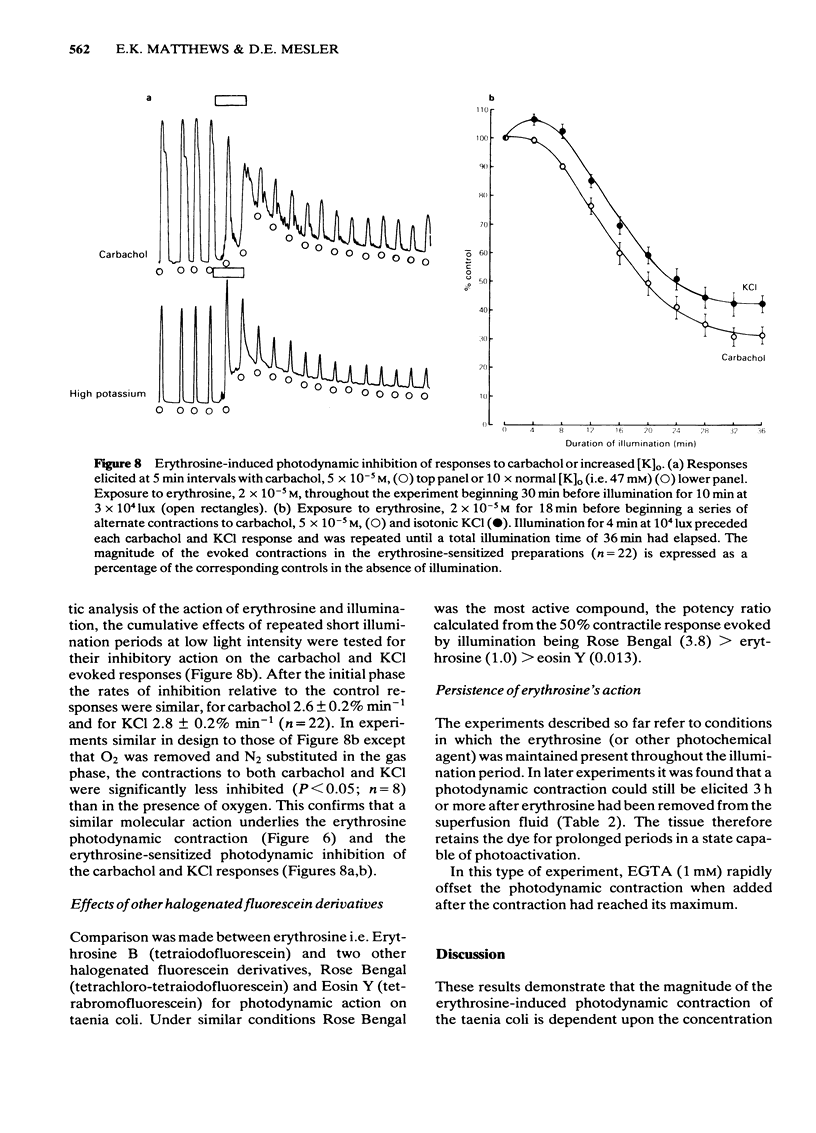
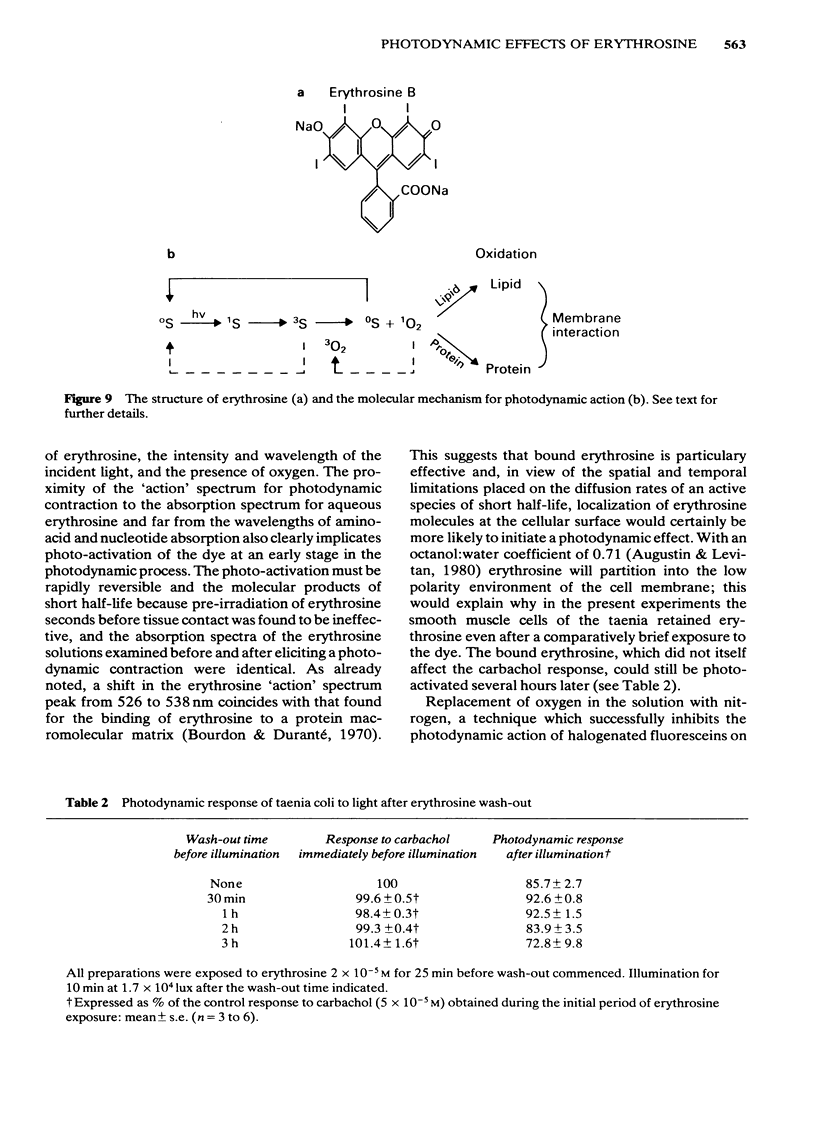
Photon activation of the halogenated fluorescein derivative erythrosine caused a marked calcium-dependent contraction of the smooth muscle cells of the guinea-pig taenia coli superfused in vitro. Neither high intensity illumination alone (up to 5 X 10(4) lux) nor erythrosine alone (up to 2 X 10(-4) M) altered the tone of the taenia or its ability to respond to carbachol (5 X 10(-5) M); photo-irradiation of erythrosine before tissue contact was also ineffective. The magnitude of the photodynamic contraction was dependent upon the concentration of erythrosine, the intensity and wavelength of the incident light, and the presence of oxygen; indirect effects via neurotransmitter release or cyclo-oxygenase activation were specifically excluded. The photodynamic response was blocked by zero-[Ca]o and addition of EGTA (1 mM) but not by omission of [Mg]o or a decrease in [Cl]o or [Na]o. D600 (methoxyverapamil) 10(-5) M, or a ten fold increase in [Mg]o, to 11.3 mM, partly inhibited the photodynamic contraction at low, but not high, light intensities. These observations are consistent with the following sequence of events: (i) photo-activation of the erythrosine molecule, (ii) the generation of highly reactive singlet oxygen, (iii) local peroxidation of cell membrane proteolipid, (iv) increased membrane permeability to Ca2+, (v) the influx of Ca2+ and, (vi) muscle contraction. It is concluded that the photodynamic action of erythrosine presents a novel method for modulation of membrane calcium permeability, and hence [Ca]i, not only in smooth muscle but possibly in other cells as well, e.g., secretory, epithelial and myocardial cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Jr, Levitan H. Neurotransmitter release from a vertebrate neuromuscular synapse affected by a food dye. Science. 1980 Mar 28;207(4438):1489–1490. doi: 10.1126/science.6244619. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Levitan H. Neurotransmitter release and nerve terminal morphology at the frog neuromuscular junction affected by the dye Erythrosin B. J Physiol. 1983 Jan;334:47–63. doi: 10.1113/jphysiol.1983.sp014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Levitan H. Presynaptic effect of Erythrosin B at the frog neuromuscular junction: ion and photon sensitivity. J Physiol. 1983 Jan;334:65–77. doi: 10.1113/jphysiol.1983.sp014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Membrane potential and excitation-contraction coupling in smooth muscle. Fed Proc. 1982 Oct;41(12):2879–2882. [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M., Wu C. Y. A food dye, erythrosine B, increases membrane permeability to calcium and other ions. Biochim Biophys Acta. 1981 Oct 20;648(1):49–54. doi: 10.1016/0005-2736(81)90122-x. [DOI] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the action of adrenaline, adenosine triphosphate and carbachol on guinea-pig taenia caeci. J Physiol. 1982 Apr;325:423–439. doi: 10.1113/jphysiol.1982.sp014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. A. Erythrosine B (red dye No. 3) mediated oxidation-reduction in brain membranes. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1305–1311. doi: 10.1016/0006-291x(80)90093-5. [DOI] [PubMed] [Google Scholar]
- Foote C. S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968 Nov 29;162(3857):963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- Grossweiner L. I. Molecular mechanisms in photodynamic action. Photochem Photobiol. 1969 Sep;10(3):183–191. doi: 10.1111/j.1751-1097.1969.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Imai S., Takeda K. Actions of calcium and certain multivalent cations on potassium contracture of guinea-pig's taenia coli. J Physiol. 1967 May;190(1):155–169. doi: 10.1113/jphysiol.1967.sp008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepka A. G., Grossweiner L. I. Photodynamic inactivation of lysozyme by eosin. Photochem Photobiol. 1973 Jul;18(1):49–61. doi: 10.1111/j.1751-1097.1973.tb06392.x. [DOI] [PubMed] [Google Scholar]
- Lafferman J. A., Silbergeld E. K. Erythrosin B inhibits dopamine transport in rat caudate synaptosomes. Science. 1979 Jul 27;205(4404):410–412. doi: 10.1126/science.451609. [DOI] [PubMed] [Google Scholar]
- Logan W. J., Swanson J. M. Erythrosin B inhibition of neurotransmitter accumulation by rat brain homogenate. Science. 1979 Oct 19;206(4416):363–364. doi: 10.1126/science.39341. [DOI] [PubMed] [Google Scholar]
- Lowe D. A., Matthews E. K., Richardson B. P. The calcium antagonistic effects of cyproheptadine on contraction, membrane electrical events and calcium influx in the guinea-pig taenia coli. Br J Pharmacol. 1981 Nov;74(3):651–663. doi: 10.1111/j.1476-5381.1981.tb10476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman R. B., Ferris R. M., Tang F. L., Vogel R. A., Kilts C. D., Lipton M. A., Smith D. A., Mueller R. A., Breese G. R. Erythrosine (Red No. 3) and its nonspecific biochemical actions: what relation to behavioral changes? Science. 1980 Feb 1;207(4430):535–537. doi: 10.1126/science.7352264. [DOI] [PubMed] [Google Scholar]
- Pooler J. P., Valenzeno D. P. The role of singlet oxygen in photooxidation of excitable cell membranes. Photochem Photobiol. 1979 Nov;30(5):581–584. doi: 10.1111/j.1751-1097.1979.tb07183.x. [DOI] [PubMed] [Google Scholar]
- Riemer J., Dörfler F., Mayer C. J., Ulbrecht G. Calcium-antagonistic effects on the spontaneous activity of guinea-pig taenia coli. Pflugers Arch. 1974;351(3):241–258. doi: 10.1007/BF00586921. [DOI] [PubMed] [Google Scholar]
- Saida K. Intracellular Ca release in skinned smooth muscle. J Gen Physiol. 1982 Aug;80(2):191–202. doi: 10.1085/jgp.80.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout M. A., Diecke F. P. 45Ca distribution and transport in saponin skinned vascular smooth muscle. J Pharmacol Exp Ther. 1983 Apr;225(1):102–111. [PubMed] [Google Scholar]


