Abstract
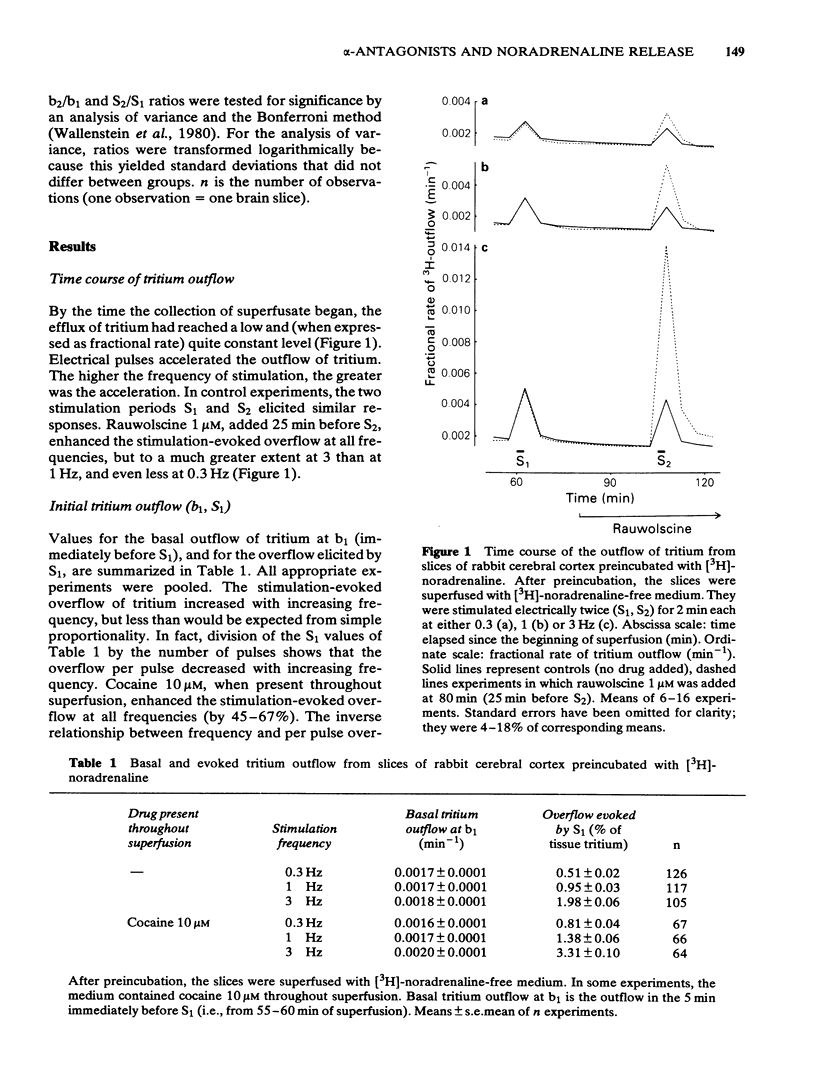
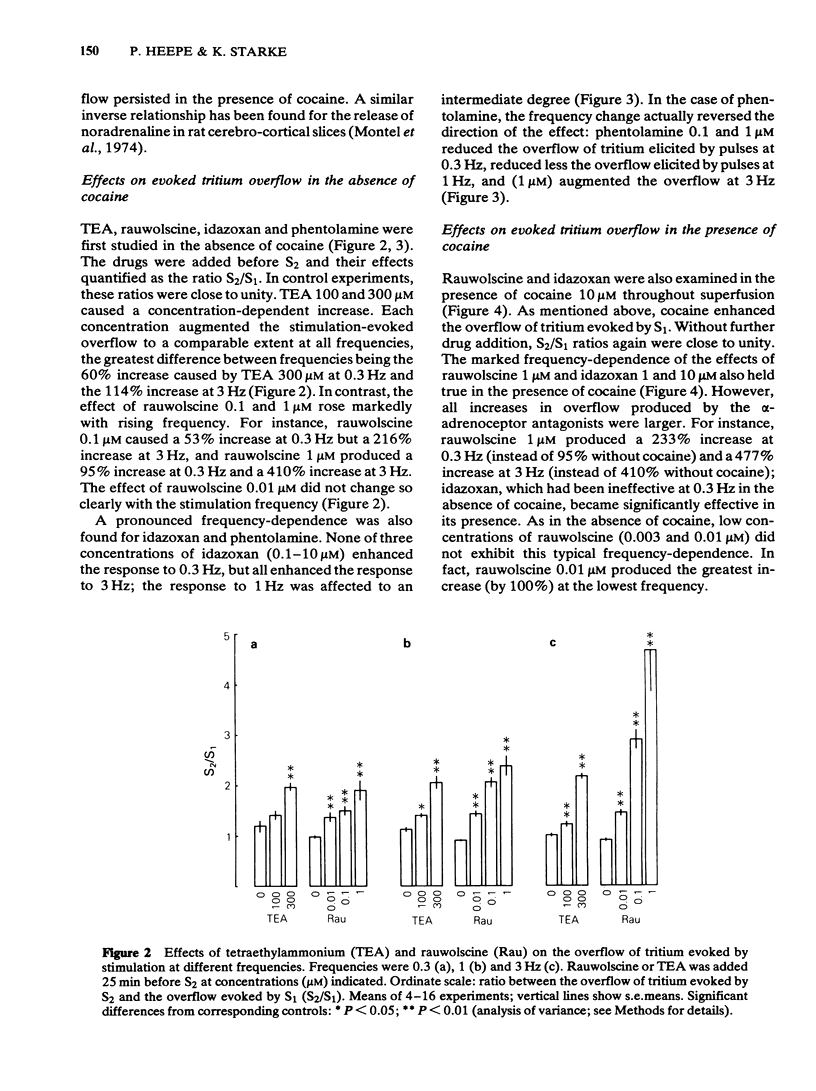
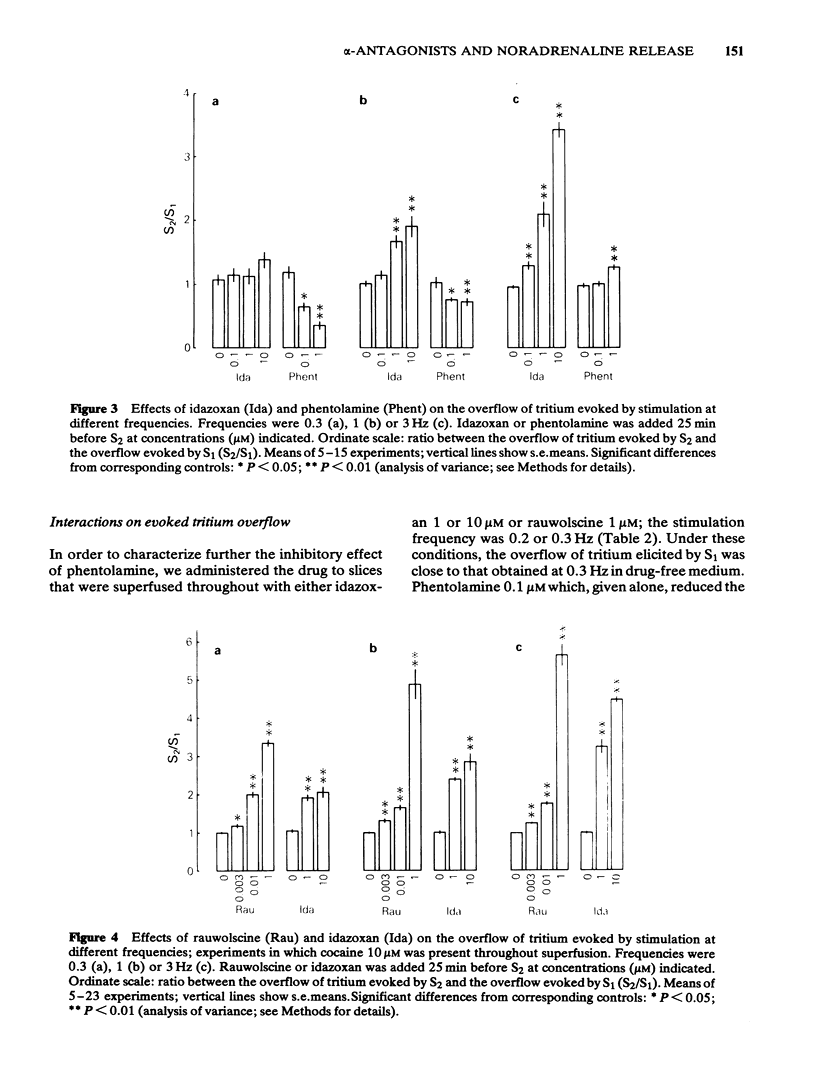
Slices of rabbit cerebral cortex were preincubated with [3H]-noradrenaline and then superfused and stimulated electrically twice for 2 min each (S1, S2) at various frequencies (0.2-3 Hz). The stimulation-evoked overflow of tritium (S1) increased with increasing frequency and was higher when cocaine (10 microM) was present. In the absence of cocaine, tetraethylammonium (TEA; 100 and 300 microM), added before S2, increased the stimulation-evoked overflow of tritium to about the same extent, irrespective of the frequency. In contrast, rauwolscine (0.1 and 1 microM) and idazoxan (0.1-10 microM) increased the evoked overflow much more, the higher the frequency of stimulation. Phentolamine (0.1 and 1 microM) reduced the overflow elicited at 0.3 and 1 Hz, and (1 microM) caused an increase only at 3 Hz. In slices superfused throughout with cocaine 10 microM, rauwolscine (1 microM) and idazoxan (1 and 10 microM) again increased the evoked overflow of tritium more, the higher the frequency of stimulation. For a given frequency, rauwolscine and idazoxan enhanced the evoked overflow to a greater extent in the presence than in the absence of cocaine. Idazoxan (1 and 10 microM) and rauwolscine (1 microM) counteracted the inhibition that phentolamine (0.1 microM) produced at low frequency. The increases caused by rauwolscine (1 microM) and TEA (300 microM) were approximately additive, but those caused by rauwolscine (1 microM) and idazoxan (10 microM) were not. The effects of rauwolscine, idazoxan and phentolamine depend on the experimental conditions (frequency, cocaine) in a manner compatible with the operation of a presynaptic alpha 2-adrenoceptor-mediated autoinhibition of noradrenaline release.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF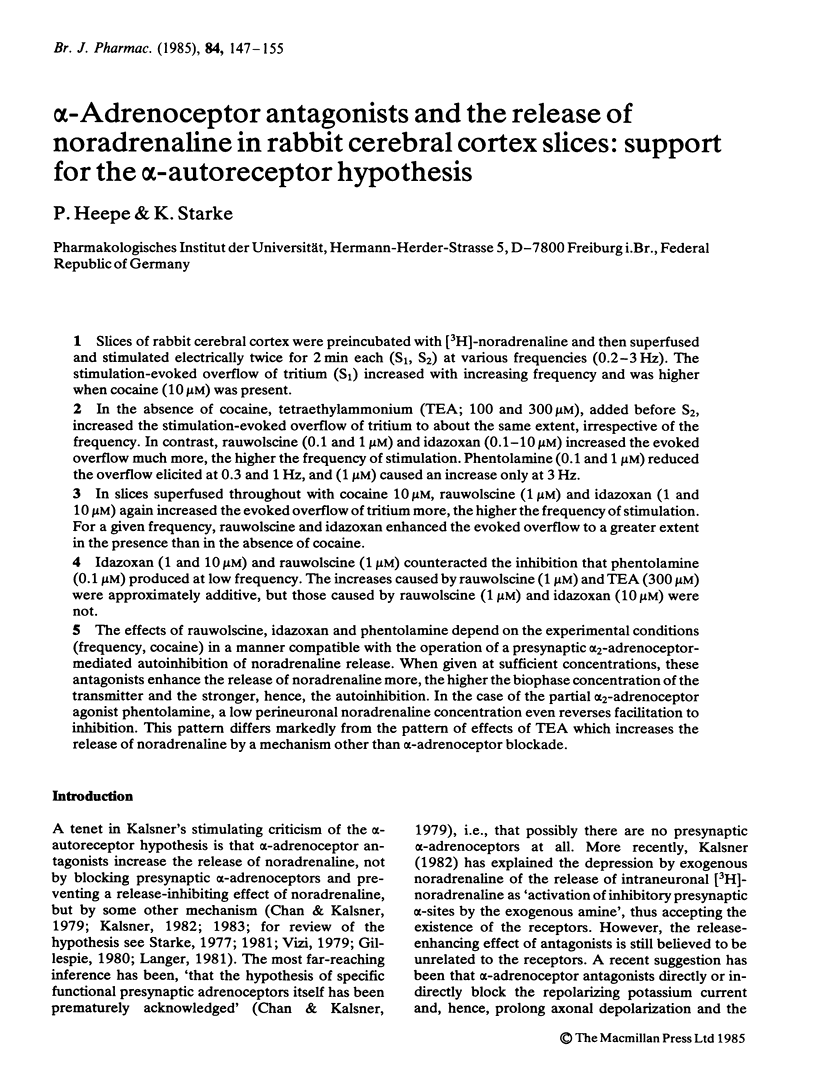




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Bobik A., Jackman G. P., Kopin I. J., Korner P. I. Role of auto-inhibitory feed-back in cardiac sympathetic transmission assessed by simultaneous measurements of changes in 3H-efflux and atrial rate in guinea-pig atrium. Br J Pharmacol. 1984 Jan;81(1):201–214. doi: 10.1111/j.1476-5381.1984.tb10762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Lew M. J. Phentolamine--an unexpected agonist in the rabbit. Br J Pharmacol. 1984 Mar;81(3):423–425. doi: 10.1111/j.1476-5381.1984.tb10094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch-Schwelk W., Starke K., Steppeler A. Experimental conditions required for the enhancement by alpha-adrenoceptor antagonists of noradrenaline release in the rabbit ear artery. Br J Pharmacol. 1983 Mar;78(3):543–551. doi: 10.1111/j.1476-5381.1983.tb08814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J., Drew G. M., Hilditch A. Presynaptic alpha-adrenoceptors: do exogenous and neuronally released noradrenaline act at different sites? Br J Pharmacol. 1984 Mar;81(3):457–464. doi: 10.1111/j.1476-5381.1984.tb10098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. A., Koella W. P. Feedback control of noradrenaline release as a function of noradrenaline concentration in the synaptic cleft in cortical slices of the rat. Brain Res. 1980 May 12;189(2):437–448. doi: 10.1016/0006-8993(80)90103-1. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Kalsner S. An examination of the negative feedback function of presynaptic adrenoceptors in a vascular tissue. Br J Pharmacol. 1979 Nov;67(3):401–407. doi: 10.1111/j.1476-5381.1979.tb08694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu L. X., Hoffmann I. S. Operational characteristics of the inhibitory feedback mechanism for regulation of dopamine release via presynaptic receptors. J Pharmacol Exp Ther. 1982 Nov;223(2):497–501. [PubMed] [Google Scholar]
- Farah M. B., Adler-Graschinsky E., Langer S. Z. Possible physiological significance of the initial step in the catabolism of noradrenaline in the central nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(2):119–131. doi: 10.1007/BF00499921. [DOI] [PubMed] [Google Scholar]
- Fuder H., Bath F., Wiebelt H., Muscholl E. Autoinhibition of noradrenaline release from the rat heart as a function of the biophase concentration. Effects of exogenous alpha-adrenoceptor agonists, cocaine, and perfusion rate. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jan;325(1):25–33. doi: 10.1007/BF00507050. [DOI] [PubMed] [Google Scholar]
- Ginesi L. M., Munday K. A., Noble A. R. Direct effect of lithium on active and inactive renin secretion. Br J Pharmacol. 1983 Jan;78(1):3–4. doi: 10.1111/j.1476-5381.1983.tb09354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. M., Knobloch L. C., Malick J. B. Electrophysiological demonstration of both alpha 2-agonist and antagonist properties of RX 781094. Eur J Pharmacol. 1983 Jul 15;91(1):101–105. doi: 10.1016/0014-2999(83)90368-0. [DOI] [PubMed] [Google Scholar]
- Hedler L., Starke K., Steppeler A. Release of [3H]-amezinium from cortical noradrenergic axons: a model for the study of the alpha-autoreceptor hypothesis. Br J Pharmacol. 1983 Apr;78(4):645–653. doi: 10.1111/j.1476-5381.1983.tb09415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S. Evidence against the unitary hypothesis of agonist and antagonist action at presynaptic adrenoceptors. Br J Pharmacol. 1982 Oct;77(2):375–380. doi: 10.1111/j.1476-5381.1982.tb09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S. Yohimbine and prolongation of stimulation pulse duration alter similarly 3H-transmitter efflux in heart: an alternative to the negative feedback hypothesis. Br J Pharmacol. 1983 Aug;79(4):985–992. doi: 10.1111/j.1476-5381.1983.tb10545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. The variation of acetylcholine release from myenteric neurones with stimulation frequency and train length. Role of presynaptic muscarine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1983 Sep;324(2):130–133. doi: 10.1007/BF00497018. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Limberger N., Starke K. Further study of prerequisites for the enhancement by alpha-adrenoceptor antagonists of the release of noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):240–246. doi: 10.1007/BF00495950. [DOI] [PubMed] [Google Scholar]
- Marshall I. Stimulation-evoked release of [3H]-noradrenaline by 1, 10 or 100 pulses and its modification through presynaptic alpha 2-adrenoceptors. Br J Pharmacol. 1983 Jan;78(1):221–231. doi: 10.1111/j.1476-5381.1983.tb09383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montel H., Starke K., Weber F. Influence of morphine and naloxone on the release of noradrenaline from rat brain cortex slices. Naunyn Schmiedebergs Arch Pharmacol. 1974;283(4):357–369. doi: 10.1007/BF00501109. [DOI] [PubMed] [Google Scholar]
- Reichenbacher D., Reimann W., Starke K. alpha-Adrenoceptor-mediated inhibition of noradrenaline release in rabbit brain cortex slices. Receptor properties and role of the biophase concentration of noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1982 Apr;319(1):71–77. doi: 10.1007/BF00491481. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H., Gayk W., Merker R. Comparison of the effects of clonidine on pre- and postsynaptic adrenoceptors in the rabbit pulmonary artery. Alpha-sympathomimetic inhibition of Neurogenic vasoconstriction. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(2):133–150. doi: 10.1007/BF00501149. [DOI] [PubMed] [Google Scholar]
- Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1981;21:7–30. doi: 10.1146/annurev.pa.21.040181.000255. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Facilitation and receptor-mediated regulation of noradrenaline secretion by control of recruitment of varicosities as well as by control of electro-secretory coupling. Neuroscience. 1978;3(12):1147–1155. doi: 10.1016/0306-4522(78)90135-5. [DOI] [PubMed] [Google Scholar]
- Taube H. D., Starke K., Borowski E. Presynaptic receptor systems on the noradrenergic neurones of rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1977 Sep;299(2):123–141. doi: 10.1007/BF00498554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- limberger N., Starke K. Partial agonist effect of 2-[2-(1,4-benzodioxanyl)]-2-imidazoline (RX 781 094) at presynaptic alpha 2-adrenoceptors in rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1983 Sep;324(1):75–78. doi: 10.1007/BF00647842. [DOI] [PubMed] [Google Scholar]


