Abstract
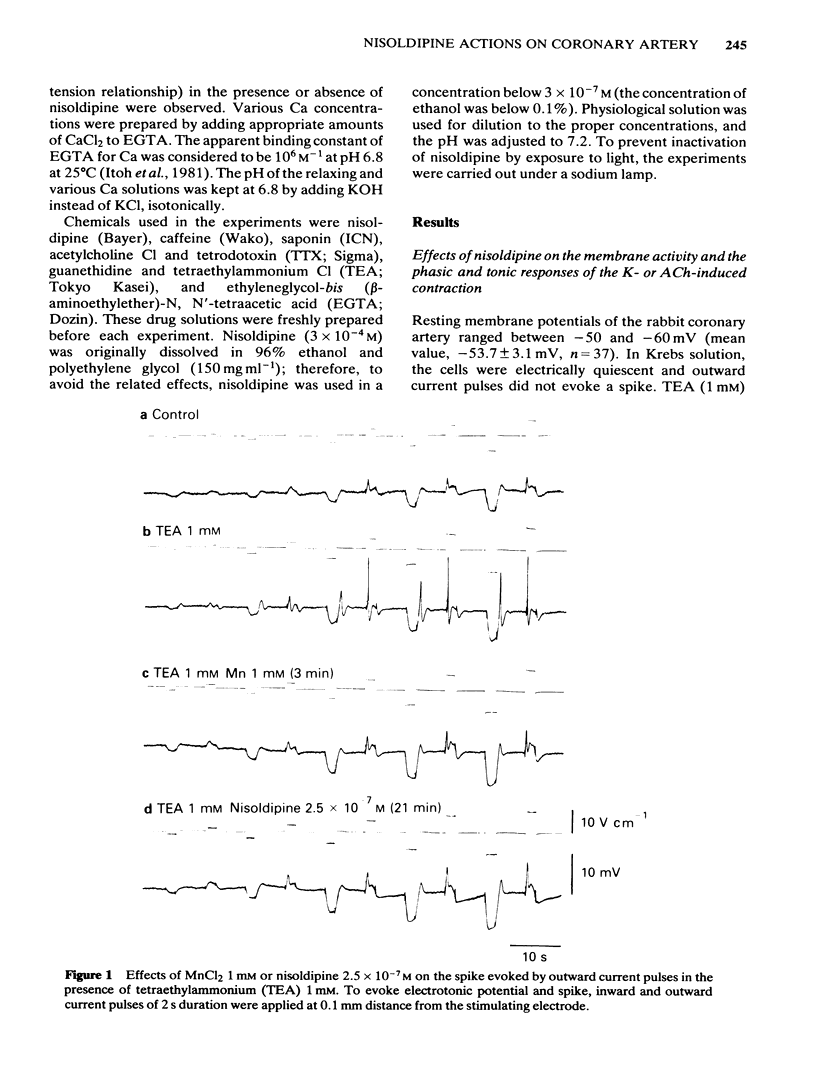
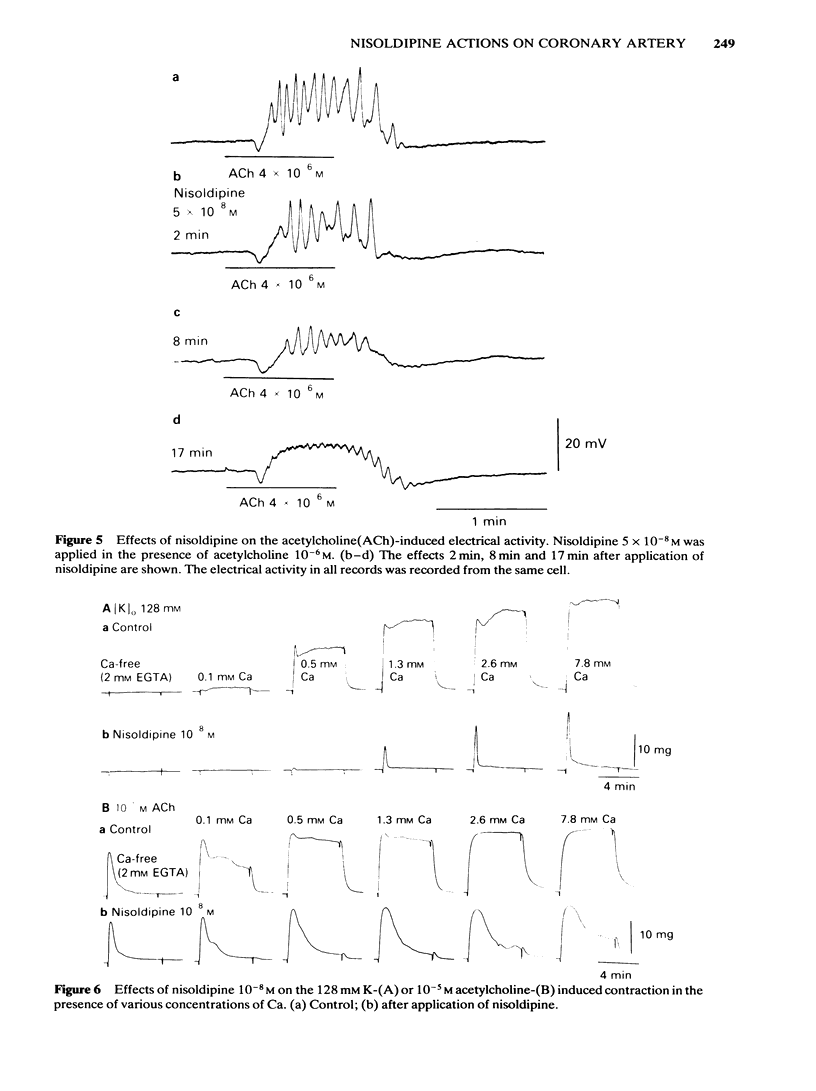
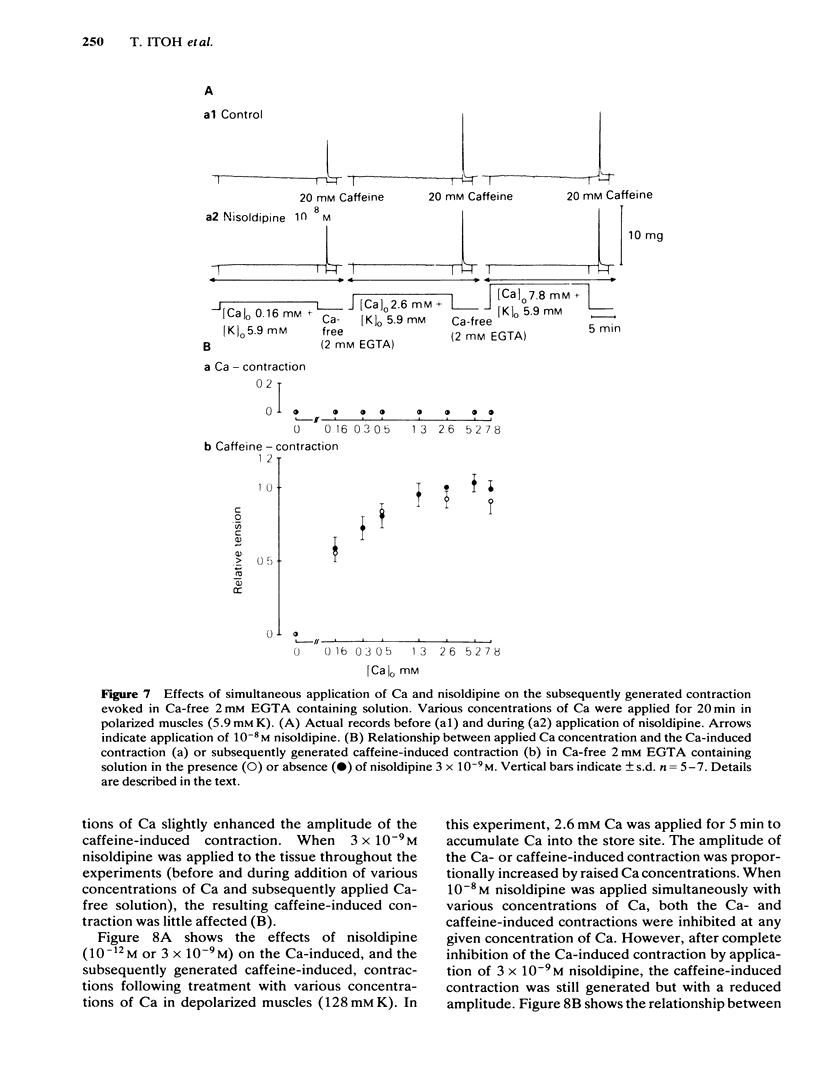
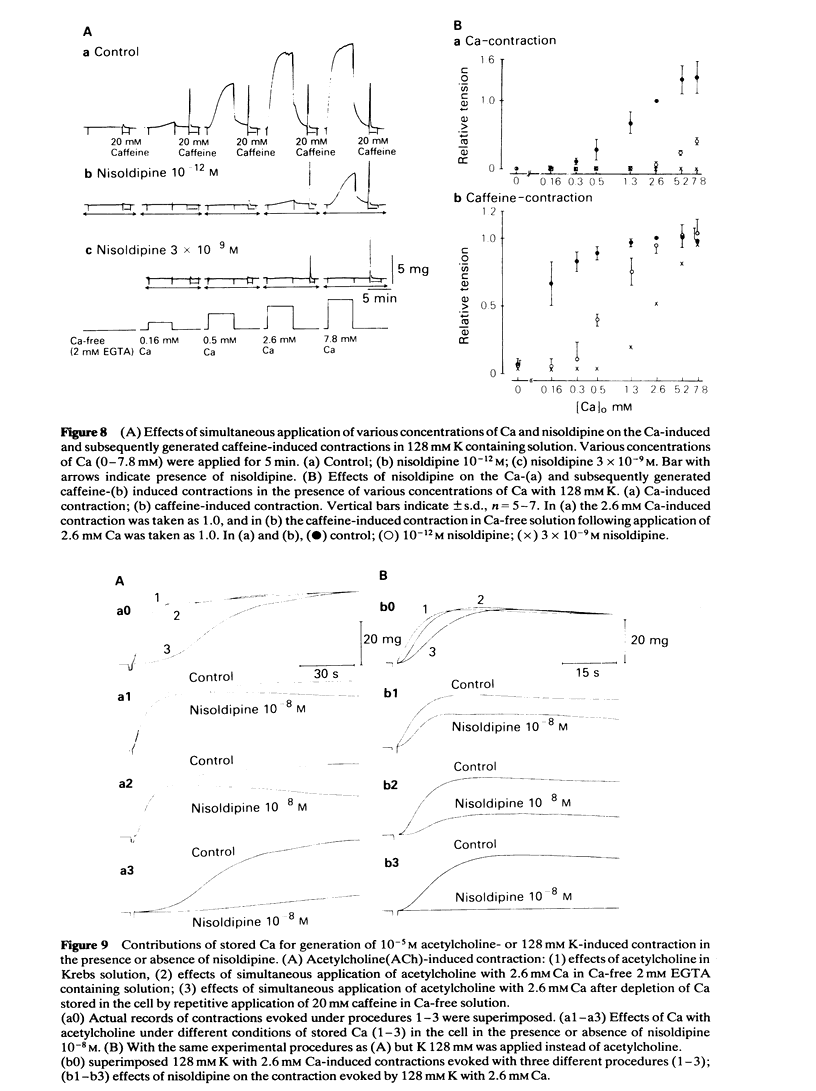
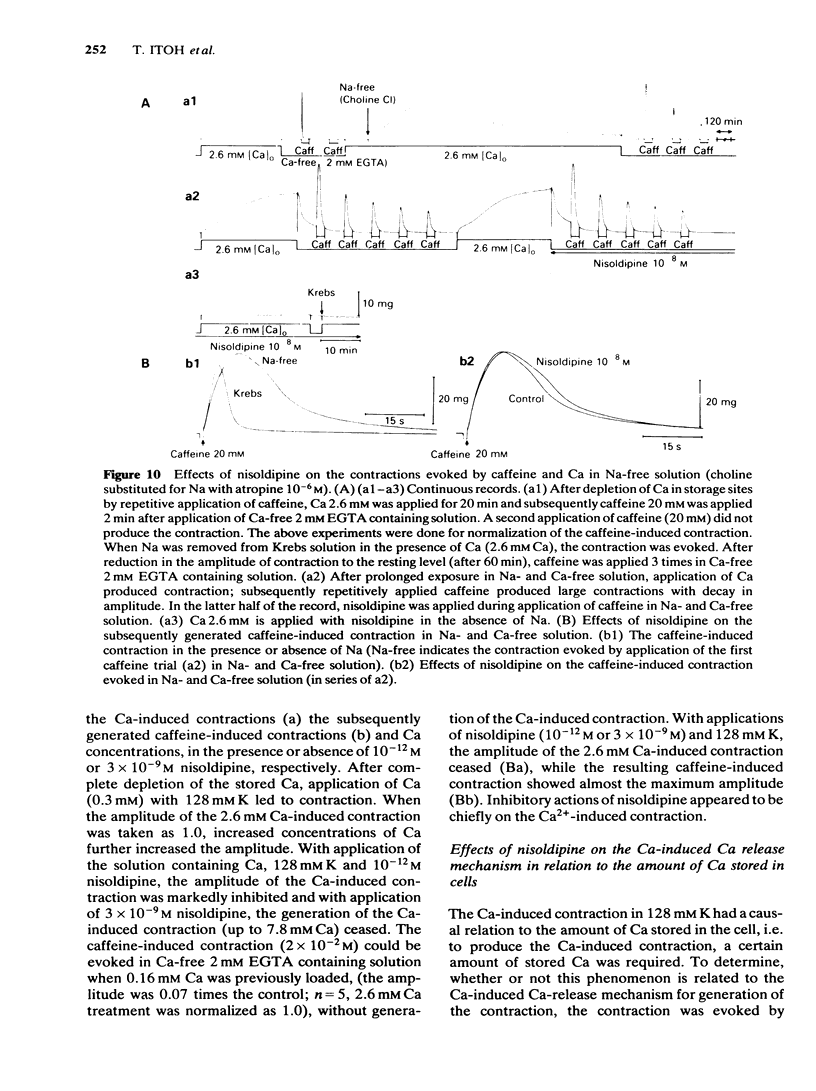
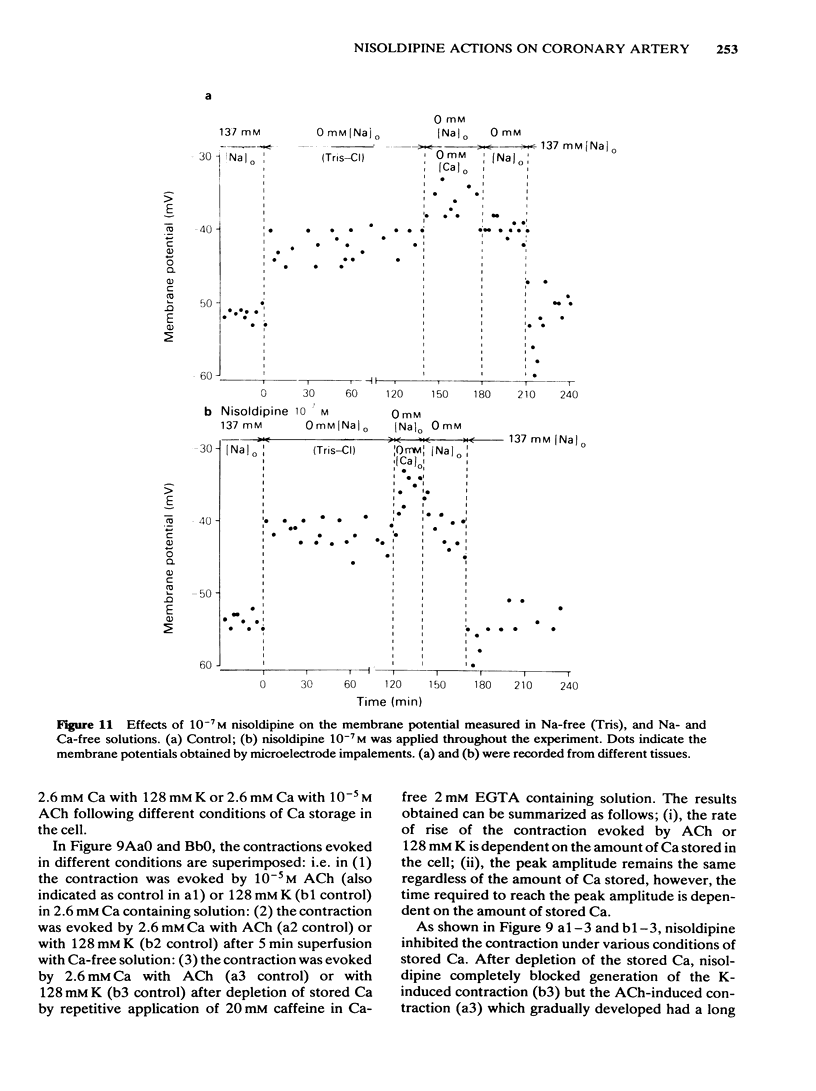
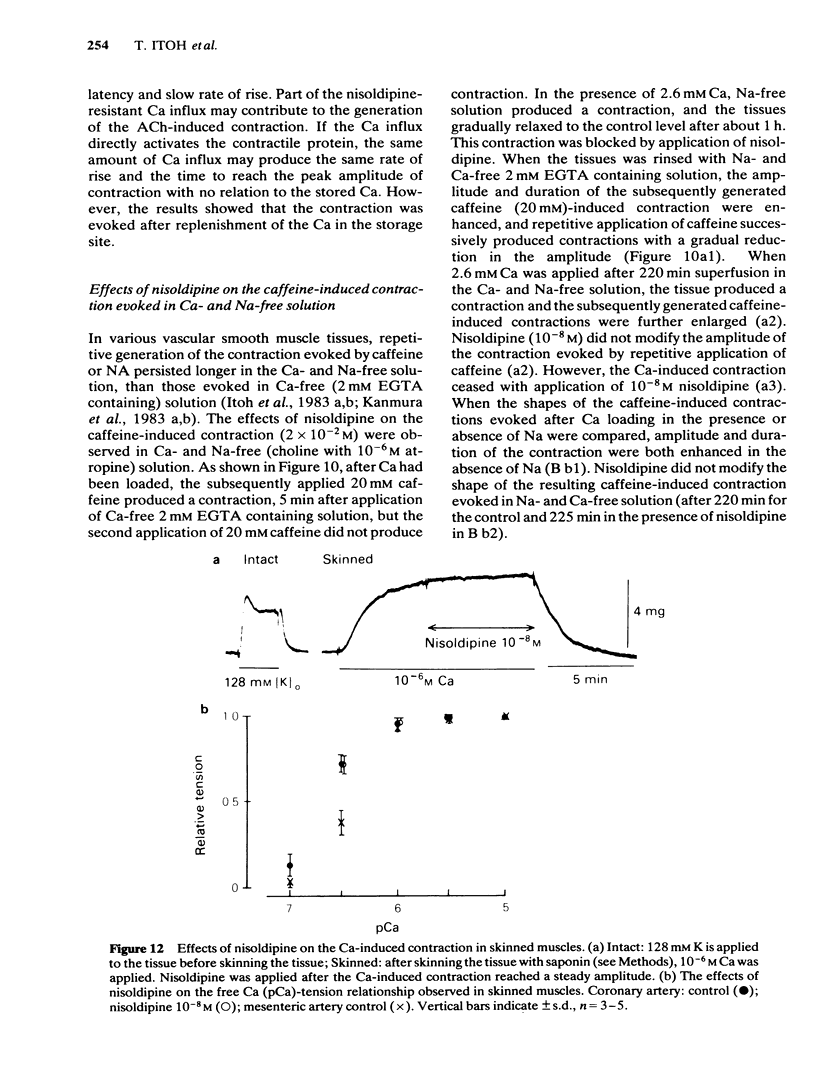
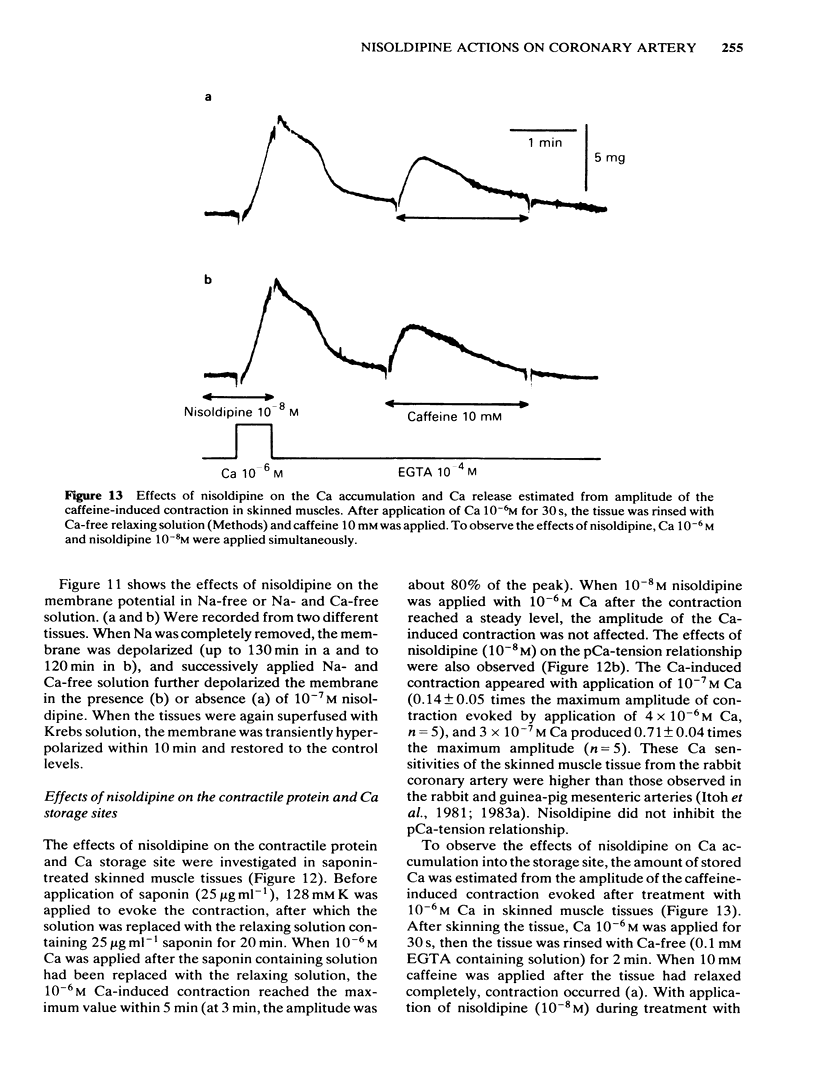
In smooth muscles of the rabbit coronary artery, nisoldipine inhibited the phasic and tonic responses of the contraction induced by 128 mM K (the IC50 values were 4 X 10(-8) M and 1 X 10(-13) M, respectively). This agent also inhibited the tonic response of the acetylcholine (ACh) (10(-5) M)-induced contraction (the IC50 value was 3 X 10(-10) M), but only slightly inhibited the phasic response (in 10(-7) M, 0.86 times the control). Nisoldipine (less than 10(-7) M) had no effect on the K-induced depolarization of the membrane at any given concentration. This drug (5 X 10(-8) M) did inhibit the oscillatory potential changes and spike potential evoked on the ACh-induced slow depolarization. After depletion of stored Ca from the polarized muscles (5.9 mM K), muscle cells accumulated Ca by application of 2.6 mM Ca without generation of contraction, i.e. a subsequently applied 20 mM caffeine produced the contraction in Ca-free solution. Nisoldipine (less than 10(-7) M) had little effect on this accumulation of Ca. The rate of rise and time to reach the maximum amplitude of the 128 mM K- or ACh-induced contraction (in 2.6 mM Ca) depended on the amount of stored Ca in cells. Nisoldipine (10(-8) M) consistently inhibited the Ca-induced contraction evoked in depolarized muscles (128 mM K), regardless of the amount of stored Ca. However, this agent (10(-8) M) did not inhibit the Ca release from storage sites evoked by activation of the muscarinic receptor. After prolonged superfusion (over 120 min) with Na- and Ca-free solution (guanethidine and atropine were present), application of 2.6 mM Ca produced contraction which was inhibited by 10(-8) M nisoldipine, while the depolarization induced by application of these solutions was not inhibited by nisoldipine. In saponin-skinned muscles, nisoldipine had no effect on the contractile proteins, as estimated from the pCa-tension relationship, or on the Ca accumulation into the Ca release from the Ca storage sites, as estimated from the caffeine-induced contraction. It is concluded that nisoldipine possesses a selective inhibitory action on voltage-dependent Ca influx, when the Ca channel is activated by depolarization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977 May;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F. Maintenance of ionic composition. Br Med Bull. 1979 Sep;35(3):227–234. doi: 10.1093/oxfordjournals.bmb.a071582. [DOI] [PubMed] [Google Scholar]
- Cauvin C., Loutzenhiser R., Van Breemen C. Mechanisms of calcium antagonist-induced vasodilation. Annu Rev Pharmacol Toxicol. 1983;23:373–396. doi: 10.1146/annurev.pa.23.040183.002105. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Grün G., Fleckenstein A. Die elektromechanische Entkoppelung der glatten Gefässmuskulatur als Grundprinzip der Coronardilatation durch 4-(2'-Nitrophenyl)-2,6-dimethyl-1,4-dihydropyridin-3,5-dicarbonsäure-dimethylester (BAY a 1040, Nifedipine. Arzneimittelforschung. 1972 Feb;22(2):334–344. [PubMed] [Google Scholar]
- Hirata M., Itoh T., Kuriyama H. Effects of external cations on calcium efflux from single cells of the guinea-pig taenia coli and porcine coronary artery. J Physiol. 1981 Jan;310:321–336. doi: 10.1113/jphysiol.1981.sp013552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Ueno H. Mechanisms of the nitroglycerine-induced vasodilation in vascular smooth muscles of the rabbit and pig. J Physiol. 1983 Oct;343:233–252. doi: 10.1113/jphysiol.1983.sp014890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y., Itoh T., Suzuki H., Ito Y., Kuriyama H. Effects of nifedipine on smooth muscle cells of the rabbit mesenteric artery. J Pharmacol Exp Ther. 1983 Jul;226(1):238–248. [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Makita Y., Kanmura Y., Itoh T., Suzuki H., Kuriyama H. Effects of nifedipine derivatives on smooth muscle cells and neuromuscular transmission in the rabbit mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol. 1983 Dec;324(4):302–312. doi: 10.1007/BF00502628. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. Receptor occupancy dose--response curve suggests that phosphatidyl-inositol breakdown may be intrinsic to the mechanism of the muscarinic cholinergic receptor. FEBS Lett. 1976 Oct 15;69(1):1–5. doi: 10.1016/0014-5793(76)80640-0. [DOI] [PubMed] [Google Scholar]


