Abstract
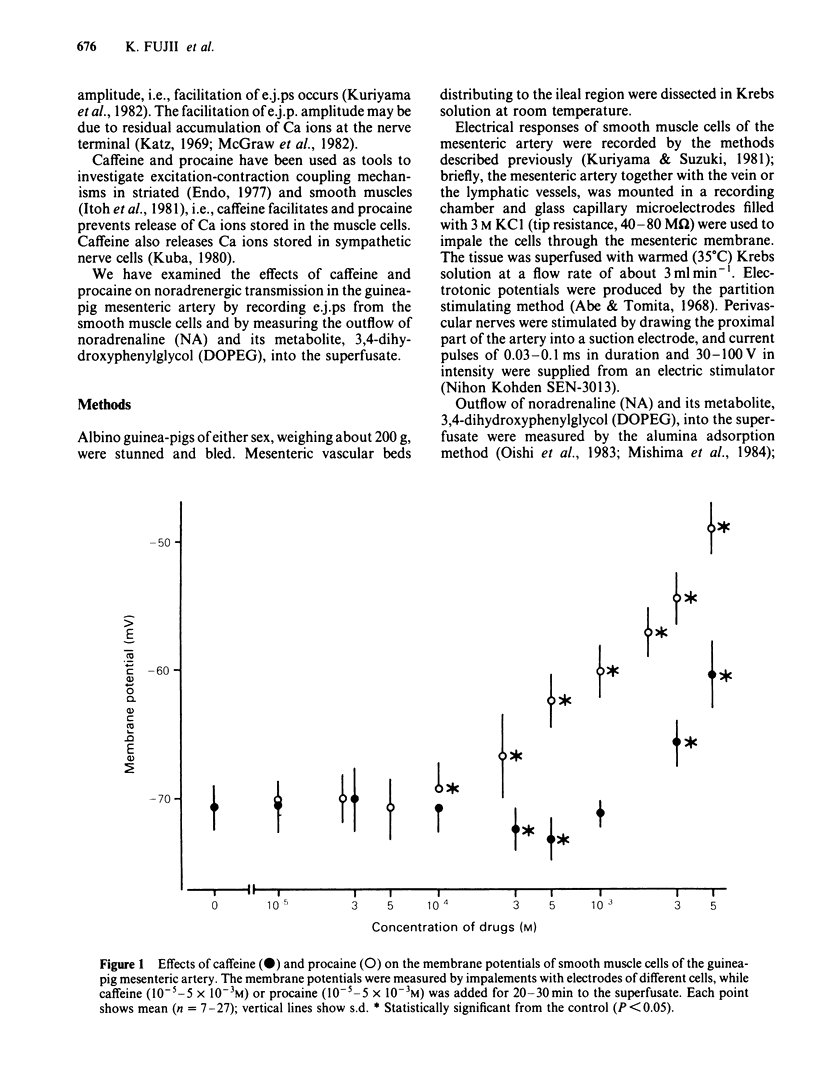
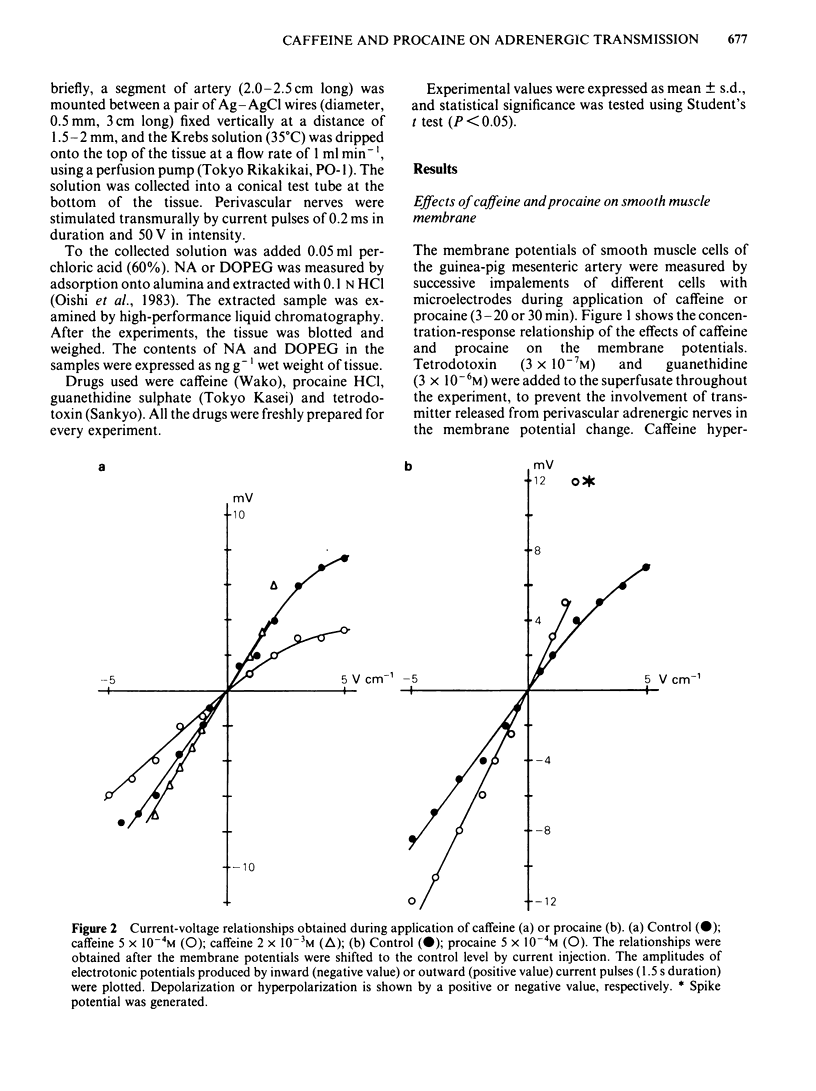
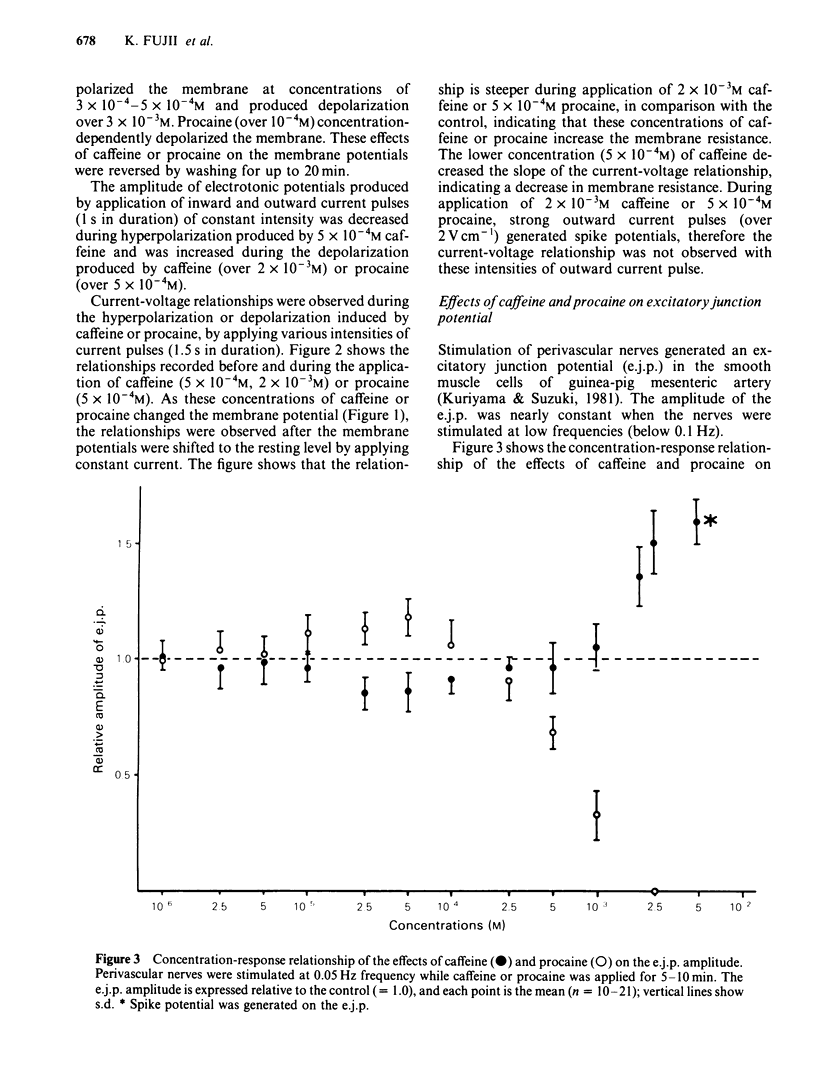
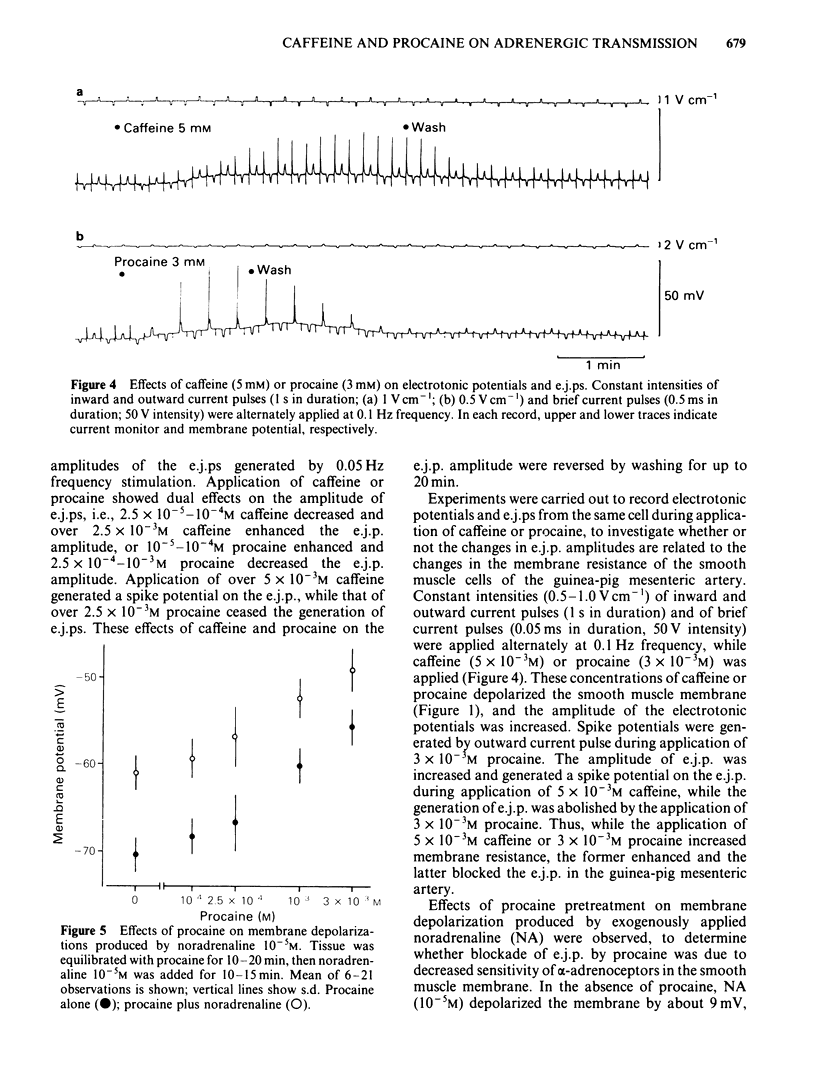
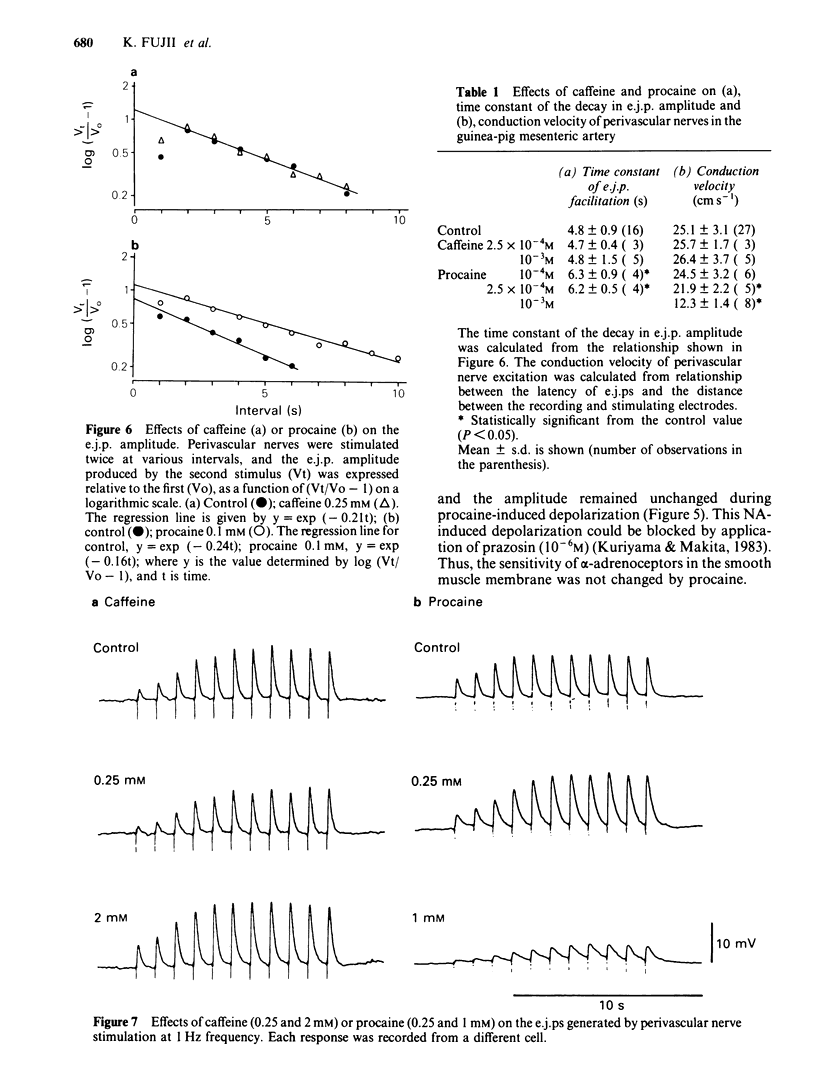
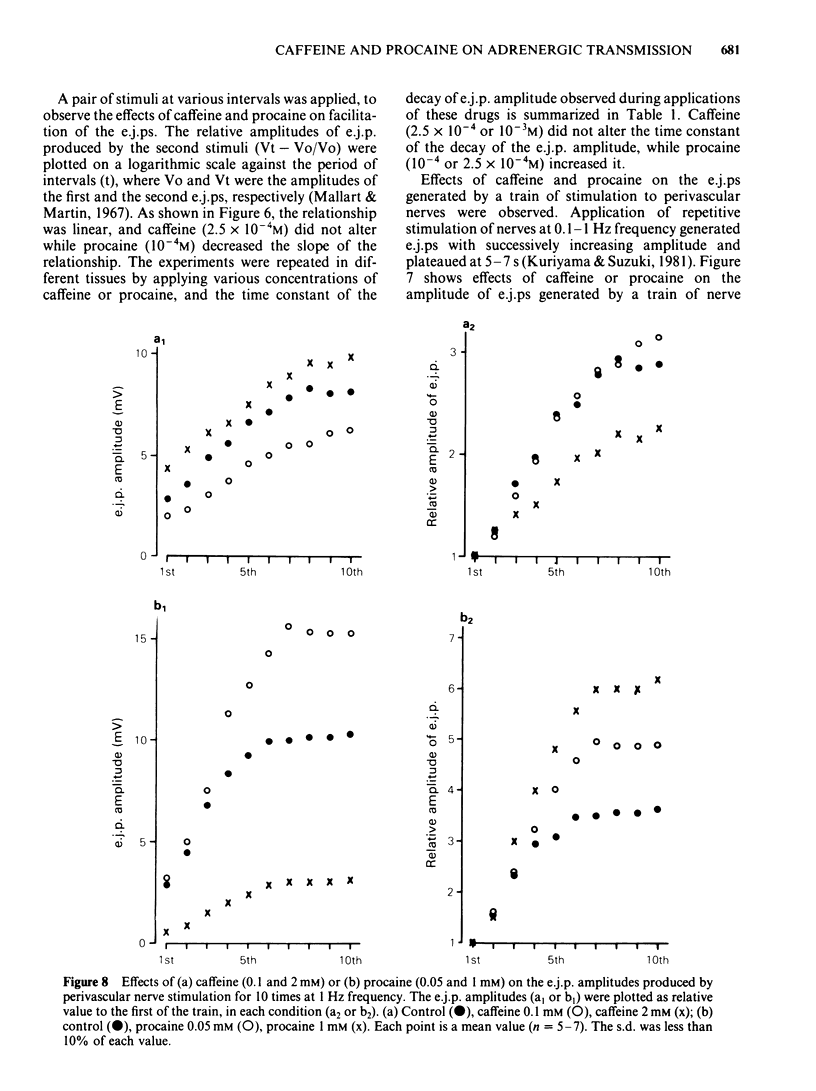
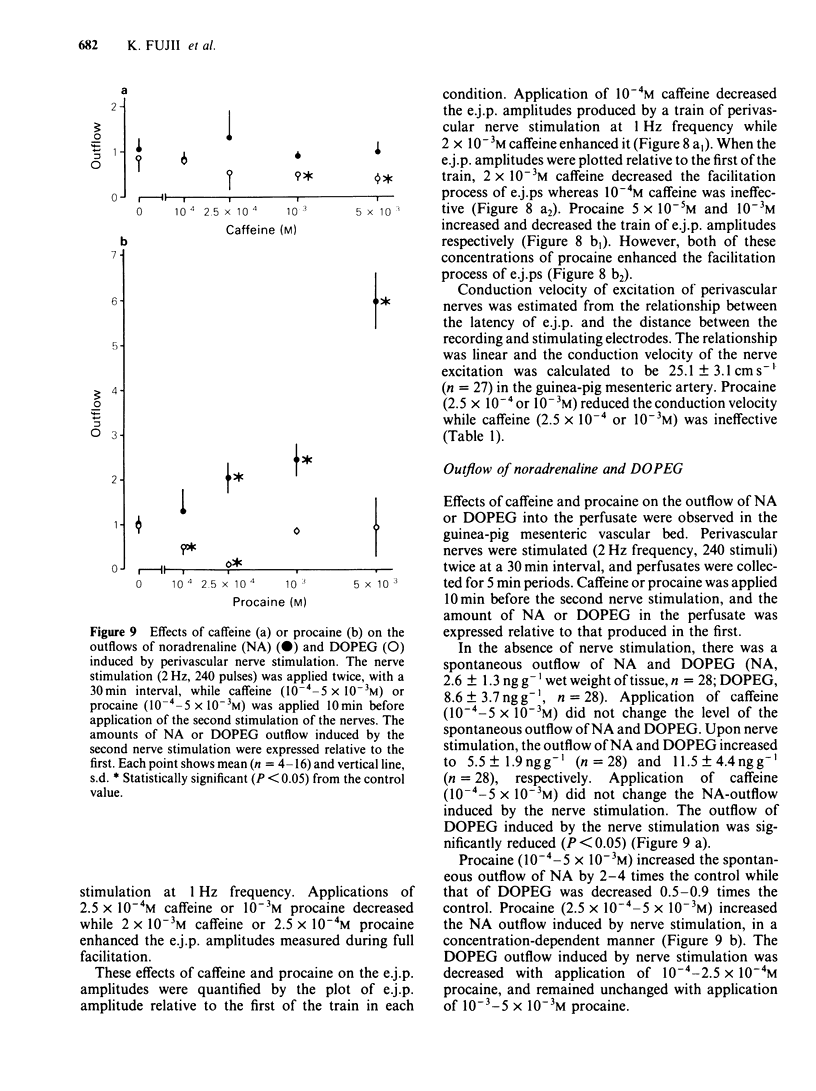
The effects of caffeine and procaine on noradrenergic transmission in the guinea-pig mesenteric artery were investigated by recording electrical responses of smooth muscle cells and by measuring the outflow of noradrenaline (NA) and 3,4-dihydroxyphenylglycol (DOPEG) induced by perivascular nerve stimulation. Caffeine possessed dual actions on the membrane, i.e., at low concentrations (2.5 X 10(-4)-5 X 10(-4)M), it hyperpolarized the membrane and decreased the membrane resistance and at high concentrations (over 2.5 X 10(-3)M) it depolarized the membrane and increased the membrane resistance. Procaine (over 10(-4)M) consistently depolarized the membrane and increased the membrane resistance. The amplitude of the excitatory junction potential (e.j.p.) produced by perivascular nerve stimulation was increased by low concentrations of procaine (2.5 X 10(-5)-10(-4)M) or high concentrations (10(-3)-5 X 10(-3)M) of caffeine and was decreased by low concentrations of caffeine (2.5 X 10(-5)-10(-4)M) or high concentrations of procaine (5 X 10(-4)-10(-3)M). Higher concentrations of caffeine (over 5 X 10(-3)M) induced a spike potential on the e.j.p., while higher concentrations of procaine (over 2.5 X 10(-3)M) inhibited the generation of e.j.ps. Facilitation of e.j.ps produced by repetitive stimulation of perivascular nerves remained unchanged by caffeine, while it was enhanced by procaine at any given concentration (caffeine 2.5 X 10(-4)-10(-3)M; procaine 10(-4)-10(-3)M). The membrane depolarization produced by exogenously applied NA (10(-5)M) was not blocked by pretreatment with procaine. Conduction velocity of perivascular nerve excitation remained unchanged by application of caffeine (up to 5 X 10(-3)M), and was reduced by application of procaine (over 2.5 X 10(-4)M). Outflow of NA during perivascular nerve stimulation remained unchanged by caffeine (10(-4)-3 X 10(-3)M), while it was enhanced by procaine (over 2.5 X 10(-4)M). The outflow of DOPEG was slightly reduced by caffeine (10(-3)-5 X 10(-3)M) and by lower concentrations of procaine (10(-4)-2.5 X 10(-4)M) but was not altered by higher concentrations of procaine (10(-3)-5 X 10(-3)M). It is concluded that in the guinea-pig mesenteric artery, high concentrations of caffeine (over 10(-3)M) increased the e.j.p. amplitude which might be due to an increase in membrane resistance of the smooth muscle cells. No marked effect of caffeine was observed on transmitter release from the nerve terminals. Procaine (over 2.5 X 10(-4)M) increased transmitter release from perivascular nerves and blocked the re-uptake mechanism of released NA.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blayney L., Thomas H., Muir J., Henderson A. Action of caffeine on calcium transport by isolated fractions of myofibrils, mitochondria, and sarcoplasmic reticulum from rabbit heart. Circ Res. 1978 Oct;43(4):520–526. doi: 10.1161/01.res.43.4.520. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Kuriyama H. Effects of agents that modulate potassium permeability on smooth muscle cells of the guinea-pig basilar artery. Br J Pharmacol. 1983 May;79(1):23–35. doi: 10.1111/j.1476-5381.1983.tb10491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graefe K. H., Henseling M. Neuronal and extraneuronal uptake and metabolism of catecholamines. Gen Pharmacol. 1983;14(1):27–33. doi: 10.1016/0306-3623(83)90058-7. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M. General features of electrical and mechanical properties of smooth muscle cells in the guinea-pig abdominal aorta. Pflugers Arch. 1982 Mar;393(1):109–117. doi: 10.1007/BF00582402. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. Modulation of noradrenergic transmission in the guinea-pig mesenteric artery: an electrophysiological study. J Physiol. 1983 Feb;335:609–627. doi: 10.1113/jphysiol.1983.sp014554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suyama A. Multiple actions of cocaine on neuromuscular transmission and smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1983 Apr;337:631–654. doi: 10.1113/jphysiol.1983.sp014646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Mishima S., Miyahara H., Suzuki H. Transmitter release modulated by alpha-adrenoceptor antagonists in the rabbit mesenteric artery: a comparison between noradrenaline outflow and electrical activity. Br J Pharmacol. 1984 Oct;83(2):537–547. doi: 10.1111/j.1476-5381.1984.tb16518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Oishi R., Mishima S., Kuriyama H. Determination of norepinephrine and its metabolites released from rat vas deferens using high-performance liquid chromatography with electrochemical detection. Life Sci. 1983 Feb 28;32(9):933–940. doi: 10.1016/0024-3205(83)90922-0. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]


