Abstract
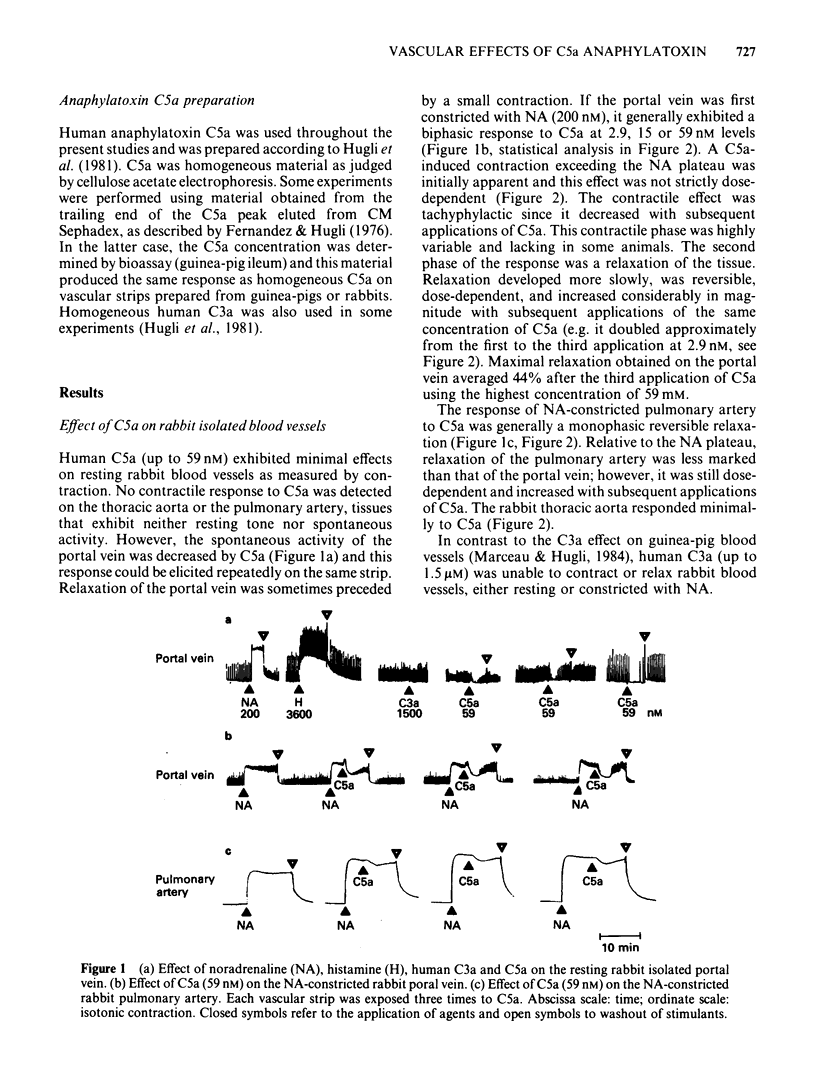
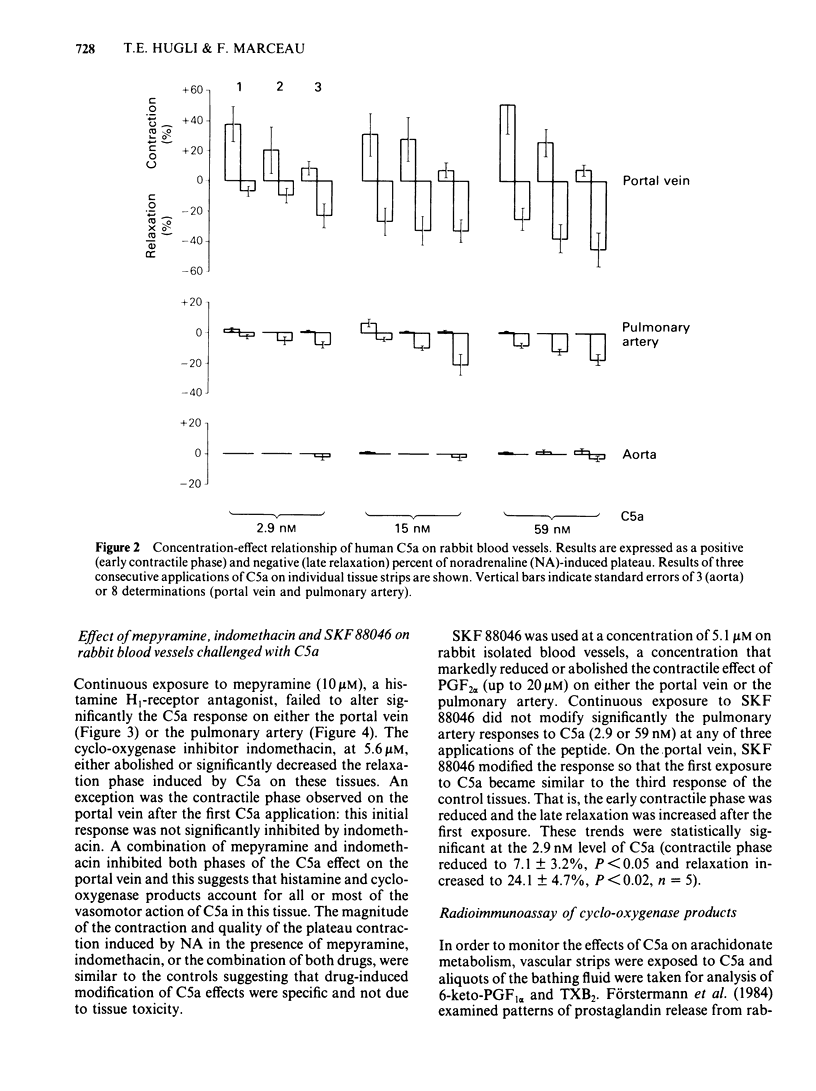
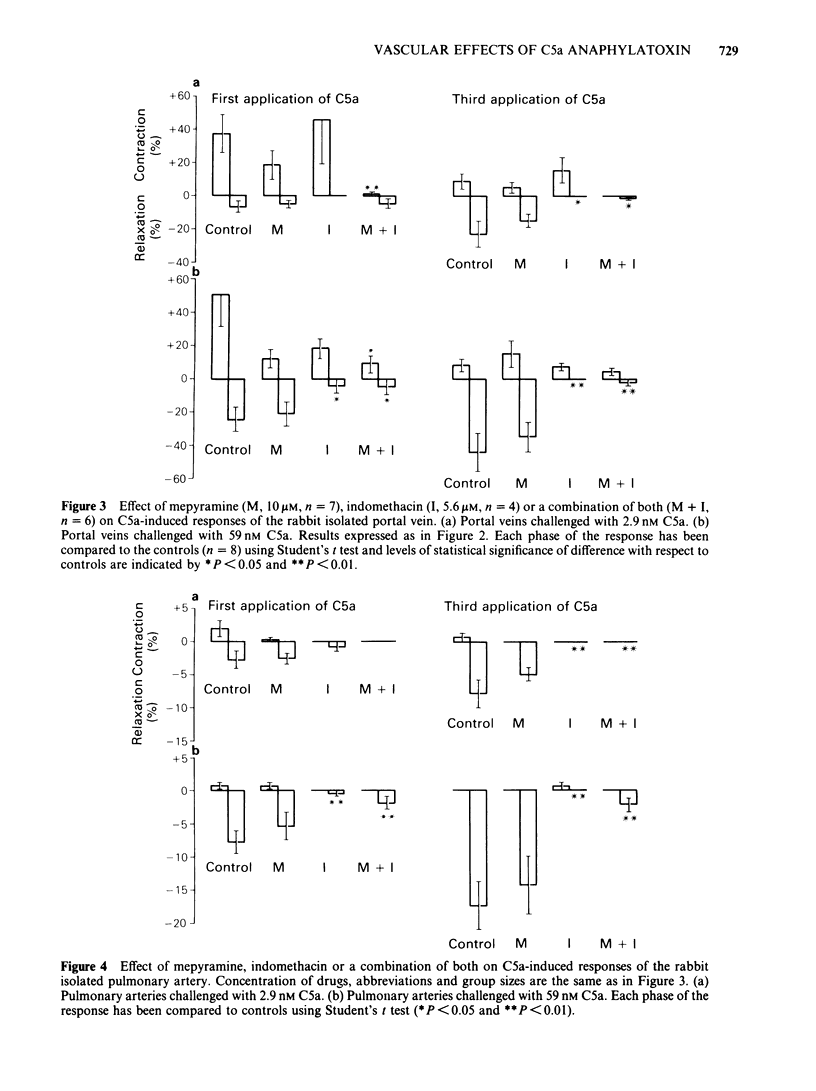
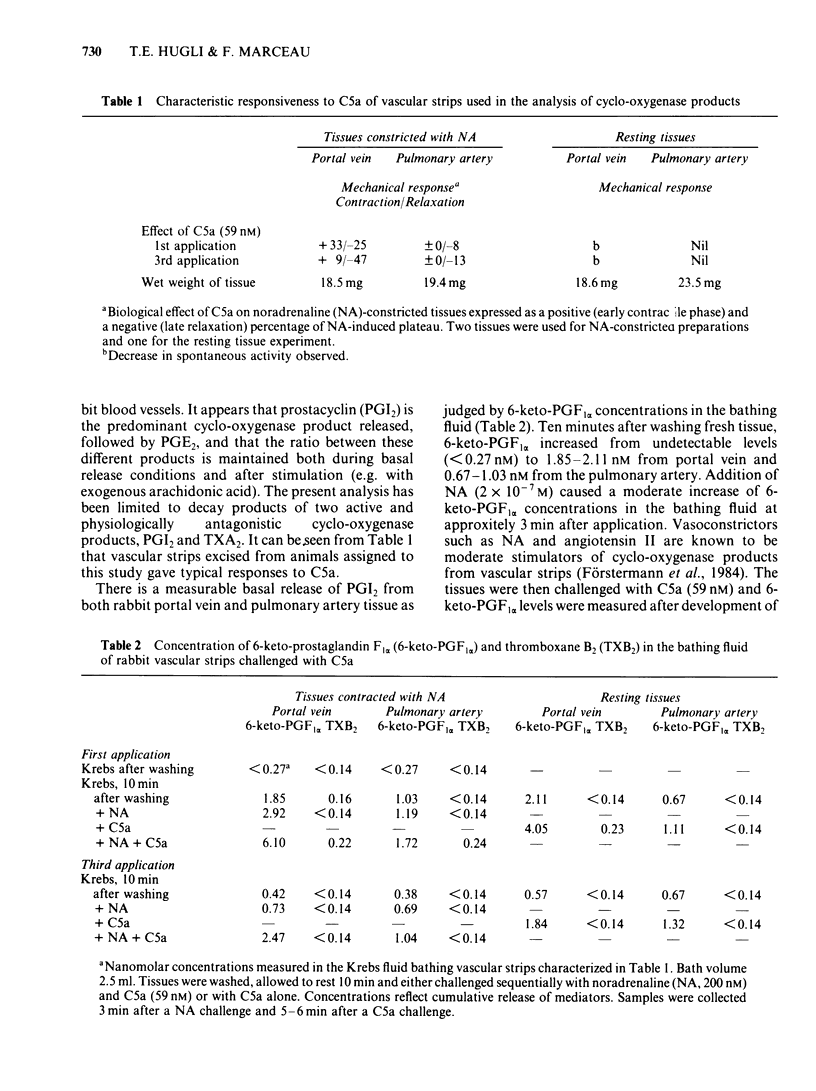
Strips of rabbit blood vessels were suspended in vitro and responses to complement peptides C3a and C5a were recorded isotonically. Human C3a (up to 1.5 microM) was inactive on rabbit vascular strips. Human C5a (2.9-59 nM) decreased spontaneous activity of the rabbit portal vein under resting baseline tension. The C5a relaxed strips of portal vein and pulmonary artery that were precontracted with noradrenaline (NA, 200 nM). On the portal vein, C5a-induced relaxation was preceded by a transient contractile phase which decreased with repeated applications of C5a. The magnitude of C5a-induced relaxation of both vessels increased with repeated stimulation by C5a. Maximal levels of relaxation for the third application of C5a at 59 nM averaged 44% and 17% of the NA-induced contraction plateau in portal vein and pulmonary artery, respectively. Strips of rabbit aorta responded minimally to C5a. Indomethacin (5.6 microM) significantly inhibited C5a-induced relaxation of the portal vein and pulmonary artery but had no effect on the early contractile response of the portal vein. Mepyramine (10 microM) failed to modify the C5a response from either vessel, but it reduced the contractile phase of the C5a response on the portal vein when applied in conjunction with indomethacin. The drug SKF 88046, an end organ antagonist of thromboxane (TX) A2 and some contractile prostaglandins, reduced the contractile phase and increased relaxation of the portal vein to C5a but did not modify the response of the pulmonary artery. Radioimmunoassays for 6-keto-prostaglandin F1 alpha (6-keto-PGF1 alpha) and TXB2 were performed on the fluid bathing rabbit isolated blood vessels.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodammer G., Vogt W. Actions of anaphylatoxin on circulation and respiration of the guinea pig. Int Arch Allergy Appl Immunol. 1967;32(5):417–428. doi: 10.1159/000229953. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Förstermann U., Hertting G., Neufang B. The importance of endogenous prostaglandins other than prostacyclin, for the modulation of contractility of some rabbit blood vessels. Br J Pharmacol. 1984 Apr;81(4):623–630. doi: 10.1111/j.1476-5381.1984.tb16127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon G., Regoli D., Rioux F. A new bioassay for glucagon. Br J Pharmacol. 1978 Sep;64(1):99–108. doi: 10.1111/j.1476-5381.1978.tb08646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Identification of classical anaphylatoxin as the des-Arg form of the C5a molecule: evidence of a modulator role for the oligosaccharide unit in human des-Arg74-C5a. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1833–1837. doi: 10.1073/pnas.78.3.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R., Sackeyfio A. C. The nature of adrenergic mechanisms involved in anaphylatoxin activity in the guinea-pig. Br J Pharmacol. 1972 Oct;46(2):260–269. doi: 10.1111/j.1476-5381.1972.tb06871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Gerard C., Kawahara M., Scheetz M. E., 2nd, Barton R., Briggs S., Koppel G., Russell S. Isolation of three separate anaphylatoxins from complement-activated human serum. Mol Cell Biochem. 1981 Dec 4;41:59–66. doi: 10.1007/BF00225297. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Suzuki H., Kuriyama H. Prostaglandin action on the main pulmonary artery and portal vein of the rabbit. Jpn J Physiol. 1976;26(6):681–692. doi: 10.2170/jjphysiol.26.681. [DOI] [PubMed] [Google Scholar]
- Marceau F., Hugli T. E. Effect of C3a and C5a anaphylatoxins on guinea-pig isolated blood vessels. J Pharmacol Exp Ther. 1984 Sep;230(3):749–754. [PubMed] [Google Scholar]
- Pavek K., Piper P. J., Smedegård G. Anaphylatoxin-induced shock and two patterns of anaphylactic shock: hemodynamics and mediators. Acta Physiol Scand. 1979 Apr;105(4):393–403. doi: 10.1111/j.1748-1716.1979.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Rampart M., Van Hove C., Bult H., Claeys M., Herman A. G. Mechanism of complement-induced stimulation of prostacyclin production by isolated rabbit peritoneum. Prostaglandins. 1983 Feb;25(2):245–261. doi: 10.1016/0090-6980(83)90108-9. [DOI] [PubMed] [Google Scholar]
- Regal J. F. C5a-induced aortic contraction: effect of an antihistamine and inhibitors of arachidonate metabolism. J Pharmacol Exp Ther. 1982 Jan;220(1):102–107. [PubMed] [Google Scholar]
- Regal J. F., Pickering R. J. C5a-induced tracheal contraction: effect of an SRS-A antagonist and inhibitors of arachidonate metabolism. J Immunol. 1981 Jan;126(1):313–316. [PubMed] [Google Scholar]
- Stimler N. P., Bach M. K., Bloor C. M., Hugli T. E. Release of leukotrienes from guinea pig lung stimulated by C5ades Arg anaphylatoxin. J Immunol. 1982 May;128(5):2247–2252. [PubMed] [Google Scholar]
- Stimler N. P., Brocklehurst W. E., Bloor C. M., Hugli T. E. Anaphylatoxin-mediated contraction of guinea pig lung strips: a nonhistamine tissue response. J Immunol. 1981 Jun;126(6):2258–2261. [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]


