Abstract
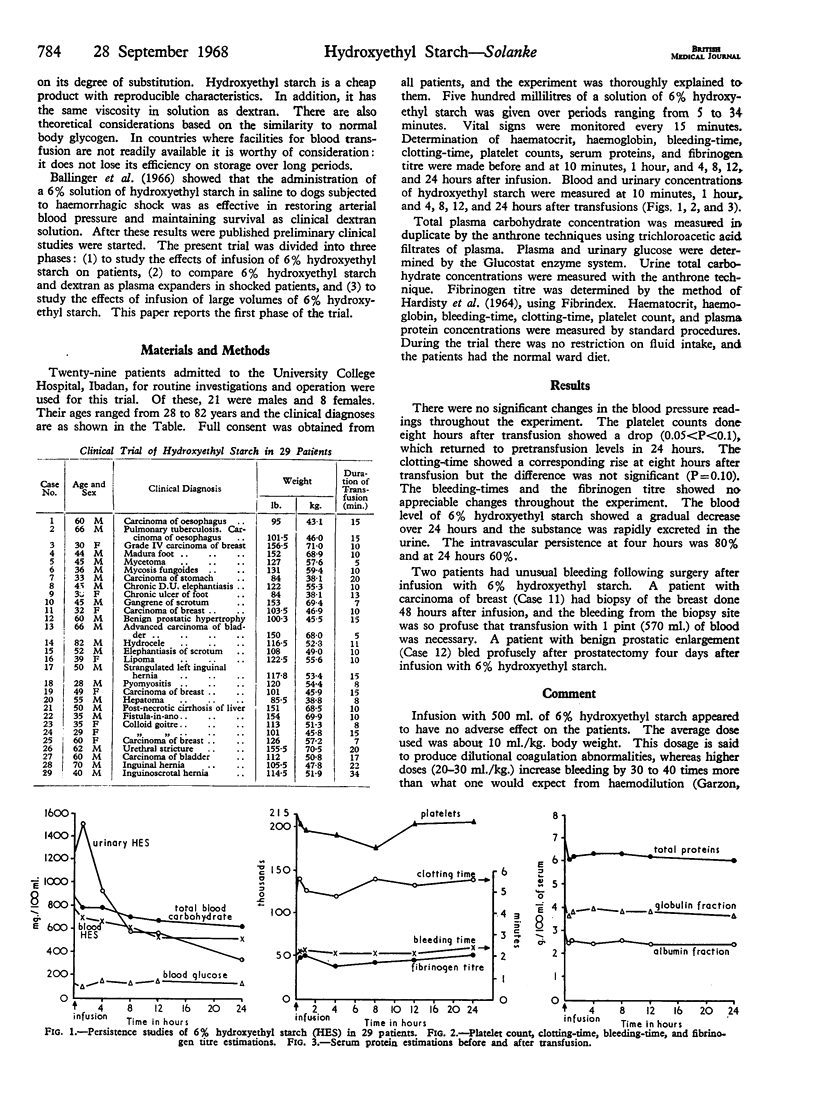
No adverse effects were seen in 29 patients given an intravenous infusion of 6% hydroxyethyl starch solution. Platelet counts had fallen by eight hours after infusion, but had reached pretransfusion levels by 24 hours. Two patients developed unusual bleeding post-operatively, which was possibly due to the infusion. Further investigations on the first stage of coagulation and prothrombin generation in patients receiving hydroxyethyl starch are required.
Full text
PDF