Abstract
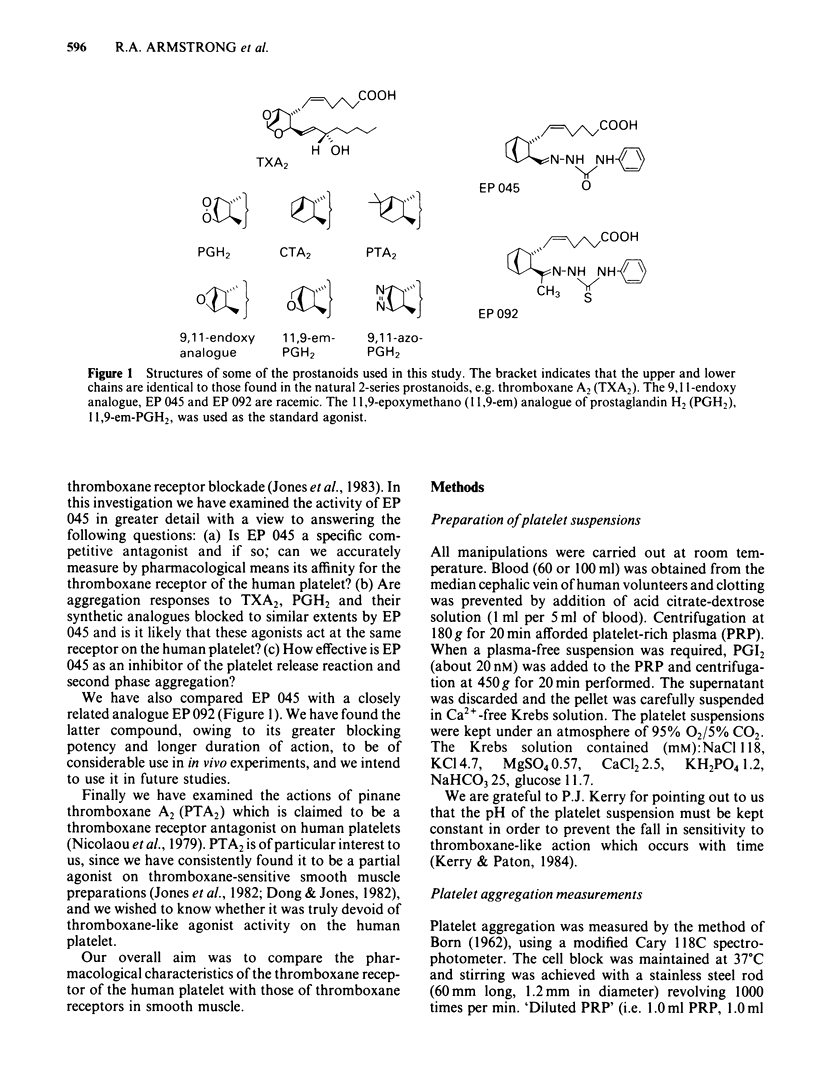
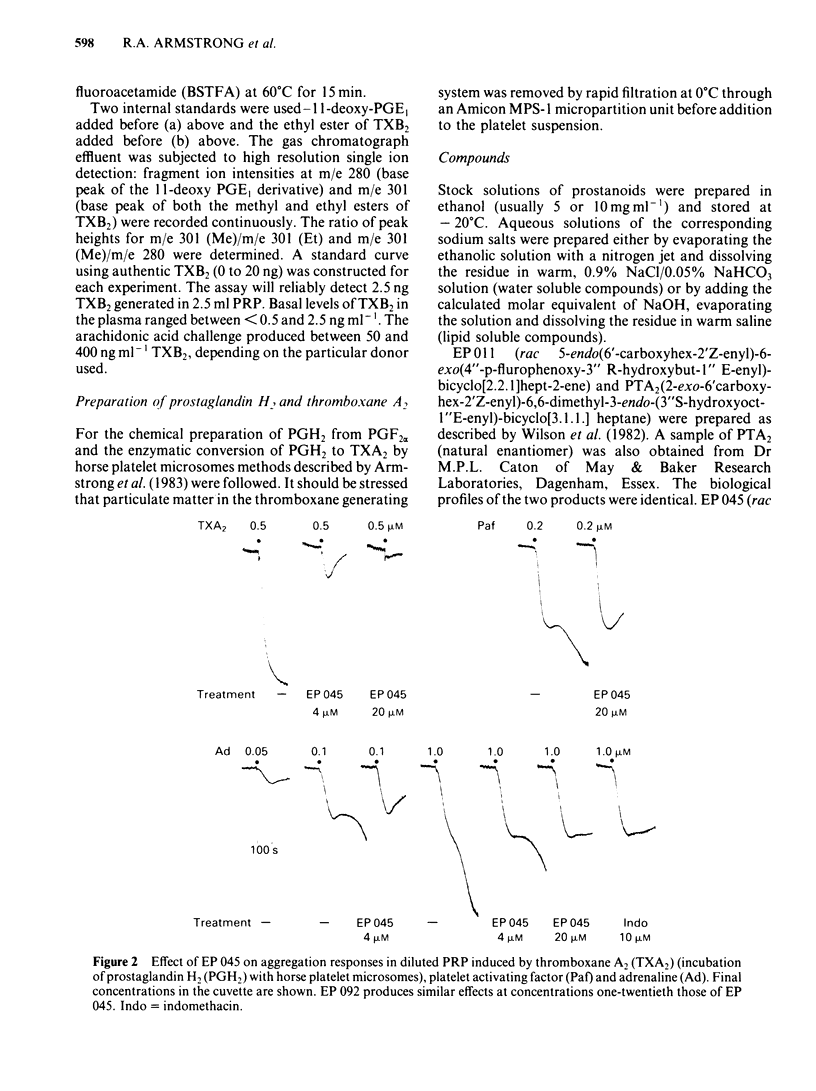
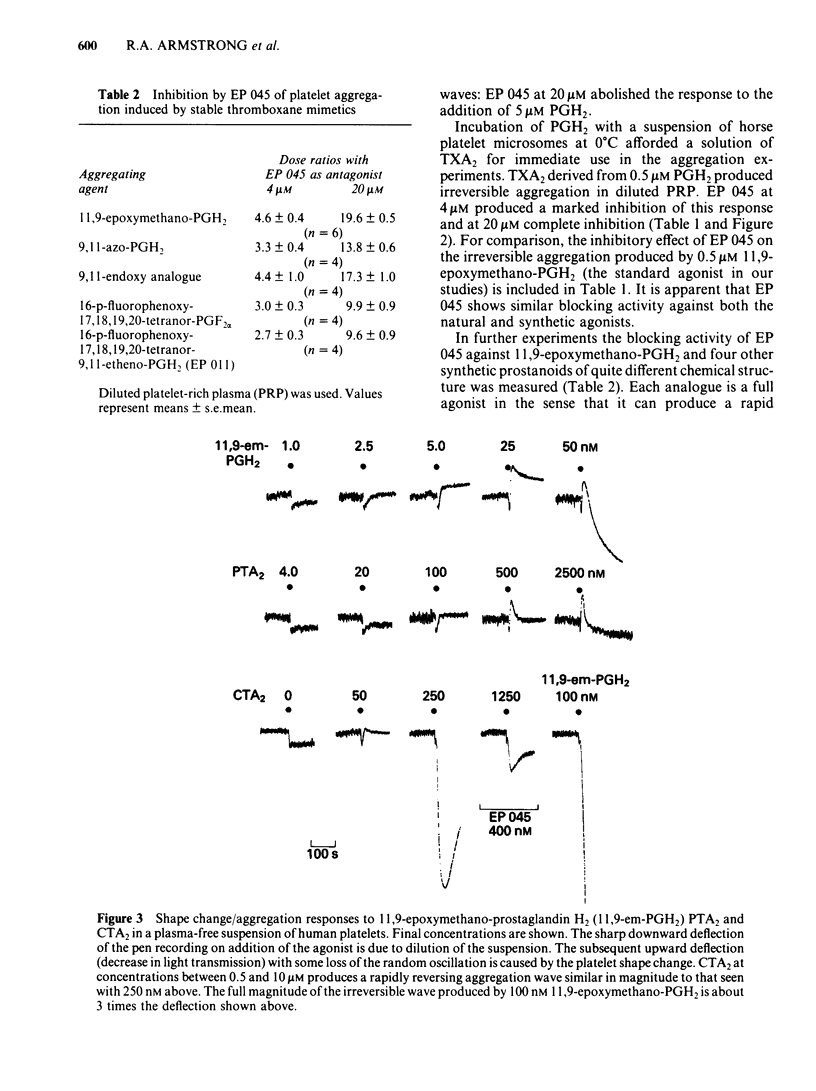
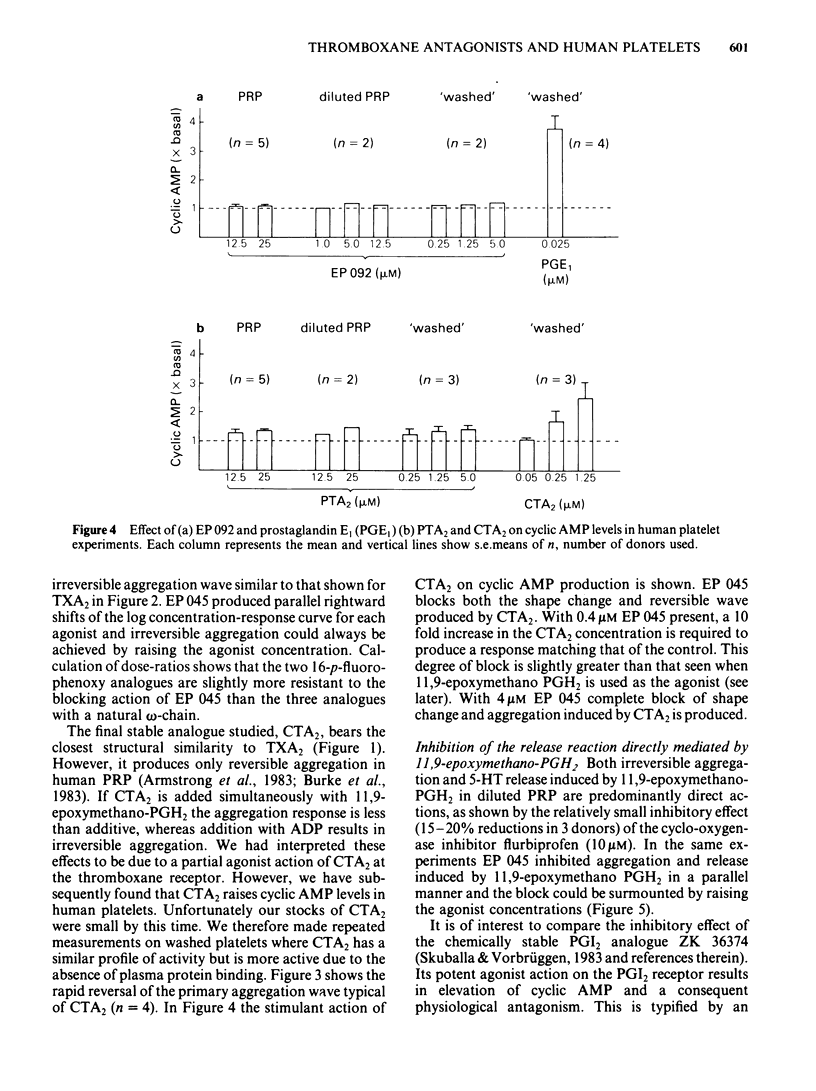
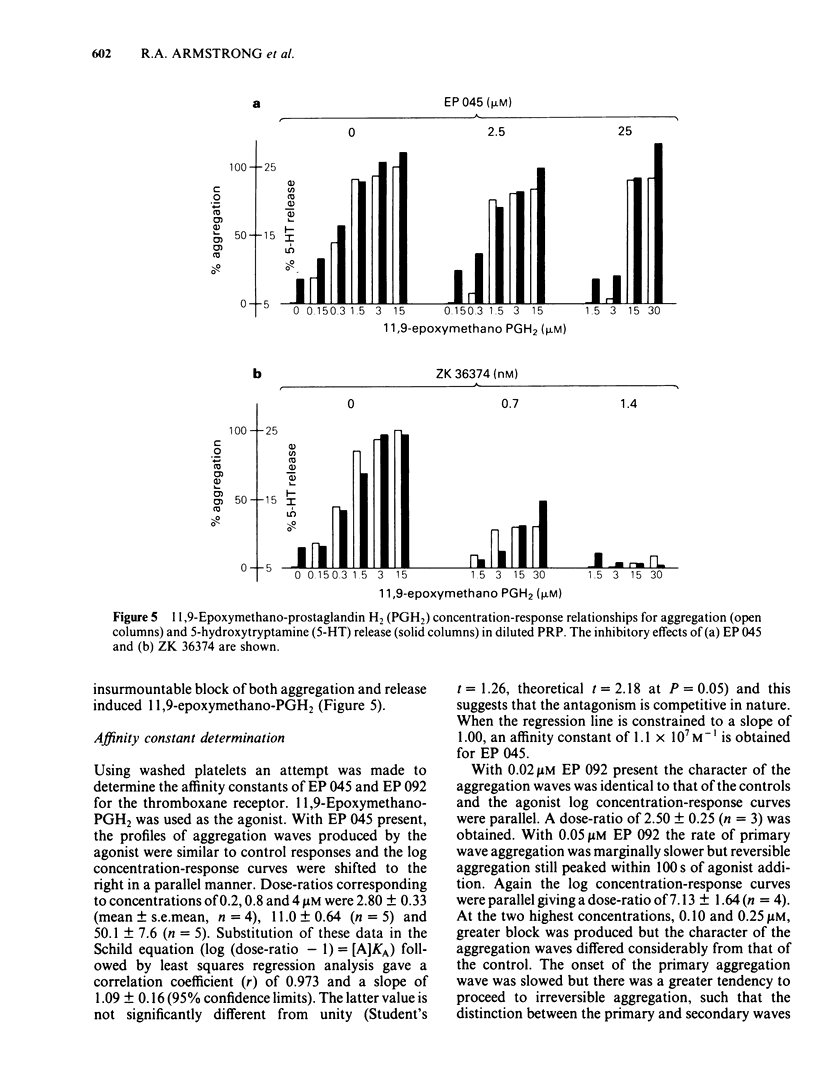
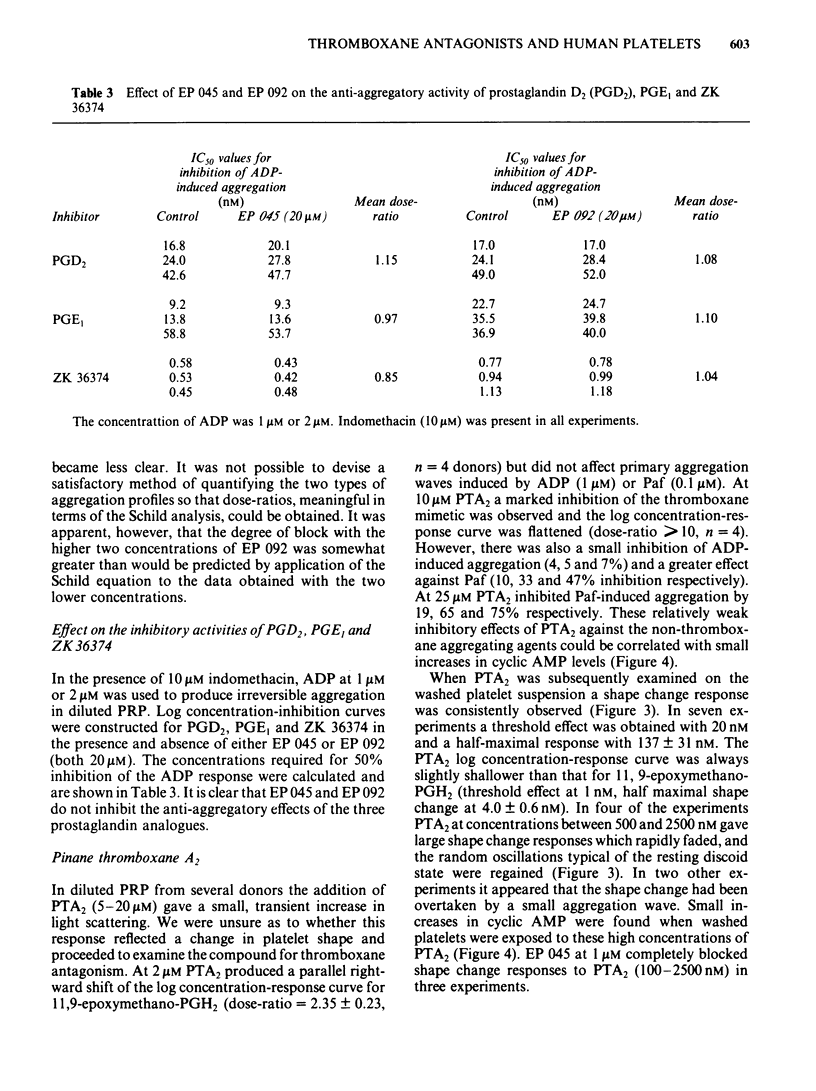
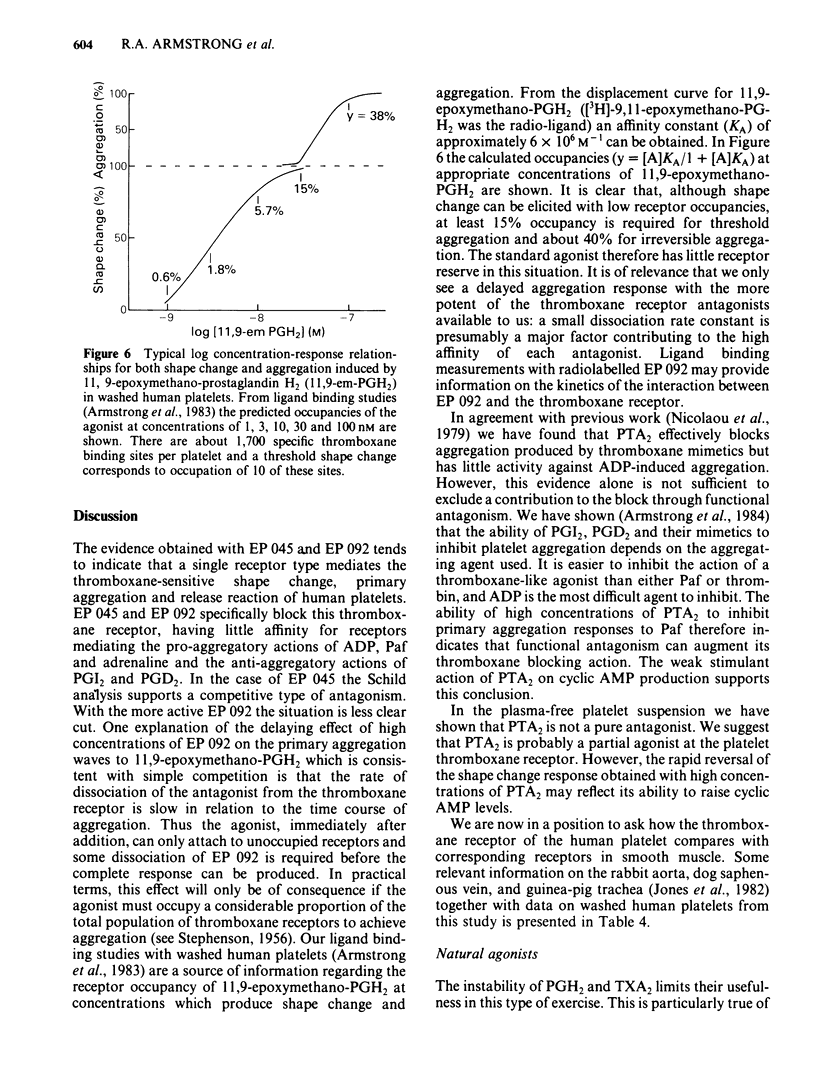
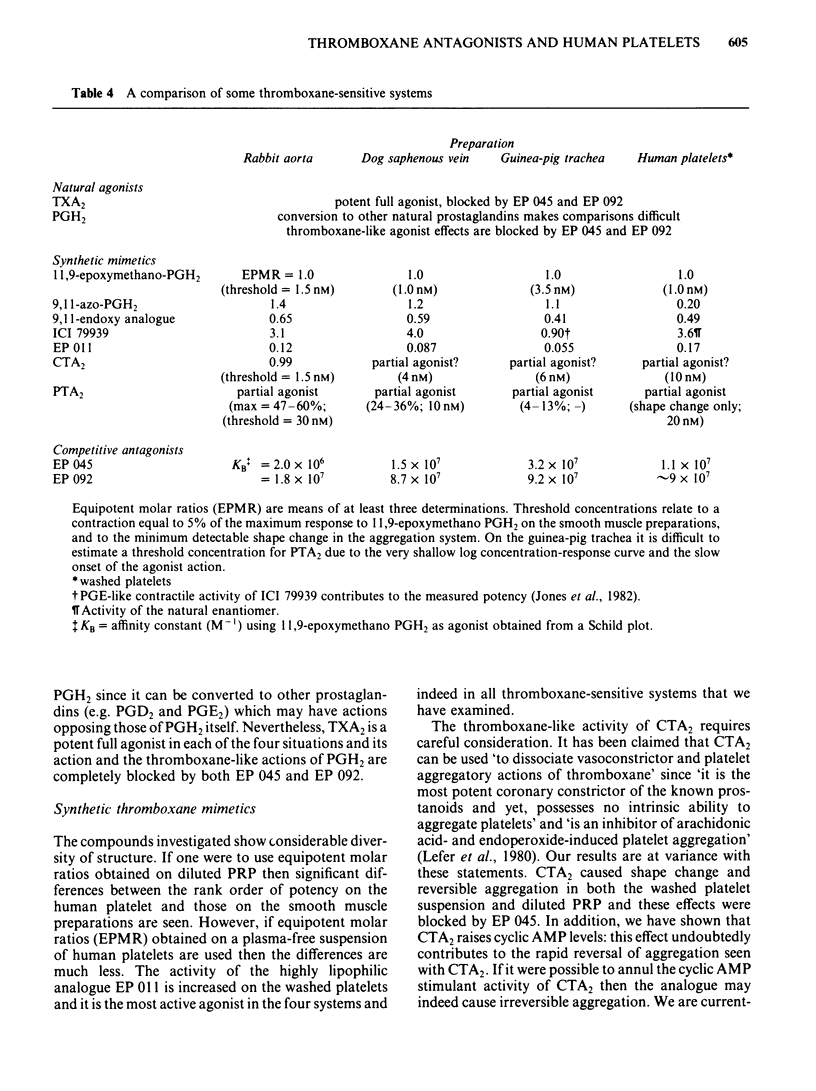
The inhibitory effects of three prostanoid analogues, EP 045, EP 092 and pinane thromboxane A2 (PTA2), on the aggregation of human platelets in vitro have been investigated. In diluted platelet-rich plasma (PRP), EP 045 (20 microM) and EP 092 (1 microM) completely inhibited irreversible aggregation responses to thromboxane A2 (TXA2), prostaglandin H2 (PGH2) and five chemically stable thromboxane mimetics, including 11,9-epoxymethano-PGH2 and 9,11-azo-PGH2. Reversible aggregation produced by the prostanoid analogue, CTA2, was also inhibited. The block of the stable agonist action was surmountable. In plasma-free platelet suspensions EP 045 and EP 092 were more potent antagonists. Schild analysis indicated a competitive type of antagonism for EP 045 (affinity constant of 1.1 X 10(7) M-1); the nature of the EP 092 block is not clear. Primary aggregation waves induced by ADP, platelet activating factor (Paf) and adrenaline were unaffected by EP 045 and EP 092, whereas the corresponding second phases of aggregation were suppressed. Aggregation and 5-hydroxytryptamine (5-HT) release induced by either PGH2 or 11,9-epoxymethano-PGH2 were inhibited in a parallel manner by EP 045. Inhibition of thromboxane biosynthesis is not involved in these effects. EP 045 and EP 092 did not raise adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels in the platelet suspensions. In plasma-free platelet suspensions PTA2 produced a shape change response which could be blocked by EP 045. PTA2, therefore, has a thromboxane-like agonist action. The block of the aggregatory action of 11,9-epoxymethano-PGH2 by PTA2 appears to be mainly due to competition at the thromboxane receptor. However, PTA2 produced a slight rise in cyclic AMP levels; this could be due to a very weak stimulant action on either PGI2 or PGD2 receptors present in the human platelet. Functional antagonism by PTA2 may therefore augment its thromboxane receptor blocking activity. The results are discussed in terms of (a) the specificity of antagonism produced by EP 045, EP 092 and PTA2, (b) the validity of affinity constant determinations for receptor antagonists when aggregation is the biological response, and (c) the characteristics of the human platelet thromboxane receptor in comparison with those of thromboxane receptors in smooth muscle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. A., Jones R. L., Wilson N. H. Ligand binding to thromboxane receptors on human platelets: correlation with biological activity. Br J Pharmacol. 1983 Aug;79(4):953–964. doi: 10.1111/j.1476-5381.1983.tb10541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S. E., Lefer A. M., Nicolaou K. C., Smith G. M., Smith J. B. Responsiveness of platelets and coronary arteries from different species to synthetic thromboxane and prostaglandin endoperoxide analogues. Br J Pharmacol. 1983 Feb;78(2):287–292. doi: 10.1111/j.1476-5381.1983.tb09393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. J., Jones R. L. Effects of prostaglandins and thromboxane analogues on bullock and dog iris sphincter preparations. Br J Pharmacol. 1982 May;76(1):149–155. doi: 10.1111/j.1476-5381.1982.tb09200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes M., Russell W., Walpole A. L. Potent luteolytic agents related to prostaglandin F2alpha. Nature. 1974 Jul 26;250(464):330–331. doi: 10.1038/250330a0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Peesapati V., Wilson N. H. Antagonism of the thromboxane-sensitive contractile systems of the rabbit aorta, dog saphenous vein and guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):423–438. doi: 10.1111/j.1476-5381.1982.tb09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Wilson N. H., Armstrong R. A., Peesapati V., Smith G. M. Effects of thromboxane antagonist EP 045 on platelet aggregation. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:345–350. [PubMed] [Google Scholar]
- Kerry P. J., Paton C. J. Increased sensitivity of arachidonic acid-induced platelet aggregation in the presence of carbon dioxide. Br J Pharmacol. 1984 Jan;81(1):125–130. doi: 10.1111/j.1476-5381.1984.tb10752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Packham M. A., Reimers H. J., Cazenave J. P., Mustard J. F. Mechanisms of platelet shape change, aggregation, and release induced by collagen, thrombin, or A23,187. J Lab Clin Med. 1977 Oct;90(4):707–719. [PubMed] [Google Scholar]
- Lefer A. M., Smith E. F., 3rd, Araki H., Smith J. B., Aharony D., Claremon D. A., Magolda R. L., Nicolaou K. C. Dissociation of vasoconstrictor and platelet aggregatory activities of thromboxane by carbocyclic thromboxane A2, a stable analog of thromboxane A2. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1706–1710. doi: 10.1073/pnas.77.3.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Nicolaou K. C., Magolda R. L., Smith J. B., Aharony D., Smith E. F., Lefer A. M. Synthesis and biological properties of pinane-thromboxane A2, a selective inhibitor of coronary artery constriction, platelet aggregation, and thromboxane formation. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2566–2570. doi: 10.1073/pnas.76.6.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock W. K., Armstrong R. A., Brydon L. J., Jones R. L., MacIntyre D. E. Thromboxane-induced phosphatidate formation in human platelets. Relationship to receptor occupancy and to changes in cytosolic free calcium. Biochem J. 1984 May 1;219(3):833–842. doi: 10.1042/bj2190833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. J., Parry M. J., Hawkeswood E., Cross P. E., Dickinson R. P. UK-37, 248, a novel, selective thromboxane synthetase inhibitor with platelet anti-aggregatory and anti-thrombotic activity. Thromb Res. 1981 Jul 1;23(1-2):145–162. doi: 10.1016/0049-3848(81)90247-4. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuballa W., Vorbrüggen H. Synthesis of ciloprost (ZK 36 374): a chemically stable and biologically potent prostacyclin analog. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:299–305. [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of an intermediate in prostaglandin biosynthesis and its association with the platelet release reaction. J Clin Invest. 1974 May;53(5):1468–1472. doi: 10.1172/JCI107695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague P. W., Heikes J. E., Harris D. N., Greenberg R. 7-Oxabicyclo[2.2.1]heptane analogs as modulators of the thromboxane A2 and prostacyclin receptors. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:337–343. [PubMed] [Google Scholar]
- Svensson J., Hamberg M., Samuelsson B. On the formation and effects of thromboxane A2 in human platelets. Acta Physiol Scand. 1976 Nov;98(3):285–294. doi: 10.1111/j.1748-1716.1976.tb10313.x. [DOI] [PubMed] [Google Scholar]
- Willis A. L., Vane F. M., Kuhn D. C., Scott C. G., Petrin M. An endoperoxide aggregator (Lass), formed in platelets in response to thrombotic stimuli: purification, identification and unique biological significance. Prostaglandins. 1974 Dec 25;8(6):453–507. doi: 10.1016/0090-6980(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Wilson N. H., Peesapati V., Jones R. L., Hamilton K. Synthesis of prostanoids with bicyclo[2.2.1]heptane, bicyclo[3.1.1]heptane, and bicyclo[2.2.2]octane ring systems. Activities of 15-hydroxy epimers on human platelets. J Med Chem. 1982 May;25(5):495–500. doi: 10.1021/jm00347a004. [DOI] [PubMed] [Google Scholar]


