Abstract
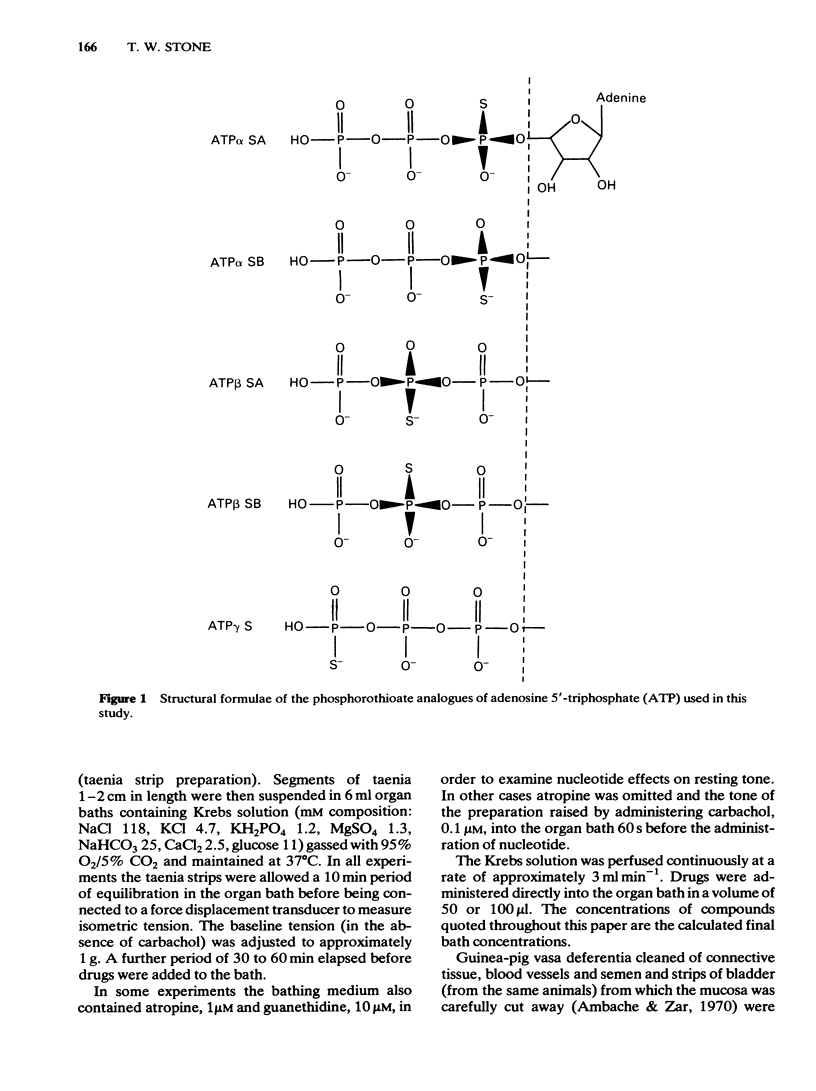
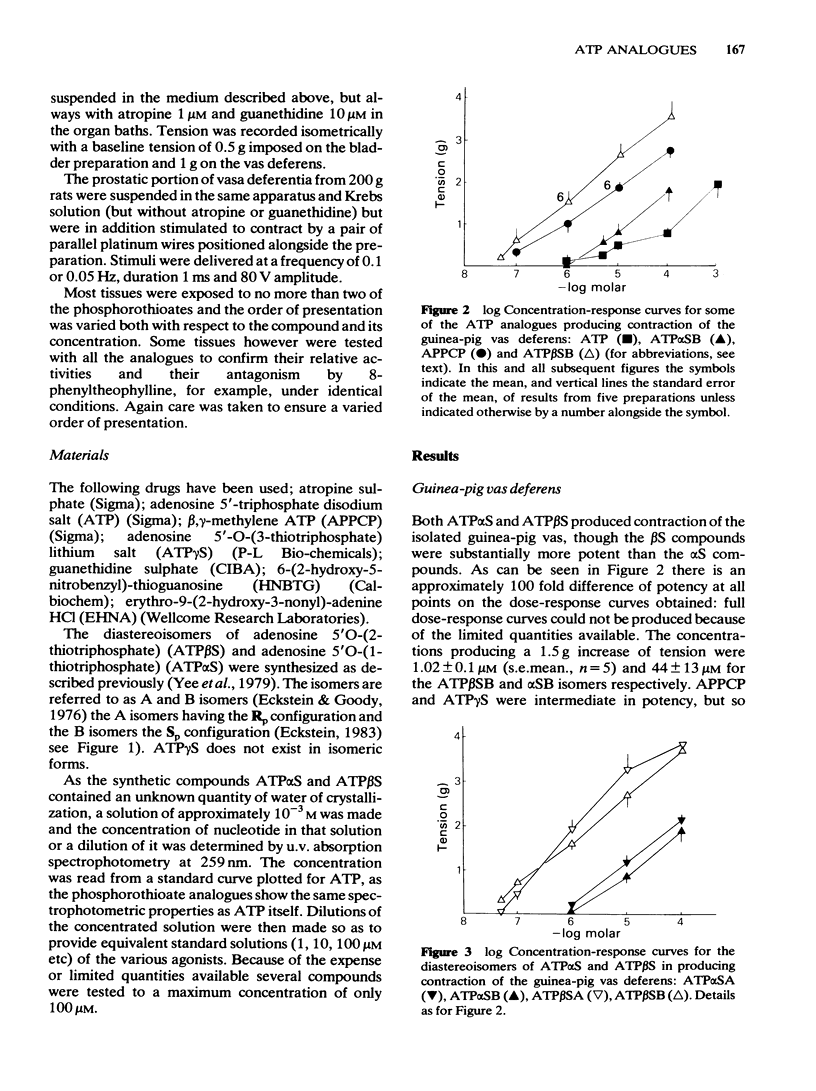
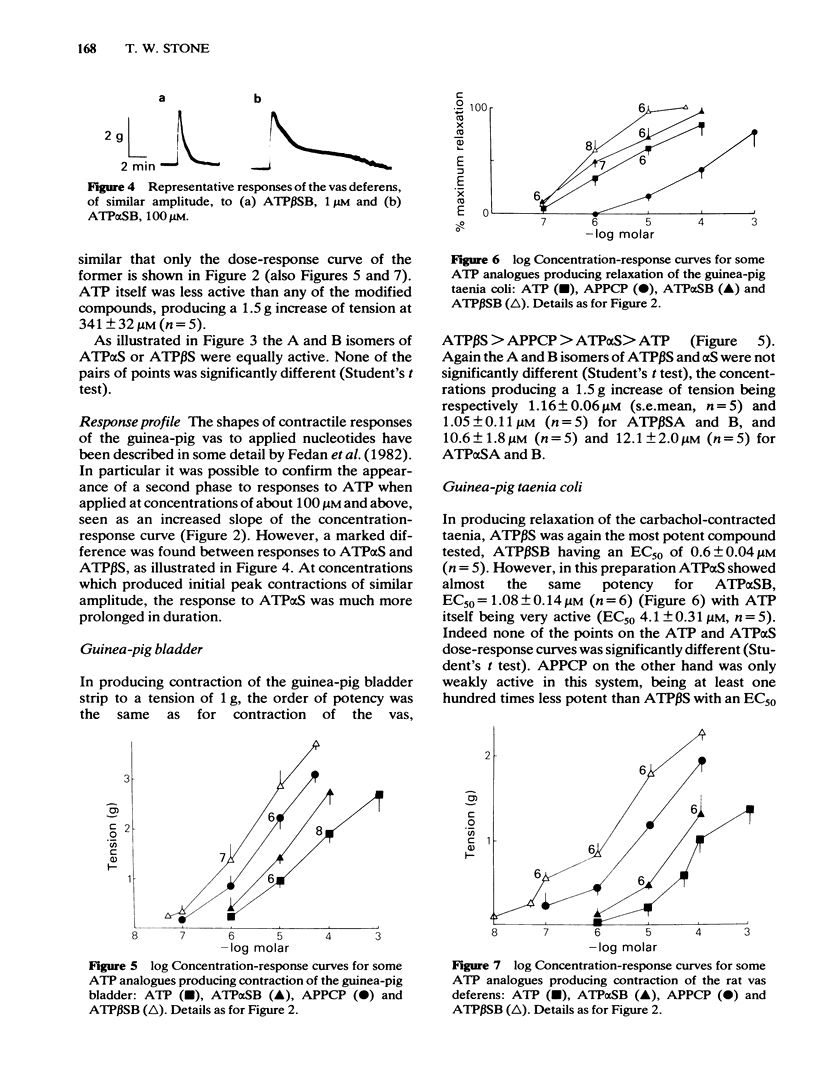
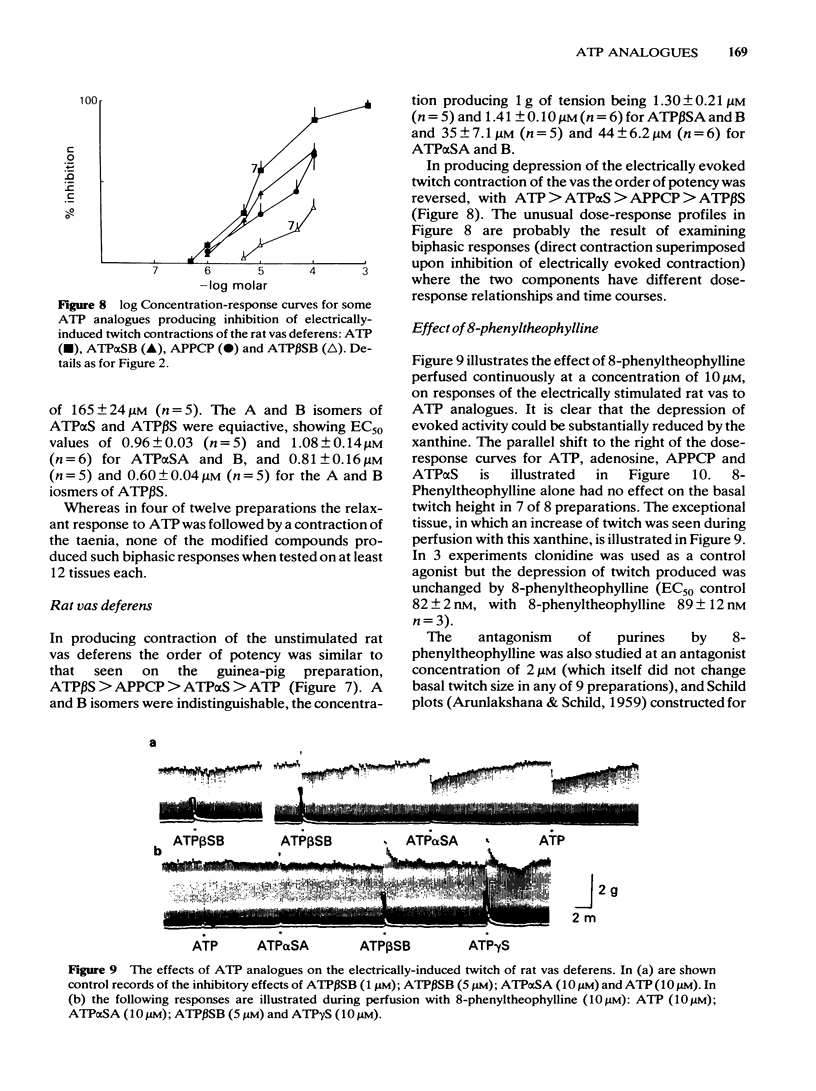
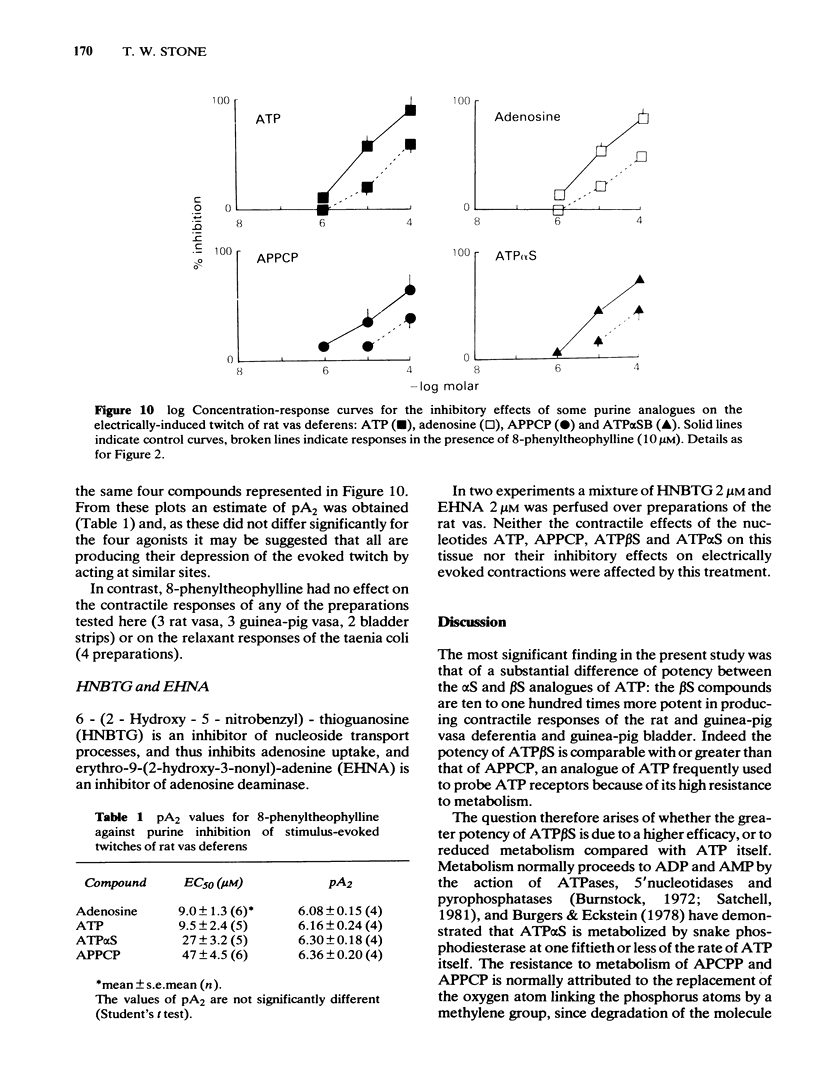
Phosphorothioate analogues of adenosine 5'-triphosphate (ATP) have been tested on the rat and guinea-pig vas deferens, the guinea-pig taenia coli and urinary bladder. Adenosine 5'0-(2-thiotriphosphate) (ATP beta S) was more active than adenosine 5'0(1-thiotriphosphate) (ATP alpha S) and ATP in producing contractile responses on the vas deferens of rat and guinea-pig, and guinea-pig bladder, though the difference of potency was less marked for producing relaxation of the carbachol-contracted taenia coli. No differences were observed between the A and B diastereoisomers of ATP alpha S or ATP beta S. Contractions of the vas deferens produced by ATP alpha S were of much longer duration than those produced by ATP beta S. When tested against electrically-evoked twitch responses of the vas deferens the order of potencies was reversed with ATP being most active and ATP beta S least active. These inhibitory effects were blocked by 8-phenyl-theophylline. The calculated pA2 values for ATP, adenosine, beta, gamma-methylene ATP (APPCP) and ATP alpha S were similar, suggesting a common site of action. The results do not reveal any stereoselectivity among the tissues tested, for the diastereoisomers of ATP phosphorothioates; the observed differences of potency may be due to differences between ATP alpha S and ATP beta S in their rates of metabolism to adenosine. The different response profiles to the phosphorothioates may however reflect some differences of receptor mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. The structural conformation of the polyphosphate chain of the ATP molecule is critical for its promotion of prostaglandin biosynthesis. Eur J Pharmacol. 1981 Jan 5;69(1):81–86. doi: 10.1016/0014-2999(81)90604-x. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. Absolute configuration of the diastereomers of adenosine 5'-O-(1-thiotriphosphate): consequences for the stereochemistry of polymerization by DNA-dependent RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4798–4800. doi: 10.1073/pnas.75.10.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Paddle B., Staszewska-Barczak J. Evidence that prostaglandin is responsible for the 'rebound contraction' following stimulation of non-adrenergic, non-cholinergic ('purinergic') inhibitory nerves. Eur J Pharmacol. 1975 Apr;31(2):360–362. doi: 10.1016/0014-2999(75)90060-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cusack N. J., Meldrum L. A. Effects of phosphorothioate analogues of ATP, ADP and AMP on guinea-pig taenia coli and urinary bladder. Br J Pharmacol. 1984 Jun;82(2):369–374. doi: 10.1111/j.1476-5381.1984.tb10771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapal J., Loubatieres-Mariani M. M. Effects of phosphate-modified adenine nucleotide analogues on insulin secretion from perfused rat pancreas. Br J Pharmacol. 1981 May;73(1):105–110. doi: 10.1111/j.1476-5381.1981.tb16778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J., Burnstock G., Vanhoutte P. M. Modulation of the evoked release of noradrenaline in canine saphenous vein via presynaptic receptors for adenosine but not ATP. Eur J Pharmacol. 1979 May 15;55(4):401–405. doi: 10.1016/0014-2999(79)90115-8. [DOI] [PubMed] [Google Scholar]
- Dipolo R. The influence of nucleotides on calcium fluxes. Fed Proc. 1976 Dec;35(14):2579–2582. [PubMed] [Google Scholar]
- Eckstein F., Goody R. S. Synthesis and properties of diastereoisomers of adenosine 5'-(O-1-thiotriphosphate) and adenosine 5'-(O-2-thiotriphosphate). Biochemistry. 1976 Apr 20;15(8):1685–1691. doi: 10.1021/bi00653a015. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P., Colby J., Westfall D. P. Contribution by purines to the neurogenic response of the vas deferens of the guinea pig. Eur J Pharmacol. 1981 Jan 5;69(1):41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., Westfall D. P., O'Donnell J. P. Comparison of the effects of arylazido aminopropionyl ATP (ANAPP3), an ATP antagonist, on responses of the smooth muscle of the guinea-pig vas deferens to ATP and related nucleotides. Eur J Pharmacol. 1982 Dec 3;85(3-4):277–290. doi: 10.1016/0014-2999(82)90215-1. [DOI] [PubMed] [Google Scholar]
- Ferrero J. D., Frischknecht R. Different effector mechanisms for ATP and adenosine hyperpolarization in the guinea-pig taenia coli. Eur J Pharmacol. 1983 Jan 28;87(1):151–154. doi: 10.1016/0014-2999(83)90063-8. [DOI] [PubMed] [Google Scholar]
- Frew R., Lundy P. M. Effect of arylazido aminopropionyl ATP (ANAPP3), on ATP responses of isolated guinea pig smooth muscle. Life Sci. 1982 Jan 18;30(3):259–267. doi: 10.1016/0024-3205(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- Maguire M. H., Satchell D. G. The contribution of adenosine to the inhibitory actions of adenine nucleotides on the guinea-pig taenia coli: studies with phosphate-modified adenine nucleotide analogs and dipyridamole. J Pharmacol Exp Ther. 1979 Dec;211(3):626–631. [PubMed] [Google Scholar]
- Niedergerke R., Page S. Two physiological agents that appear to facilitate calcium discharge from the sarcoplasmic reticulum in frog heart cells: adrenalin an ATP. Proc R Soc Lond B Biol Sci. 1981 Nov 13;213(1192):325–344. doi: 10.1098/rspb.1981.0069. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Maguire M. H. Evidence for separate receptors for ATP and adenosine in the guinea-pig taenia coli. Eur J Pharmacol. 1982 Jul 30;81(4):669–672. doi: 10.1016/0014-2999(82)90358-2. [DOI] [PubMed] [Google Scholar]
- Satchell D. G. Nucleotide pyrophosphatase antagonizes responses to adenosine 5'-triphosphate and non-adrenergic, non-cholinergic inhibitory nerve stimulation in the guinea-pig isolated taenia coli. Br J Pharmacol. 1981 Oct;74(2):319–321. doi: 10.1111/j.1476-5381.1981.tb09973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M., Pinkas R., Raz A. Evidence for different purinergic receptors for ATP and ADP in rabbit kidney and heart. Eur J Pharmacol. 1981 Sep 11;74(2-3):167–173. doi: 10.1016/0014-2999(81)90527-6. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Differential blockade of ATP, noradrenaline and electrically evoked contractions of the rat vas deferens by nifedipine. Eur J Pharmacol. 1981 Sep 24;74(4):373–376. doi: 10.1016/0014-2999(81)90058-3. [DOI] [PubMed] [Google Scholar]
- Su C. Purinergic neurotransmission and neuromodulation. Annu Rev Pharmacol Toxicol. 1983;23:397–411. doi: 10.1146/annurev.pa.23.040183.002145. [DOI] [PubMed] [Google Scholar]
- Taylor D. A., Wiese S., Faison E. P., Yarbrough G. G. Pharmacological characterization of purinergic receptors in the rat vas deferens. J Pharmacol Exp Ther. 1983 Jan;224(1):40–45. [PubMed] [Google Scholar]
- Westfall D. P., Fedan J. S., Colby J., Hogaboom G. K., O'Donnell J. P. Evidence for a contribution by purines to the neurogenic response of the guinea-pig urinary bladder. Eur J Pharmacol. 1983 Mar 4;87(4):415–422. doi: 10.1016/0014-2999(83)90080-8. [DOI] [PubMed] [Google Scholar]
- Yee D., Armstrong V. W., Eckstein F. Mechanistic studies on deoxyribonucleic acid dependent ribonucleic acid polymerase from Escherichia coli using phosphorothioate analogues. 1. Initiation and pyrophosphate exchange reactions. Biochemistry. 1979 Sep 18;18(19):4116–4120. doi: 10.1021/bi00586a009. [DOI] [PubMed] [Google Scholar]


