Abstract
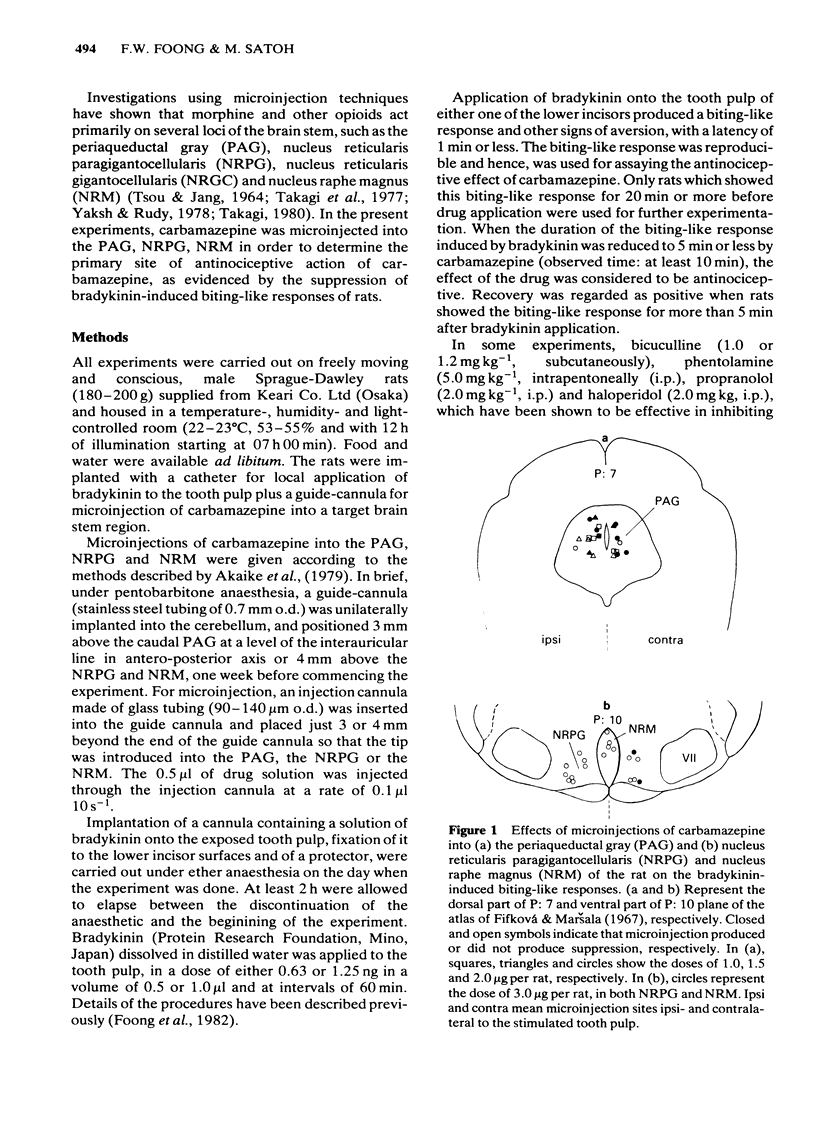
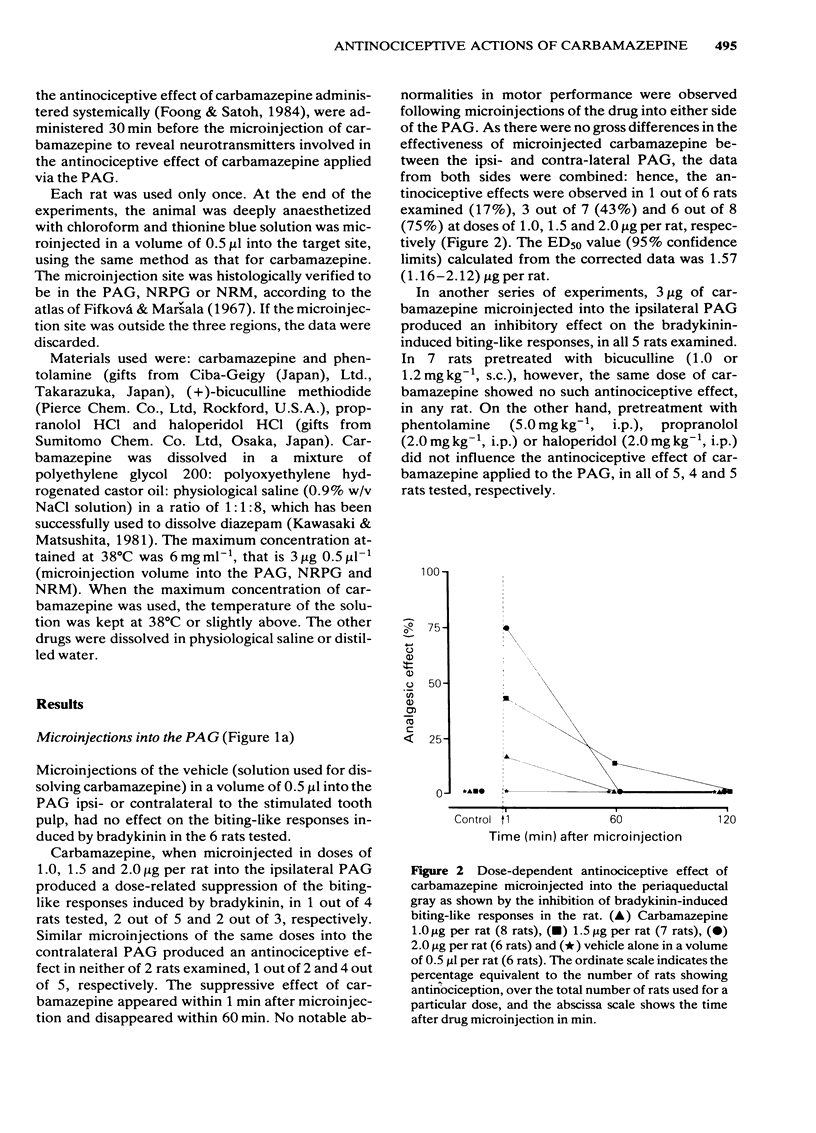
Using freely moving and conscious rats, the antinociceptive effects of microinjections of carbamazepine, into the periaqueductal gray (PAG), nucleus reticularis paragigantocellularis (NRPG) and nucleus raphé magnus (NRM) on the biting-like responses induced by bradykinin applied to the tooth pulp, were investigated to determine the primary site of action of this drug. Microinjections of carbamazepine into the PAG ipsi- and contralateral to the stimulated tooth pulp produced dose-dependent suppressive effects on the biting-like responses within 1 min. The ED50 was 1.57 micrograms per rat, that is about 1,500 times less than that for carbamazepine administered systemically. The antinociceptive effect of carbamazepine administered into the PAG was inhibited by pretreatment with bicuculline but not by phentolamine, propranolol and haloperidol. Microinjections of carbamazepine into the NRPG and NRM were rarely effective in the production of antinociception at doses used (up to 3 micrograms per rat). These results suggest that the PAG is one of the primary target sites for the antinociceptive activity of carbamazepine, and that GABAergic systems are involved this action of carbamazepine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Shibata T., Satoh M., Takagi H. Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology. 1978 Sep;17(9):775–778. doi: 10.1016/0028-3908(78)90093-x. [DOI] [PubMed] [Google Scholar]
- Beitz A. J. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982 Jan;7(1):133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Belin M. F., Aguera M., Tappaz M., McRae-Degueurce A., Bobillier P., Pujol J. F. GABA-accumulating neurons in the nucleus raphe dorsalis and periaqueductal gray in the rat: a biochemical and radioautographic study. Brain Res. 1979 Jul 13;170(2):279–297. doi: 10.1016/0006-8993(79)90107-0. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Oliveras J. L., Besson J. M. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979 Jul 6;170(1):95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- Figueiras R., Buño W., Jr, García-Austt E., Delgado J. M. Periaqueductal gray inhibition of trigeminal subnucleus caudalis unitary responses evoked by dentine and nonnoxious facial stimulation. Exp Neurol. 1983 Jul;81(1):34–49. doi: 10.1016/0014-4886(83)90155-3. [DOI] [PubMed] [Google Scholar]
- Foong F. W., Satoh M. Analgesic potencies of non-narcotic, narcotic and anesthetic drugs as determined by the bradykinin-induced biting-like responses in rats. Jpn J Pharmacol. 1983 Oct;33(5):933–938. doi: 10.1254/jjp.33.933. [DOI] [PubMed] [Google Scholar]
- Foong F. W., Satoh M. Neurotransmitter-blocking agents influence antinociceptive effects of carbamazepine, baclofen, pentazocine and morphine on bradykinin-induced trigeminal pain. Neuropharmacology. 1984 Jun;23(6):633–636. doi: 10.1016/0028-3908(84)90143-6. [DOI] [PubMed] [Google Scholar]
- Foong F. W., Satoh M., Takagi H. A newly devised reliable method for evaluating analgesic potencies of drugs on trigeminal pain. J Pharmacol Methods. 1982 Jun;7(4):271–278. doi: 10.1016/0160-5402(82)90080-8. [DOI] [PubMed] [Google Scholar]
- Fromm G. H., Chattha A. S., Terrence C. F., Glass J. D. Role of inhibitory mechanisms in trigeminal neuralgia. Neurology. 1981 Jun;31(6):683–687. doi: 10.1212/wnl.31.6.683. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Matsushita A. Sensitive depressant effect of benzodiazepines on the crossed extensor reflex in chloralose-anesthetized rats. Life Sci. 1981 Mar 23;28(12):1391–1398. doi: 10.1016/0024-3205(81)90414-8. [DOI] [PubMed] [Google Scholar]
- Satoh M., Foong F. W. A mechanism of carbamazepine-analgesia as shown by bradykinin-induced trigeminal pain. Brain Res Bull. 1983 Mar;10(3):407–409. doi: 10.1016/0361-9230(83)90113-2. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Dubner R., Greenwood L. F., Lucier G. E. Descending influences of periaqueductal gray matter and somatosensory cerebral cortex on neurones in trigeminal brain stem nuclei. Can J Physiol Pharmacol. 1976 Feb;54(1):66–69. doi: 10.1139/y76-010. [DOI] [PubMed] [Google Scholar]
- TSOU K., JANG C. S. STUDIES ON THE SITE OF ANALGESIC ACTION OF MORPHINE BY INTRACEREBRAL MICRO-INJECTION. Sci Sin. 1964 Jul;13:1099–1109. [PubMed] [Google Scholar]
- Takagi H., Satoh M., Akaike A., Shibata T., Kuraishi Y. The nucleus reticularis gigantocellularis of the medulla oblongata is a highly sensitive site in the production of morphine analgesia in the rat. Eur J Pharmacol. 1977 Sep 1;45(1):91–92. doi: 10.1016/0014-2999(77)90064-4. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978 Apr;4(4):299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- Yokota T., Hashimoto S. Periaqueductal gray and tooth pulp afferent interaction on units in caudal medulla oblongata. Brain Res. 1976 Dec 3;117(3):508–512. doi: 10.1016/0006-8993(76)90758-7. [DOI] [PubMed] [Google Scholar]


