Abstract
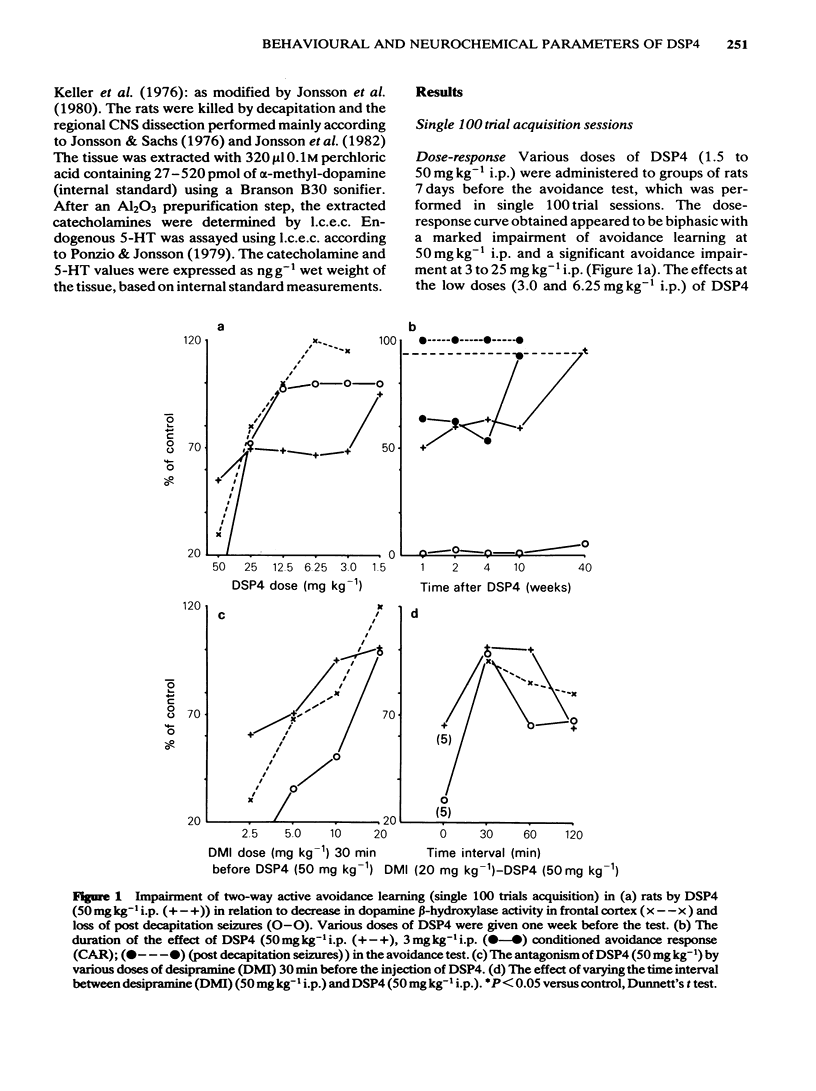
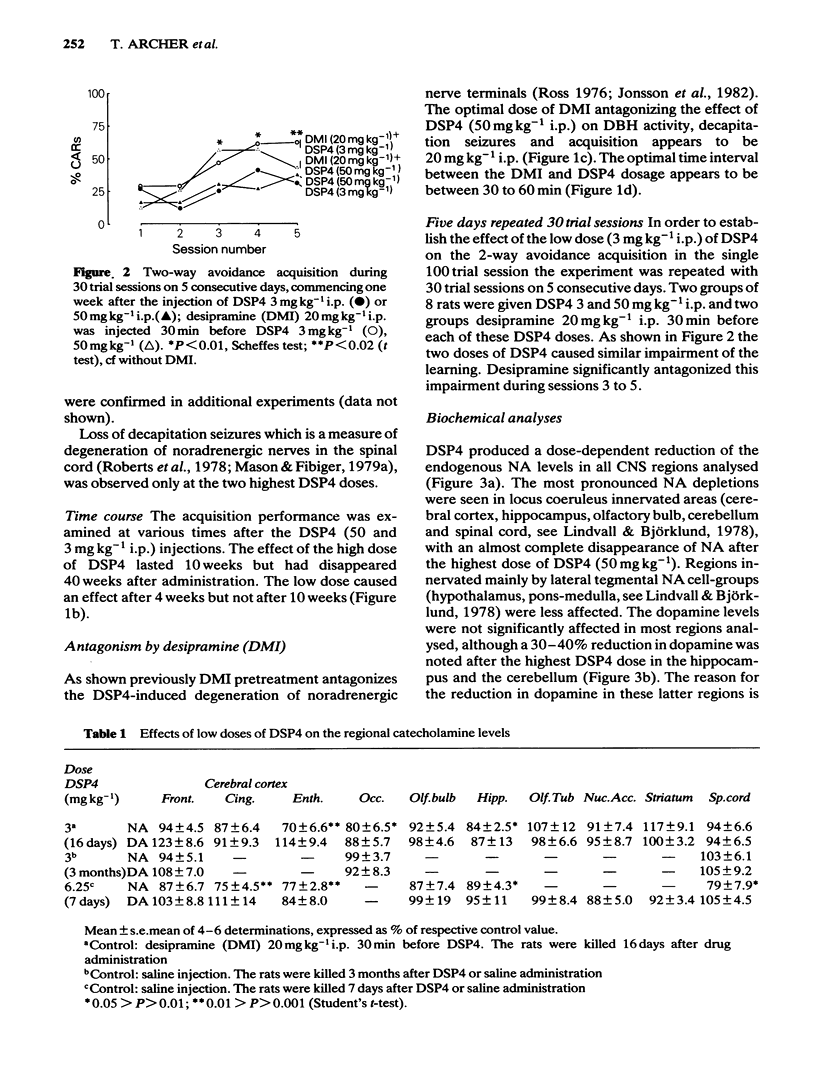
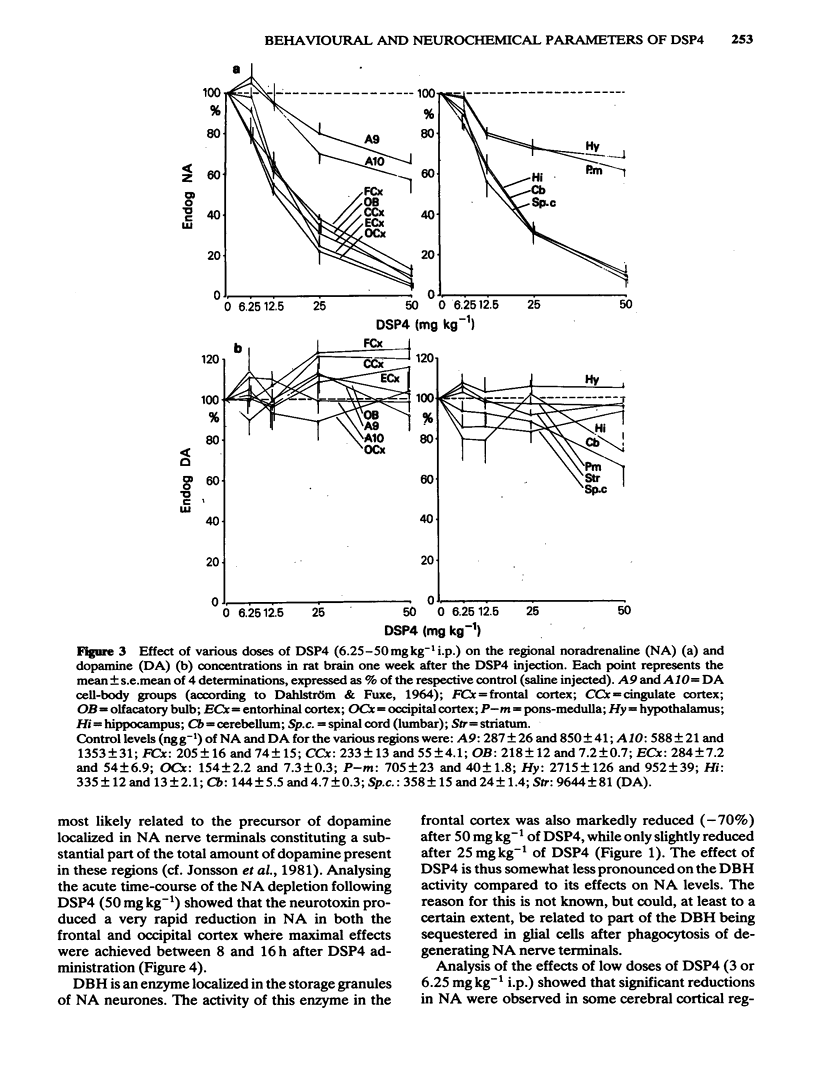
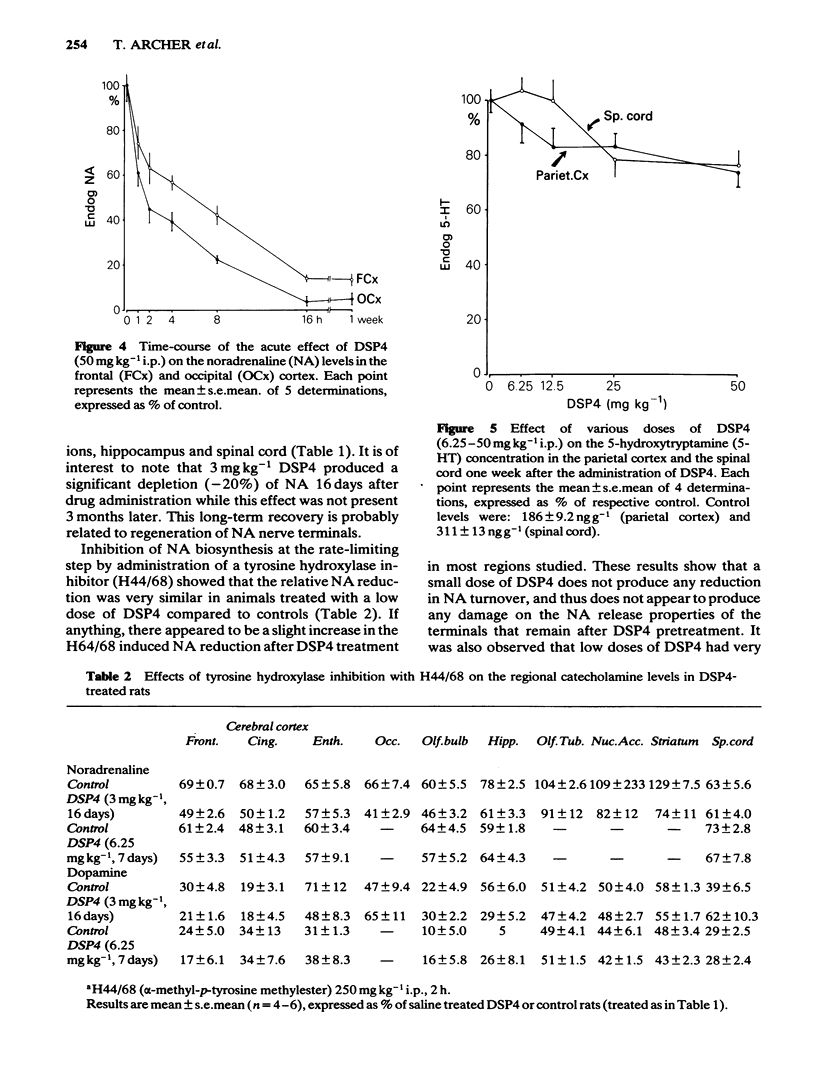
The effects of various doses of DSP4 on two-way active avoidance acquisition in rats and on central noradrenaline neurones were compared. Doses of DSP4 from 3 mg kg-1 i.p. and upwards injected one week before the onset of the avoidance trials significantly impaired two-way avoidance learning. The learning impairment caused by DSP4 (50 mg kg-1 i.p.) lasted for at least 10 weeks. Desipramine (20 mg kg-1) injected either 30 or 60 min before DSP4 (50 mg kg-1) antagonized the active avoidance impairment. A high dose of DSP4 (50 mg kg-1 i.p.) produced profound decreases in dopamine-beta-hydroxylase activity in the frontal cortex and in the concentrations of noradrenaline in various brain regions indicating degeneration of the locus coeruleus noradrenaline system. Low doses of DSP4 (3 and 6 mg kg-1 i.p.) produced small but significant decrease in the concentrations of noradrenaline (NA) in some regions, e.g. cerebral cortex, hippocampus, olfactory bulb and spinal cord. The avoidance impairment caused by the low dose of DSP4 (3 mg kg-1) was absent when rats were tested 10 weeks after treatment nor was NA depletion present when NA was analysed 3 months after treatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer T., Cotic T., Järbe T. U. Attenuation of the context effect and lack of unconditioned stimulus-preexposure effect in taste-aversion learning following treatment with DSP4, the selective noradrenaline neurotoxin. Behav Neural Biol. 1982 Jun;35(2):159–173. doi: 10.1016/s0163-1047(82)91173-6. [DOI] [PubMed] [Google Scholar]
- Archer T. DSP4 (N-2-chloroethyl-N-ethyl-2-bromobenzylamine), a new noradrenaline neurotoxin, and stimulus conditions affecting acquisition of two-way active avoidance. J Comp Physiol Psychol. 1982 Jun;96(3):476–490. doi: 10.1037/h0077896. [DOI] [PubMed] [Google Scholar]
- Archer T., Mohammed A. K., Ross S. B., Söderberg U. T-maze learning, spontaneous activity and food intake recovery following systemic administration of the noradrenaline neurotoxin, DSP4. Pharmacol Biochem Behav. 1983 Jul;19(1):121–130. doi: 10.1016/0091-3057(83)90320-9. [DOI] [PubMed] [Google Scholar]
- Archer T., Ogren S. O., Fuxe K., Agnati L. F., Eneroth P. On the interactive role of central noradrenaline neurons and corticosterone in two-way active avoidance acquisition in the rat. Neurosci Lett. 1981 Dec 23;27(3):341–346. doi: 10.1016/0304-3940(81)90454-7. [DOI] [PubMed] [Google Scholar]
- Archer T., Ogren S. O., Johansson G., Ross S. B. DSP4-induced two-way active avoidance impairment in rats: involvement of central and not peripheral noradrenaline depletion. Psychopharmacology (Berl) 1982;76(4):303–309. doi: 10.1007/BF00449115. [DOI] [PubMed] [Google Scholar]
- Archer T. The role of noradrenaline in learned behaviors: studies using DSP4. Scand J Psychol. 1982;Suppl 1:61–71. doi: 10.1111/j.1467-9450.1982.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Axelrod J. Dopamine- -hydroxylase in the rat brain: developmental characteristics. J Neurochem. 1972 Feb;19(2):449–459. doi: 10.1111/j.1471-4159.1972.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Crow T. J., Longden A., Smith A., Wendlandt S. Pontine tegmental lesions, monoamine neurons and varieties of learning. Behav Biol. 1977 Jun;20(2):184–196. doi: 10.1016/s0091-6773(77)90747-7. [DOI] [PubMed] [Google Scholar]
- Jaim-Etcheverry G., Zieher L. M. DSP-4: a novel compound with neurotoxic effects on noradrenergic neurons of adult and developing rats. Brain Res. 1980 Apr 28;188(2):513–523. doi: 10.1016/0006-8993(80)90049-9. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Hallman H., Ponzio F., Ross S. DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine)--a useful denervation tool for central and peripheral noradrenaline neurons. Eur J Pharmacol. 1981 Jun 19;72(2-3):173–188. doi: 10.1016/0014-2999(81)90272-7. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Hallman H., Sundström E. Effects of the noradrenaline neurotoxin DSP4 on the postnatal development of central noradrenaline neurons in the rat. Neuroscience. 1982;7(11):2895–2907. doi: 10.1016/0306-4522(82)90112-9. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Sachs C. Regional changes in [3H]-noradrenaline uptake, catecholamines and catecholamine synthetic and catabolic enzymes in rat brain following neonatal 6-hydroxydopamine treatment. Med Biol. 1976 Aug;54(4):286–297. [PubMed] [Google Scholar]
- Keller R., Oke A., Mefford I., Adams R. N. Liquid chromatographic analysis of catecholamines routine assay for regional brain mapping. Life Sci. 1976 Oct 1;19(7):995–1003. doi: 10.1016/0024-3205(76)90290-3. [DOI] [PubMed] [Google Scholar]
- Mason S. T., Fibiger H. C. Noradrenaline and avoidance learning in the rat. Brain Res. 1979 Feb 2;161(2):321–333. doi: 10.1016/0006-8993(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Mason S. T., Fibiger H. C. Physiological function of descending noradrenaline projections to the spinal cord: role in post-decapitation convulsions. Eur J Pharmacol. 1979 Jul 15;57(1):29–34. doi: 10.1016/0014-2999(79)90100-6. [DOI] [PubMed] [Google Scholar]
- Mobley P. L., Sulser F. Adrenal corticoids regulate sensitivity of noradrenaline receptor-coupled adenylate cyclase in brain. Nature. 1980 Aug 7;286(5773):608–609. doi: 10.1038/286608a0. [DOI] [PubMed] [Google Scholar]
- Mobley P. L., Sulser F. Adrenal steroids affect the norepinephrine-sensitive adenylate cyclase system in the rat limbic forebrain. Eur J Pharmacol. 1980 Jul 25;65(2-3):321–322. doi: 10.1016/0014-2999(80)90412-4. [DOI] [PubMed] [Google Scholar]
- Ogren S. O., Archer T., Ross S. B. Evidence for a role of the locus coeruleus noradrenaline system in learning. Neurosci Lett. 1980 Dec;20(3):351–356. doi: 10.1016/0304-3940(80)90173-1. [DOI] [PubMed] [Google Scholar]
- Roberts D. C., Mason S. T., Fibiger H. C. Selective depletion of spinal noradrenaline abolishes post-decapitation convulsions. Life Sci. 1978 Dec 11;23(24):2411–2413. doi: 10.1016/0024-3205(78)90299-0. [DOI] [PubMed] [Google Scholar]
- Ross S. B. Long-term effects of N-2-chlorethyl-N-ethyl-2-bromobenzylamine hydrochloride on noradrenergic neurones in the rat brain and heart. Br J Pharmacol. 1976 Dec;58(4):521–527. doi: 10.1111/j.1476-5381.1976.tb08619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. B., Renyl A. L. On the long-lasting inhibitory effect of N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP 4) on the active uptake of noradrenaline. J Pharm Pharmacol. 1976 May;28(5):458–459. doi: 10.1111/j.2042-7158.1976.tb04659.x. [DOI] [PubMed] [Google Scholar]
- Spyraki C., Arbuthnott G. W., Fibiger H. C. The effect of DSP-4 on some positively reinforced operant behaviors in the rat. Pharmacol Biochem Behav. 1982 Feb;16(2):197–202. doi: 10.1016/0091-3057(82)90147-2. [DOI] [PubMed] [Google Scholar]


