SUMMARY
Extensive evidence suggests that 5-HT2 receptors may play a role in mental disorders including schizophrenia. In addition, several studies indicate that Gq-coupled 5-HT2A receptors are likely targets for the initiation of events leading to the hallucinogenic behavior elicited by lysergic acid diethylamide (LSD), (±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), and related drugs. However, 5-HT2A receptors couple to other G proteins in addition to Gq protein. To evaluate the role of the Gq signaling pathway in DOI-induced behaviors, we utilized two behavioral models of 5-HT2A receptor activation: induction of head-twitches by DOI, a common response to hallucinogenic drugs in rodents, and DOI elicited anxiolytic-like effects in the elevated plus maze. Experimental subjects were genetically modified mice [Gαq(−/−)] in which the Gq alpha gene was eliminated. Gαq(−/−) mice exhibited a decrease in DOI-induced head-twitches, when compared to wild-type littermates. In addition, the DOI-induced increase in anxiolytic-like behavior was abolished in Gαq(−/−) mice. These results, combined with our finding that DOI-induced FOS expression in the medial prefrontal cortex was also eliminated in Gαq(−/−) mice, suggests a key role for Gq protein in hallucinogenic drug effects.
Keywords: Hallucinogens, 5-HT2A receptors, Gq protein, Elevated plus maze, Head-twitch response, c-fos
INTRODUCTION
G-proteins transduce signals from a wide array of G-protein coupled receptors (GPCRs) initiating a plethora of second messenger cascades. Heterotrimeric G-proteins, consisting of three subunits, Gα, Gβ, Gγ, act as molecular switches between their active and inactive states in response to guanine nucleotides. The Gq/11 subfamily of G-proteins mediates the activation of phospholipase Cβ (PLCβ), which in turn catalyzes the hydrolysis of phosphatidylinositol-4,5-biphosphate (PIP2) into inositol triphosphate and diacylglycerol. Inositol triphosphate promotes intracellular calcium mobilization, while diacylglycerol stimulates the activity of protein kinase C (Exton, 1996).
5-Hydroxytryptamine2A (5-HT)2A receptors are a subtype of the 5-HT2 subfamily of receptors, known to stimulate the PLC pathway via Gαq (Chang et al., 2000). 5HT2A receptors are widely distributed in brain, including cortex, caudate nucleus, olfactory tubercle, nucleus accumbens, and hippocampus. They are believed to be involved in many peripheral, as well as, central nervous system functions such as smooth muscle contraction, platelet aggregation, cognition and mood, and in psychiatric disorders such as depression and schizophrenia (for review, see Barnes and Sharp, 1999). Furthermore, there is now vast evidence from biochemical, electrophysiological, and behavioral studies that hallucinogens, such as lysergic acid diethylamide (LSD), have a key site of action as agonists at 5-HT2A receptors in the brain (for review, see Aghajanian and Marek, 1999; Marek and Aghajanian, 1996; Nichols, 2004). Cortical pyramidal neurons constitute the major 5-HT2A receptor-expressing neurons, where the receptors are located presynaptically on glutamatergic nerve terminals as well as postsynaptically on pyramidal neurons. It has been suggested that the “positive” hallucination-like symptoms observed in acute schizophrenia may be related to a dysfunctional 5-HT2A receptor signaling system in apical dendrites of pyramidal cells (Jakab and Goldman-Rakic, 1998). In addition, clozapine, the classical atypical antipsychotic drug, is a 5-HT2A receptor antagonist (Meltzer et al., 1989). These data suggest that 5-HT2A receptors are important mediators of the behavioral effects induced by hallucinogenic drugs and behavior abnormalities in psychiatric disorders.
The hallucinogen (±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), a highly selective 5-HT2 agonist (McClue et al., 1989), has been utilized to evaluate the role of 5-HT2A receptors in many biochemical and behavioral responses in rodents (Darmani et al., 1990; Leslie et al., 1993; Mazzola-Pomietto et al., 1995). Acute administration of DOI elicits a 5-HT2A receptor-dependent induction of the immediate early gene c-fos and its protein FOS in the rat cortex (Leslie et al., 1993; Scruggs et al., 2000; Tilakaratne and Friedman, 1996). In mouse behavioral assays, DOI has been shown to exhibit anxiolytic-like properties in anxiety paradigms such as the four plates test and the elevated plus maze (Nic Dhonnchadha et al., 2003; Onaivi et al., 1995). Moreover, activation of 5-HT2A receptors by DOI evokes a head-twitch response in both rats and mice (Dursun and Handley, 1996; Kleven et al., 1997; Schreiber et al., 1995).
The precise signal transduction pathway involved in the in vivo effects of hallucinogenic drugs is unknown. The objective of the present study is to evaluate the role of Gq proteins in hallucinogen-induced in vivo effects. As a tool to assess the role of the Gq signaling pathway, we have made use of genetically modified mice in which the gene encoding for the α-subunit of Gq protein has been eliminated [Gαq(−/−)].
METHODS
Animals
Gαq(−/−) mice were generated by mating heterozygous [Gαq(+/−)] males and heterozygous females to obtain wild-type and knock-out littermates. Mice were kept on a C57BL/6x129/Sv background, and their genotype was determined by PCR of genomic DNA from tail samples as previously described (Offermanns et al., 1997). Although Gαq(−/−) mice exhibit signs of ataxia and motor incoordination, their peripheral and central nervous system morphology is largely undisturbed (Offermanns et al., 1997). Animals had free access to food and water, and were maintained on a 12:12 light/dark cycle. An equal number of male and female mice were used for experimental studies; the mice were 6–8 weeks of age at the time of testing. C57BL/6 mice (Harlan. Indianapolis, IN) were utilized for control experiments evaluating the role of 5-HT2A receptors in the in vivo effects of DOI. All experiments were done in compliance with the guide Principles of Laboratory Animal Care (NIH publication No. 85-23) and the Vanderbilt University Animal Care and Use Committee.
FOS Immunohistochemistry
Mice were anesthetized with 150 mg/kg pentobarbital i.p. before transcardiac perfusion with 30 ml of 0.1 M phosphate-buffered saline (PBS) followed by 30 ml of 4% paraformaldehyde in 0.1M PBS. Brains were removed immediately, post fixed in paraformaldehyde overnight at 4°C, and then transferred to increasing concentrations of sucrose. Coronal sections were cut at 40μm thickness on a vibratome and collected into buffer containing 30% ethylene glycol, 30% glycerol, 10% 0.1M PBS, and 30% water. Immunohistochemistry was performed on free floating sections. Sections were preincubated for 30 min in 0.3% hydrogen peroxide, washed 3 times with PBS, and then incubated for one hour in 5% goat serum/2% Triton X-100 to block non-specific binding. Sections were incubated for 48h at 4°C in anti-FOS primary antibody (Oncogene Research Products) diluted in blocking solution 1:30,000. Following 3 washes in PBS, a Vectastain Elite ABC horseradish peroxidase kit (Vector Labs) was used for the secondary antibody and avidin–biotin complex steps. The colorimetric detection reaction produced 3-3′-diaminobenzidine tetrahydrochloride (DAB) as a brown chromagen product. Sections were then washed, mounted on slides, dehydrated through a series of solutions with increasing ethanol concentrations, treated with xylenes, and coverslipped with Mounting Medium (Richard-Allan Scientific. Kalamazoo, MI)
Analysis of FOS-Li positive nuclei
Brain sections containing medial prefrontal cortex were analyzed as described previously (Gresch et al., 2002). Briefly, bright field images were taken using Openlab 2.2.5 software (Improvision. Lexington, MA) with a Coolsnap cf camera (Photometrics. Tucson, AZ) mounted on a Zeiss Axioverts S100 microscope. All settings were kept constant throughout the image collection process. Analysis and quantification of images were performed using Image J 1.33u (Wayne Rasband, NIH) in mPFC sections that correspond to AP +1.34mm, ML +0.3mm, and DV −2.25mm relative to bregma (Franklin and Paxinos, 1997). An image of 600μm × 450 μm area was analyzed for the number of FOS-Li positive nuclei. Cells with brown black nuclei were considered positive FOS-Li. These were determined by using the particle count macro in Image J 1.33u where the pixel density threshold had been set four times above background levels.
Radioligand binding
Frontal cortex was dissected and homogenized in binding buffer (50mM Tris and 10mM MgCl2, pH 7.4). The homogenate was centrifuged at 20,000g for 20 min at 4°C, and the pellet was resuspended in binding buffer. Protein concentration was determined with Bio-Rad protein assay dye reagent (Hercules, CA). Membrane preparations (200μg/sample) were incubated with [3H]-ketanserin (10nM) for 30 min at 37°C. Nonspecific binding was determined with 10μM methysergide. Following incubation, free radioligand was separated from bound by vacuum filtration through Whatman GF/C glass filters (Brandel, Gaithersburg, MD). Filters were placed in vials and counted in a liquid scintillation counter.
Elevated Plus Maze
The elevated plus maze (Hamilton-Kinder. San Diego, CA) consisted of two open arms (37.5 × 5.0 × 0.25 cm) and two closed arms (37.5 × 5.0 × 15 cm) emanating from a central platform (5cm × 5cm) to form a plus shape. The maze was built from black Plexiglas, and equipped with infrared photobeams. The entire maze was elevated 45 cm above the floor. Light beam breaks were recorded and analyzed automatically by Motor Monitor software (Hamilton-Kinder).
Animals were transported to the experiment room, and following a habituation period of 15 min, they were injected i.p. with drug, and placed back into their home cages for 30 min after DOI or 5 min after ethanol. Animals were individually placed into the central platform of the plus maze and allowed 5 min of free exploration. Time and percent time spent in the open arms were used as an index of anxiolytic-like effects, and total distance traveled was used as a measure of general activity. After each session, the plus maze was cleaned with 30% ethanol and allowed to completely air dry prior to placing the next animal for testing.
Head-twitch Response
The head-twitch response is a distinctive behavior characterized by a rapid, rotational movement of the head, ears, and neck. Mice were injected i.p and immediately transferred to a 3000 mL glass beaker lined with pinedust bedding for observation. Head-twitches were counted, in consecutive 5 min bins, for 30 minutes following drug administration by two observers (one of them blind to the treatment) with over 95% inter-rater reliability.
Drugs
Drugs were administered i.p. in an injection volume of 10 ml/kg. All drugs were diluted in 0.9% saline solution. Ethanol concentration, 15% (w/v), was obtained by diluting absolute ethanol (AAPER. Shelbyville, KY) with saline. DOI was purchased from Sigma-Aldrich, and MDL 100907 was a gift from Marion Merrell Dow (Cincinnati, Ohio).
Statistical Analyses
All data are presented as mean ± S.E.M. The effects of DOI and ethanol in the elevated plus maze, and the effects of DOI in the head-twitch response test were compared by two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests. The effects of MDL100907 were analyzed by a one-way ANOVA followed by Tukey’s multiple comparison tests.
RESULTS
Role of 5-HT2A receptors in DOI-induced behaviors
Consistent with earlier reports (Nic Dhonnchadha et al., 2003), DOI significantly increased open arm activity in the elevated plus maze. As illustrated in fig 1A, pretreatment with the highly selective 5-HT2A receptor antagonist MDL 100907 abolished the anxiolytic-like effects of DOI on elevated plus maze behavior (i.e., MDL-DOI does not differ from Sal-Sal). In an additional experiment, we showed that MDL-DOI does not differ from MDL-Sal, confirming that the effects are not mediated by some independent effect of MDL100907. As illustrated in fig 1B, DOI induced a robust head-twitch response in mice, which was also completely blocked by pretreatment with the selective 5-HT2A receptor antagonist MDL100907.
Fig. 1.
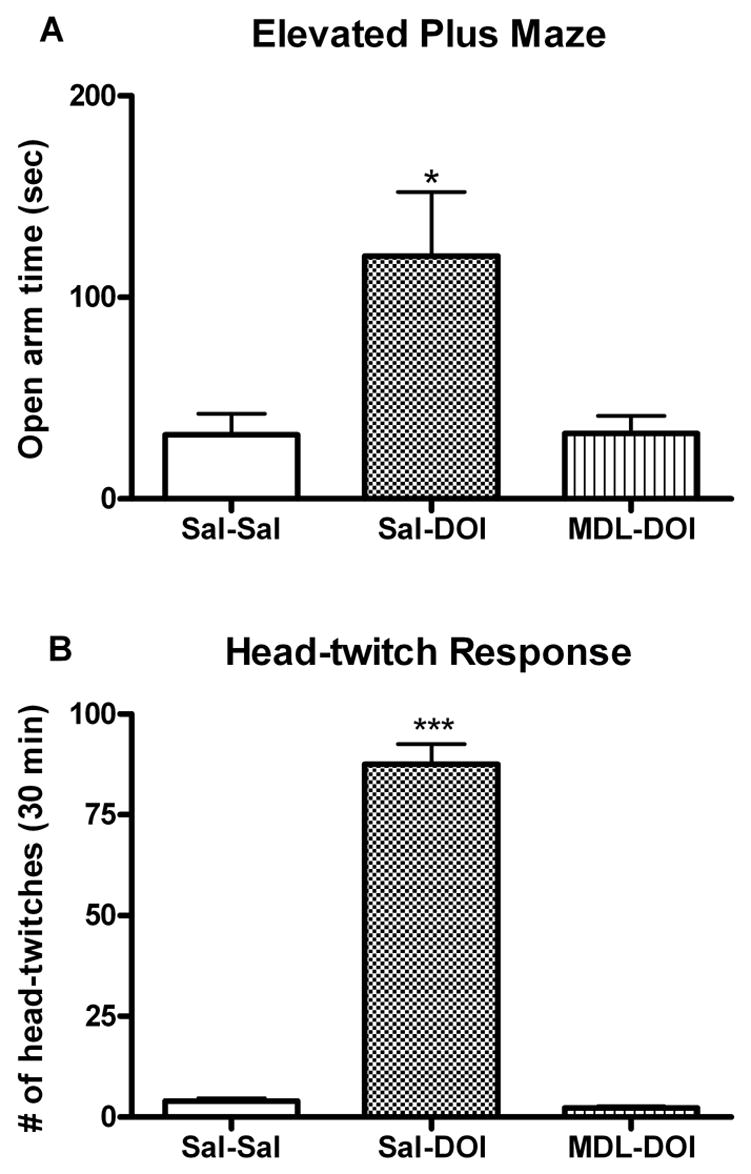
Effect of pretreatment with MDL100907 on DOI-induced behaviors. A Anxiolytic-like effects of DOI (2.5 mg/Kg, i.p. 30 min pre-test) are prevented by pretreatment with the 5-HT2A receptor antagonist MDL100907 (0.25 mg/Kg, i.p. 50 min pre-test) B DOI-induced head-twitches counted during a 30 min observation period were inhibited by pretreatment with MDL100907 (0.25 mg/Kg, i.p. 20 min pre-test). Data shown as means ± S.E.M., n= 6 per group. (*)p<0.05, (***)p<0.001 relative to saline control group determined by a one-way ANOVA.
Characterization of DOI-induced FOS expression in mice
The induction of FOS by DOI is a reliable marker for 5-HT2A receptor activation. The effect of DOI on FOS expression has not been previously evaluated in C57BL/6 mice. We therefore performed control pharmacological experiments to establish the potency of DOI to induce FOS expression in the medial prefrontal cortex (mPFC). DOI (0, 0.3, 3, 10 mg/kg, i.p. 3 hours pre-test) elicits a dose-dependent induction of FOS-Li nuclei in the mPFC (fig. 2A) with an EC50 value of 5.6 mg/kg. Pretreatment with the 5-HT2A receptor antagonist MDL 100907 blocked the ability of DOI (5mg/kg) to induce FOS-Li expression in the mPFC (fig. 2B). In an additional experiment, we found that treatment of mice with MDL 100907 alone had no effect on FOS-like positive cells in the mPFC (15.4±5 for Sal-Sal vs 21.0±2 for MDL-Sal; n=6).
Fig. 2.
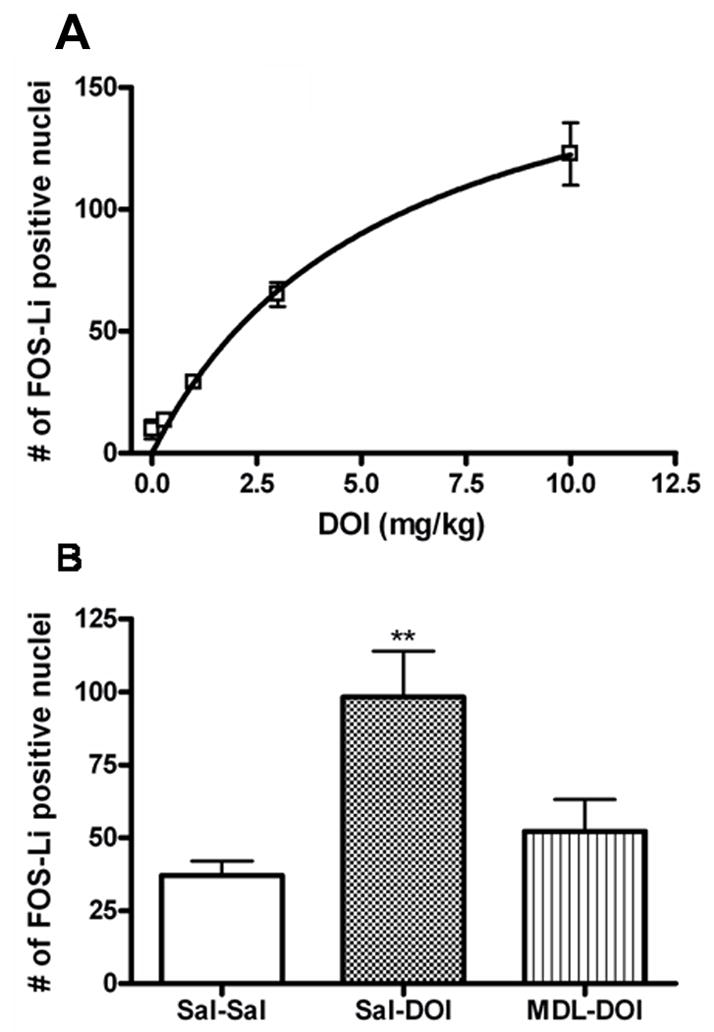
Quantitative analysis of FOS-Li positive nuclei in the mPFC of C57BL/6 mice. A Dose response of DOI-induced FOS expression in the mPFC, n=3. B DOI (2.5 mg/Kg, i.p.) induced FOS-Li expression is inhibited by pretreatment with MDL100907 (0.25mg/kg, i.p. 20 min pre-DOI). Data shown as means ± S.E.M., n= 6. (**) p<0.01 relative to saline (Sal-Sal) determined by a one-way ANOVA.
Gαq is required for the anxiolytic-like effect of DOI in the elevated plus maze
The anxiolytic-like effect of DOI was compared in the elevated plus maze using wild-type and Gαq(−/−) mice. Although DOI elicited an increase in both time spent in the open arms (p<0.01) (fig. 3A) and percent open arm time (p<0.01) (fig. 3B) in wild-type littermates, this effect of DOI was abolished in mice deficient for Gαq. DOI treatment did not alter percent open arm entries in either genotype. As a positive control, ethanol (1.5 g/kg, i.p. 5 min pre-test) was shown to significantly increase the time spent in the open arms (fig. 3A), as well as percent open arm time (fig. 3B), in both wild-type and Gαq knockout mice. These effects were independent of activity changes; there was no difference in total distance traveled between Gαq(−/−) and wild-type littermates following any of the treatment conditions (fig. 3C).
Fig. 3.
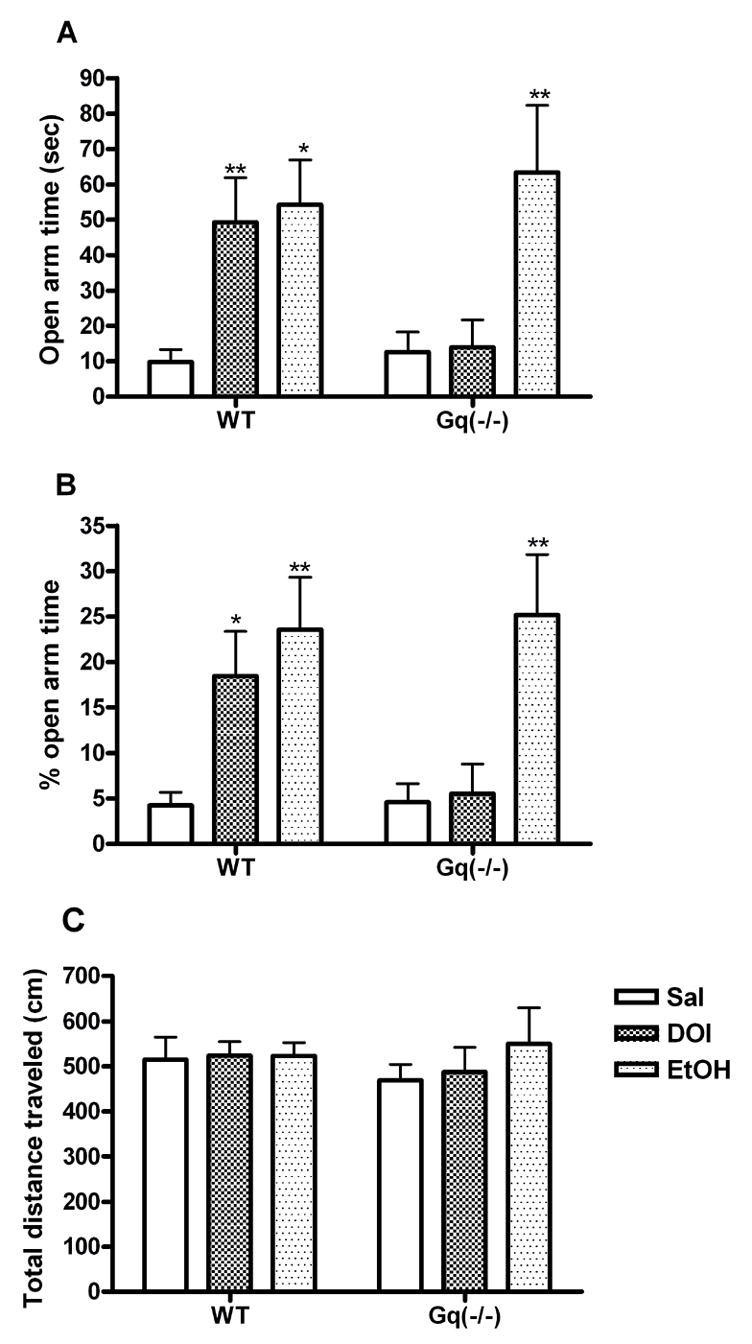
Anxiolytic-like activity of DOI on the elevated plus maze is absent in mice deficient for Gαq. A Time spent in the open arms, and B % time spent in the open arms, as a percentage of time spent in the open and close arms (center excluded) during the 5 min test session following administration of DOI (2.5 mg/kg, i.p. 30 min pre-test) or ethanol (1.5 g/kg, i.p. 5 min pre-test). C Exploratory activity measured in total distance traveled during the elevated plus maze test. Data shown as means ± S.E.M., n= 7–12 per group. (*) p<0.05, (**) p<0.01 relative to saline control group determined by a two-way ANOVA.
As an additional control, we compared [3H]-ketanserin binding in wild-type and Gαq(−/−) mice to determine whether altered 5-HT2A receptor expression could explain the behavioral difference. There was no difference in binding of a maximum concentration of [3H]-ketanserin (10nM) between Gαq(−/−) and wild-type littermates (500±40 fmole/mg protein for Gαq(−/−) vs 486±64 fmole/mg protein for WT, n=6; p>0.05)
DOI-induced head-twitch response is decreased in Gαq knockouts
Intraperitoneal administration of DOI elicited a dose-dependent head-twitch response in wild-type mice which peaked at 10 minutes (fig. 4A). This response was significantly blunted in mice deficient for Gαq (p<0.05 for 1.0 mg/kg DOI. p<0.001 for 2.5 mg/kg DOI) (fig. 4B). The percent decrease was comparable for the two doses of DOI (40% for 1mg/kg vs 35% for 2.5 mg/kg). Thus the head-twitch response appears to be less sensitive to a loss of Gαq than is the elevated plus maze. This conclusion is supported in heterozygous Gαq(+/−) mice, in which Gαq protein is reduced by 50% (Offermanns et al., 1997). The DOI-induced head-twitch response was reproduced in Gαq(+/−) mice; however, DOI effects in the elevated plus maze were completely eliminated in Gαq(+/−) knockouts (data not shown). In an additional experiment, we determined that MDL 100907 blocks the DOI-induced head-twitch response in Gαq(−/−) mice (44±14 head-twitches for Sal-DOI vs 4±1.3 head-twitches for MDL-DOI; n=6), confirming that DOI is acting through 5-HT2A receptors to elicit head twitch behavior in Gq(−/−) mice.
Fig. 4.
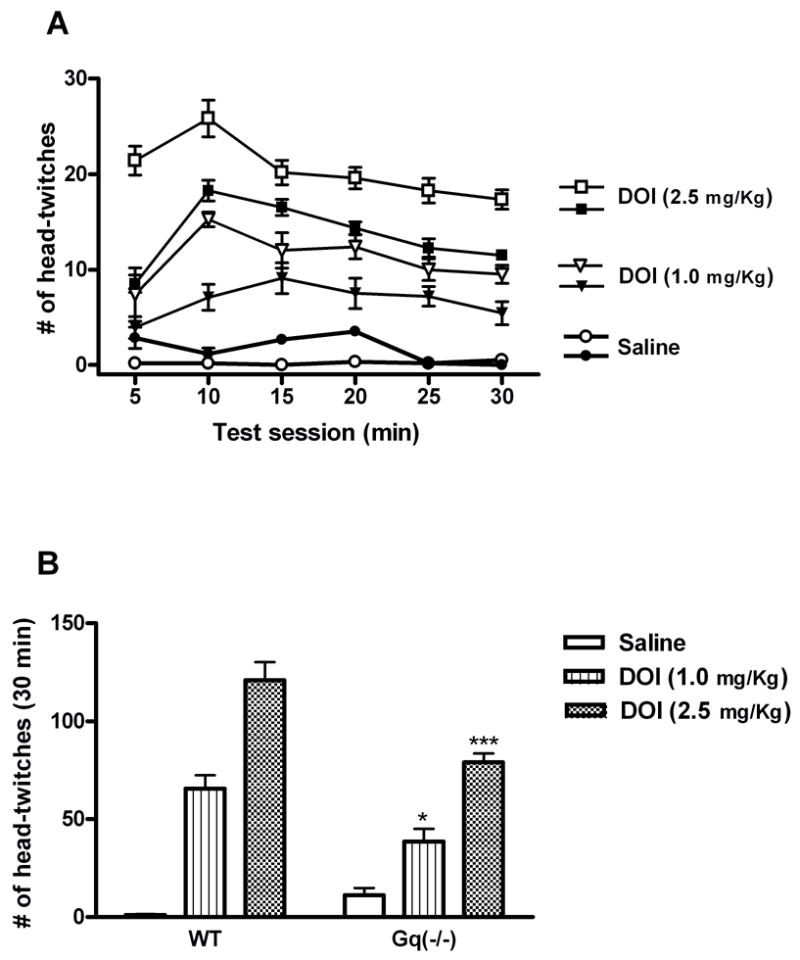
DOI-induced head-twitch response is reduced in Gαq knockouts. Mice were injected with DOI (1.0 mg/Kg or 2.5 mg/Kg, i.p.) prior to a 30 min observation period. A Number of head twitches in 5 min bins (open symbols represent WT mice, closed symbols represent Gαq(−/−) littermates). Main effect of genotype, p<0.0001. B Each column represents the total number of head-twitches counted during 30-min test time. Data shown as means ± S.E.M., n= 6–7 per group. (*) p<0.05, (***) p<0.001 relative to respective wild-type control groups determined by a two-way ANOVA..
Cortical FOS expression is reduced in Gαq(−/−) mice
Based on the previous dose response data, an EC50 dose of DOI (5 mg/kg) was utilized. DOI markedly increased the number of FOS-Li positive nuclei in the medial prefrontal cortex of wild-type mice (p<0.001). This increase was abolished in Gαq(−/−) mice (fig. 5).
Fig. 5.
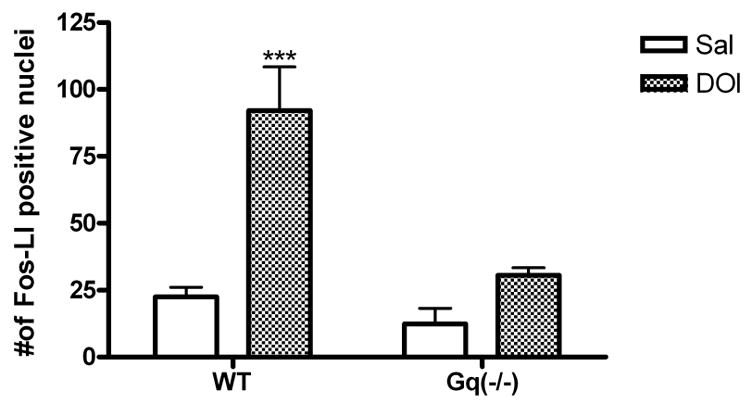
Quantitative analysis of number of FOS-Li positive nuclei in the medial prefrontal cortex of wild-type and Gαq(−/−) mice. Values are numbers of FOS-Li positive cells (mean ± S.E.M. within area of analysis; n=6). DOI-induced FOS expression in the medial prefrontal cortex is abolished in Gαq(−/−) mice. (***) p<0.001 relative to saline control group determined by a two-way ANOVA.
DISCUSSION
5-HT2A receptors are known to be a key site of action for hallucinogenic drug action in both humans and laboratory animals. There is abundant evidence from biochemical, electrophysiological, and behavioral studies in rats that hallucinogens, including LSD and DOI, are potent partial agonists at 5-HT2A receptors in the central nervous system (Aghajanian and Marek, 1999; Sanders-Bush et al., 1988). Furthermore, correlations between human hallucinogenic potency and 5-HT2A receptor binding affinity are consistent with the hypothesis that classical hallucinogens exert their hallucinogenic effects through 5-HT2A receptors (Glennon et al., 1984). This conclusion was confirmed recently in genetically modified mice lacking the 5-HT2A receptor (Gonzalez-Maeso et al., 2007). In addition, our studies with DOI in mice suggest a major role for the 5-HT2A receptor in the elevated plus maze and head-twitch response (present study), as well as in drug discrimination (Smith et al., 2003).
5-HT2A receptors are known to couple to multiple G-proteins including Gq, G11, and G13 to mediate a wide array of second messenger signaling pathways (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003; Robertson et al., 2003); however, the classical pathway associated with 5-HT2A receptor signaling is stimulation of PLCβ;via Gαq protein. In the present study, we evaluated the role of the Gαq protein in several behavioral and biochemical assays, including the elevated plus maze, head-twitch response, 5-HT2A receptor binding, and c-fos immunohistochemistry.
The current studies demonstrate that activation of the Gq signaling pathway is required for the mediation of the anxiolytic-like effects of DOI in the elevated plus maze. DOI significantly increased time spent in the open arms, as well as percent open arm time in wild-type animals; however, these effects were absent in Gαq knockout mice. Baseline performance on the maze and total exploratory activity did not differ between Gαq knockout mice and wild-type controls suggesting that deletion of the α subunit of Gq does not produce alterations in overall anxiety-related behavior. As an additional, positive control to determine whether the absence of DOI effects in Gαq knockouts was due to non-specific, ectopic effects or developmental abnormalities affecting anxiety-related behavior, we evaluated ethanol, known to exert its anxiolytic-like effects through the ionotropic GABA-A receptor system (Durcan and Lister, 1988; Prunell et al., 1994). The ability of ethanol treatment to significantly increase open arm activity in the elevated plus maze for wild-type mice was reproduced in Gαq knockouts, suggesting that the behavioral deficits in Gαq(−/−) mice are specifically related to the loss of the α-subunit of the Gq protein. Additional experiments using conditional knockouts or knockins are needed to entirely rule out developmental issues.
As with the elevated plus maze, the DOI-induced head-twitch response was blunted in Gαq(−/−) mice; however, unlike the elevated plus maze results, DOI still produced a significant head-twitch response in the knockout mice. Thus it appears that the Gq signaling pathway is not the sole mediator of the 5-HT2A receptor dependent behavioral effects of DOI. Gq and G11 proteins are close structural and functional analogs that substitute for each other in the intracellular signaling cascade. Although these two proteins coexist, Gq expression exceeds G11 throughout the brain (Milligan, 1993), including cortical and midbrain areas that mediate these behaviors. Compensatory changes, for instance, in G11 or in other components of the signaling complex, such as RGS proteins, may differ within the relevant brain sites, leading to the differential sensitivity of the two behaviors. Alternatively, some other signaling pathway, e.g., G13 activation of PLD (McGrew et al., 2002; Kurrasch-Orbaugh et al., 2003) or Gi/o activation of the erg signaling pathway (Gonzales Maeso et al., 2007), may contribute to the head-twitch behavior. How this differential behavioral sensitivity in Gq null mice relates to human hallucinogenic drug experience is not known. Recent studies of psilocybin in humans suggest that anxiety is a significant symptom (Griffiths et al., 2006), although presumably unrelated to the unique psychedelic experience. The head-twitch response is a stereotypical behavior that is a well accepted behavioral model of 5-HT2A receptor activation in rodents (Dursun and Handley, 1996; Kleven et al., 1997; Schreiber et al., 1995). Although head-twitch behavior is difficult to relate to the human hallucinogenic experience, recent evidence that the head-twitch behavior is unique to 5-HT2A receptor agonists with hallucinogenic properties (Gonzalez-Maseo et al., 2007) suggests it is a useful model. The introceptive cues, responsible for drug discrimination in rodents, are believed to model the subjective effects of drugs in humans. This is based largely on extensive studies showing that discriminative stimuli of psychoactive drugs in rats closely parallel the subjective effects reported by humans (Altman et al., 1976; Barry, 1974). Additional behavioral studies characterizing the hallucinogen-induced drug discriminative cue in Gαq(−/−) mice are planned.
The current studies also demonstrate that Gαq is required for DOI-induced FOS expression. Following DOI administration, wild-type mice exhibited a robust increase in the number of FOS-Li positive nuclei in the mPFC; however this increase was abolished in Gαq(−/−) littermates. Since the DOI-induced FOS expression is mediated by activation of 5-HT2A receptors, and 5-HT2A receptors couple to Gq protein, a logical conclusion is that these two events are directly related. However, Gq protein is coupled to many neurotransmitter receptors, making it impossible to rule out the alternative interpretation that some other receptor’s interaction with Gq is the relevant point of intervention leading to a disruption of the DOI-induced effects. Given that the 5-HT2A receptor-Gq protein pathway is the likely signaling pathway, it is interesting that Mackowiak et al. (2002) reported that activation of phospholipase A2 via 5-HT2A receptors is engaged in the mechanism of DOI-induced FOS expression in the rat cortex. Since DOI-induced FOS expression is essentially eliminated in Gαq(−/−) mice, this suggests that phospholipase A2 activation may be downstream of Gq activation in mice. Additional studies of the phospholipase C and phospholipase A2 pathways in mice deficient for Gαq may enhance our understanding of the molecular mechanisms responsible for DOI’s effects.
In conclusion, the present study provides evidence that activation of the Gq protein pathway, downstream of 5-HT2A receptors, is a key signaling mechanism involved in the mediation of hallucinogen-induced behavioral effects. One of the most striking findings is the difference in sensitivity of two behaviors in Gαq null mice. DOI fails to elicit anxiolytic-like behavior in mutant mice, whereas the head-twitch response to DOI is reduced less than 50%, suggesting that other mechanisms are equally important for mediating this behavior. It is known that different brain sites mediate these behaviors (Graeff et al., 1993; Willins and Meltzer, 1997), and it is possible that 5-HT2A receptors within these brain sites are differentially coupled to G protein signaling pathways or that different compensatory mechanisms exist. Importantly, our studies suggest that the Gq signaling pathway is required for the full expression of hallucinogen-induced behaviors.
Acknowledgments
This work was supported by grants from the NIH R01-DA05181 (E.S.B) and F31-GM 73331-01 (E.E.G). Behavioral experiments were performed in the Vanderbilt Murine Neurobehavioral Laboratory. The authors would like to thank Dr. Melvin Simon (California Institute of Technology, Pasadena, CA) for the donation of Gαq(−/−) mice that were used to establish our colony. We also thank Kathleen Patterson for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Altman JL, Albert JM, Milstein SL, Greenberg I. Drugs as the discriminative events in humans. Psychopharmacol Commun. 1976;2:327–330. [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barry H., 3rd Classification of drugs according to their discriminable effects in rats. Fed Proc. 1974;33:1814–1824. [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Chang M, Zhang L, Tam JP, Sanders-Bush E. Dissecting G protein-coupled receptor signaling pathways with membrane-permeable blocking peptides. Endogenous 5-HT(2C) receptors in choroid plexus epithelial cells. J Biol Chem. 2000;275:7021–7029. doi: 10.1074/jbc.275.10.7021. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Lister RG. Time course of ethanol’s effects on locomotor activity, exploration and anxiety in mice. Psychopharmacology (Berl) 1988;96:67–72. doi: 10.1007/BF02431535. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychopharmacology (Berl) 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- Exton JH. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in sterotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maseo J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RM. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res. 1993;58:123–131. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Strickland LV, Sanders-Bush E. Lysergic acid diethylamide-induced Fos expression in rat brain: role of serotonin-2A receptors. Neuroscience. 2002;114:707–713. doi: 10.1016/s0306-4522(02)00349-4. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. 284–292. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Assie MB, Koek W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther. 1997;282:747–759. [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem. 2003;86:980–991. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Moorman JM, Grahame-Smith DG. Lithium enhances 5-HT2A receptor-mediated c-fos expression in rat cerebral cortex. Neuroreport. 1993;5:241–244. doi: 10.1097/00001756-199312000-00014. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, Czyrak A, Wedzony K. Inhibition of arachidonic acid cascade attenuates the induction of c-Fos proteins by DOI, 5-HT2A/2C receptor agonist, in the rat cortex. Pol J Pharmacol. 54:73–76. [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Aulakh CS, Wozniak KM, Hill JL, Murphy DL. Evidence that 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced hyperthermia in rats is mediated by stimulation of 5-HT2A receptors. Psychopharmacology (Berl) 1995;117:193–199. doi: 10.1007/BF02245187. [DOI] [PubMed] [Google Scholar]
- McClue SJ, Brazell C, Stahl SM. Hallucinogenic drugs are partial agonists of the human platelet shape change response: a physiological model of the 5-HT2 receptor. Biol Psychiatry. 1989;26:297–302. doi: 10.1016/0006-3223(89)90042-5. [DOI] [PubMed] [Google Scholar]
- McGrew L, Chang MS, Sanders-Bush E. Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol. 2002;62:1339–1343. doi: 10.1124/mol.62.6.1339. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. 1989;25:390–392. [PubMed] [Google Scholar]
- Milligan G. Regional distribution and quantitative measurement of the phosphoinositidase C-linked guanine nucleotide binding proteins G11 alpha and Gq alpha in rat brain. J Neurochem. 1993;61:845–851. doi: 10.1111/j.1471-4159.1993.tb03595.x. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Bourin M, Hascoet M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res. 2003;140:203–214. doi: 10.1016/s0166-4328(02)00311-x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson RF, Inoue Y, Kano M, Simon MI. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc Natl Acad Sci U S A. 1997;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Bishop-Robinson C, Darmani NA, Sanders-Bush E. Behavioral effects of (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane, (DOI) in the elevated plus-maze test. Life Sci. 1995;57:2455–2466. doi: 10.1016/0024-3205(95)02242-9. [DOI] [PubMed] [Google Scholar]
- Prunell M, Escorihuela RM, Fernandez-Teruel A, Nunez JF, Tobena A. Anxiolytic profiles of alprazolam and ethanol in the elevated plus-maze test and the early acquisition of shuttlebox avoidance. Pharmacol Res. 1994;29:37–46. doi: 10.1016/1043-6618(94)80096-0. [DOI] [PubMed] [Google Scholar]
- Robertson DN, Johnson MS, Moggach LO, Holland PJ, Lutz EM, Mitchell R. Selective interaction of ARF1 with the carboxy-terminal tail domain of the 5-HT2A receptor. Mol Pharmacol. 2003;64:1239–1250. doi: 10.1124/mol.64.5.1239. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246:924–928. [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(±)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 2003;166:61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Friedman E. Genomic responses to 5-HT1A or 5-HT2A/2C receptor activation is differentially regulated in four regions of rat brain. Eur J Pharmacol. 1996;307:211–217. doi: 10.1016/0014-2999(96)00233-6. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]


