Abstract
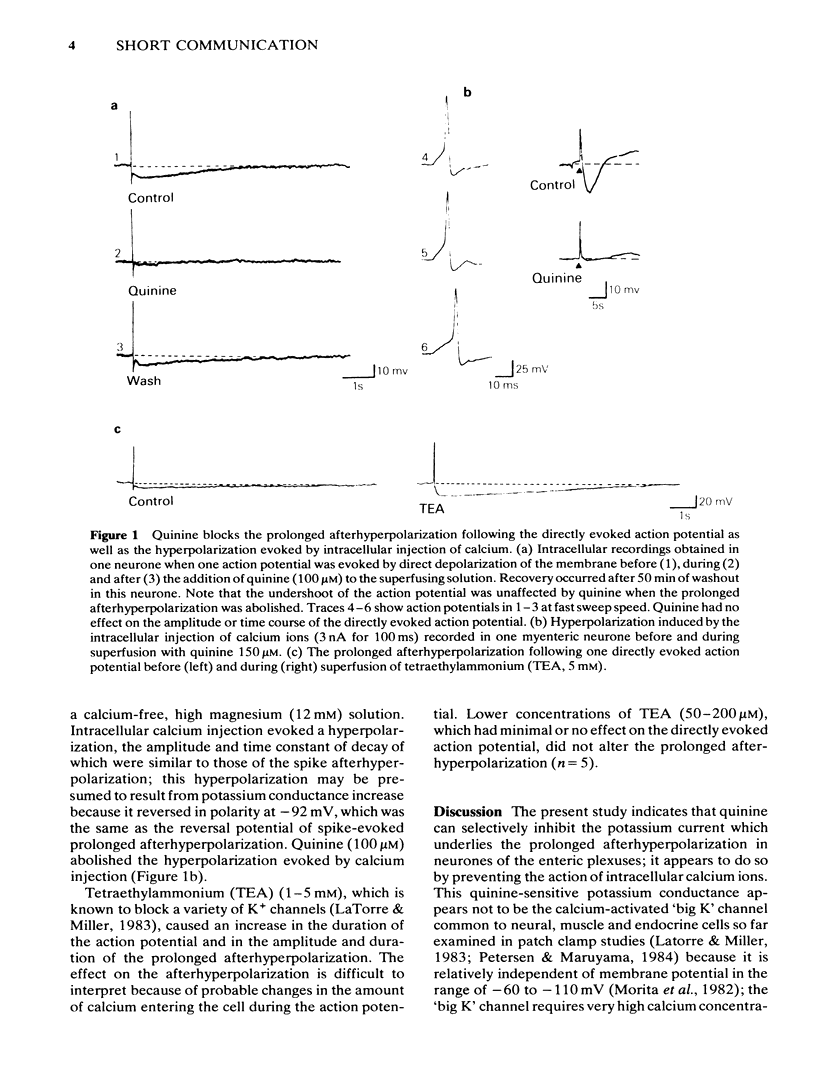
Quinine (100 microM) abolished the slow calcium-dependent afterhyperpolarization which occurs after an action potential in some neurones of the guinea-pig myenteric and submucous plexus. This occurred without any effect on the amplitude or time course of the action potential itself, or on the faster calcium-independent afterhyperpolarization. Tetraethylammonium did not reduce the slow afterhyperpolarization. Quinine also abolished the hyperpolarization which was evoked by intracellular injection of calcium ions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M., Shaw C. A potassium contribution to the response of the barnacle photoreceptor. J Physiol. 1977 Aug;270(1):151–163. doi: 10.1113/jphysiol.1977.sp011943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichstein E., Rothstein A. Effects of quinine on Ca++-induced K+ efflux from human red blood cells. J Membr Biol. 1981 Mar 15;59(1):57–63. doi: 10.1007/BF01870821. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Two types of neurones lacking synaptic input in the submucous plexus of guinea-pig small intestine. J Physiol. 1984 Jun;351:363–378. doi: 10.1113/jphysiol.1984.sp015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden J., Speckmann E. J. Effects of quinine on membrane potential and membrane currents in identified neurons of Helix pomatia. Neurosci Lett. 1981 Dec 11;27(2):139–143. doi: 10.1016/0304-3940(81)90258-5. [DOI] [PubMed] [Google Scholar]


