Abstract
Exposure to HIV type 1 (HIV-1) does not usually lead to infection. Although this could be because of insufficient virus titer, there is now abundant evidence that some individuals resist infection even when directly exposed to a high titer of HIV. This protection recently has been correlated with homozygous mutations of an HIV-1 coreceptor, namely CCR5, the receptor for the β-chemokines. Moreover, earlier results already had shown that the same chemokines markedly suppress the nonsyncitial inducing variants of HIV-1, the chief virus type transmitted from person to person. CCR5 mutation, as a unique mechanism of protection, is, however, suspect because HIV-1 variants can use other chemokine receptors as their coreceptor. Moreover, recent results have established that infection can indeed sometimes occur with such mutations. Here, we report on transient natural resistance over time of most of 128 hemophiliacs who were inoculated repeatedly with HIV-1-contaminated Factor VIII concentrate from plasma during 1980–1985 before the development of the HIV blood test. Furthermore, and remarkably, 14 subjects remain uninfected to this date, and in these subjects we found homozygous CCR5 mutations in none but in most of them overproduction of β chemokines. In vitro experiments confirmed the potent anti-HIV suppressive effect of these chemokines.
Some of the β or C-C chemokines, a subset of the relatively low molecular weight chemoattractant inflammatory cytokines, recently were found to be specific and potent inhibitors of HIV type 1 (HIV-1) (1) that likely account for the bulk, if not all, of the specific anti-nonsyncitial inducing (NSI) HIV-1 suppressive factors secreted by activated immune cells from seropositive individuals (2). They are also produced by human T cell leukemia/lymphotropic virus type-I-transformed CD8 T cell lines (1). Their protective antiviral effect is directed against most of the tested strains of HIV-1, HIV-2, and simian immunodeficiency virus but not most syncytium-inducing strains of HIV-1 and not against other viruses (1). Subsequent but independent work by Feng et al. (3) showed that the second receptor for HIV-1 is a chemokine receptor (Fusin, CXCR4), a finding quickly confirmed and extended by others to CCR5 (4) and to a lesser extent to some other chemokine receptors (5). Evidence was presented that indicates that the mechanism does not act by interference with signal transduction but by blocking HIV-1 interaction with these receptors (6). Recent results have shown that some high risk HIV-1-exposed people had homozygous mutations in the CCR5 receptor (7), suggesting that protection is due to the inability of HIV-1 to infect target cells in the absence of the receptor per se (8). However, recent studies have established that infection can sometimes occur with such mutations (9). Furthermore, as noted above, variants of HIV-1 can use more than one co-receptor. These and the earlier results on chemokine suppression of HIV (1) prompted us to study the production of C-C chemokines as an effector component of cellular activation and to investigate whether C-C chemokines production or genetic abnormality of their receptors were linked with protection against HIV-1 infection.
MATERIALS AND METHODS
Reagents.
Virus stock. HIV-1 consisted of the supernatant (SN) of a permanent cell culture infected with a laboratory NSI virus isolate (Strain Z96, reverse transcriptase activity = 800 × 103 cpm/ml) or, in some experiments, the SN of cocultures of peripheral blood mononuclear cells (PBMC) from the primary isolate (AUD) with normal activated PBMC (reverse transcriptase activity > 300 × 103 cpm/ml).
Antibodies (Abs).
Neutralizing anti-macrophage inflammatory protein (MIP)-1α, anti-MIP-1β, and anti-RANTES (MMR) Abs were purchased from R & D Systems.
Cell Cultures.
Cells. PBMC were isolated on Ficoll/Hypaque from heparinized blood.
Cell activation.
PBMC (106 cell/ml) were activated for 48 h with phytohemagglutinin (PHA)-P (3 μg/ml). Culture SN then were collected and stored at −80°C.
HIV-1 infection.
Forty eight-hour PHA-activated PBMC were pelleted and incubated at room temperature for 90 min with 1 μl of a 1:10 dilution of virus stock (NSI strain Z96 or primary isolate AUD) and washed twice. These cells were cultured for 6 days in medium supplemented with interleukin 2 (100 units/ml).
Assays.
Measure of T cell proliferation. T cell proliferation, used to evaluate immune activation of PHA-stimulated PBMC, was measured by 3H-thymidine (0.5 μCi/well) incorporation assay, which was added 18 h before cell harvest. 3H-thymidine incorporation was measured in a β-counter. All tests were carried out in triplicate, and results are expressed as crude cpm.
SSCP/HA analysis of CCR5 (formerly CKR5) among human genotypes.
The CCR5 primers were used to amplify 100 ng of DNA in the presence of α-32P-dCTP, in 10 μl, as described. The samples were digested by adding 10 μl of 2X Hinfl restriction buffer and 10 units of Hinfl and digesting at 37°C for 1 h. A 3-μl portion of the sample was run on a 5% acrylamide gel (37.5:1 acrylamide:bis-acrylamide) at 50 W for 3 h in a 4°C cold room. The gel was dried and exposed to x-ray film for 16–72 h. The PCR product was purified from a 50-μl reaction to pCRscript vector (Stratagene), according to the manufacturer’s protocol. Clones with inserts were checked by SSCP (1× SSCP = 120 mM NaCl/5 mM sodium citrate/20 mM sodium phosphate, pH 6.8) to identify the clones with altered alleles, and these clones were sequenced by using dye terminators and AmpliTaq FS enzyme (Perkin–Elmer). Sequencing reactions were run on an Applied Biosystems 373 machine. The primers CCKF4 and CCKR4 (5′-CTTCTTCATCATCCTCCTGACA, TGTAGGGAGCCCAGAAGAGA) were used to amplify the portion of the gene containing the CCR5Δ32 deletion. Products were resolved on either 2% agarose gels or 6% acrylamide gels.
Titration of C-C chemokines.
MMR produced by activated cells were measured by ELISA (kits from R & D Systems) in culture SN of PBMC after 48 h and of antigen presenting cells after 24 h.
HIV-1 titration.
HIV-1 production by in vitro-infected PBMC was assessed by p24 ELISA test.
RESULTS AND DISCUSSION
Human sera do not contain reliably measurable amounts of MMR because they aggregate, bind to glassware, stick to carbohydrate moieties present in blood, and are produced by platelets in varying amounts, which may be influenced by the method of blood withdrawal and use. However, their production by blood-derived cells can be assessed readily and reproducibly in culture by ELISA. Resting PBMC did not secrete C-C chemokines at measurable levels. By contrast, upon PHA activation, these cells release consistent amounts of MMR in the culture SN (Fig. 1a). This is also the case after immune activation with anti-CD3/interleukin 2, 1,4-phenylenediamine/tetanus toxoid, or Staphylococcus enterotoxin B. Thus, as reported for monocytes (U937) and T (Jurkatt) cell lines in which induction of MIP-1α expression was obtained only upon cellular activation (10), the C-C chemokine secretion by PBMC from normal individuals, virtually absent at the resting stage, also was induced by cell activation. Levels of production, however, were dependent on the blood donor (Fig. 1b), the type of immune cells (PBMC, purified CD4 or CD8 T cells, antigen presenting cells), the culture conditions, and the mode of stimulation (data not shown). It is noteworthy that (i) C-C chemokine secretion was an early and transient effector component of the cellular response to PBMC activation that preceded T cell proliferation. Significant amounts of MIP-1α and MIP-1β were found in the 12 ± 6-h culture SN, before DNA replication that occurred 24–48 h after activation, as measured by 3H-thymidine incorporation (Fig. 1a). After the peak of chemokine release, reached by 48 h, C-C chemokine secretion by activated PBMC (growing in the presence of interleukin 2) markedly dropped to background levels (1–2 × 103 μg/ml). (ii) Although the production levels of MMR were different in the same individual, they varied according to a similar trend from one subject to another, as if activation induced a coordinated cellular response of the three chemokines (Fig. 1b).
Figure 1.
Production of C-C chemokines by PBMC from seronegative individuals. T cell proliferation was assayed by 3H-thymidine test, and C-C chemokine production was measured by ELISA in culture SN. (a) Kinetics of T cell proliferation (—◊—) and of C-C chemokines secretion: MIP-1α (—•—), MIP-1β (—▪—), and RANTES (—▴—). Only background values of chemokines production (<0.5 ng/ml) and T cell proliferation (<500 cpm) were found for resting cells. A representative experiment is shown. (b) Variations of chemokines production by PBMC from five different seronegative donors. (c) Box plot analysis of C-C chemokine production in high risk HEH. HEH (white boxes) compared with control seronegative donors (black boxes). Box limits correspond to 50% percentile of each group, and the horizontal bar corresponds to the median. Asterisks refer to a statistical difference (P = 0.0063 for MIP-1α, P = 0.0223 for MIP-1β, and P = 0.0361 for RANTES).
These initial studies demonstrated that reproducible results for quantitative comparison of C-C chemokine production by PBMC from different subjects could be obtained. To study the possible anti-HIV role played by C-C chemokines in resistance to HIV-1 infection, the production of MMR by 48-h PHA-activated PBMC from 14 seronegative hemophiliacs highly exposed (HEH) to HIV-1 infection was investigated.
These subjects belong to a cohort of 128 hemophiliacs with follow-up at the Hemophilia Centre of Milan. Each subject of the cohort was infused with an average amount of 3,300,000 units of factor VIII and factor IX concentrates manufactured from huge pools of hepatitis C virus and HIV-1-contaminated plasma from paid U.S. plasmapheresis donors (Cutter; Hyland, Costa Mesa, CA; and Immune, Austria). Between 1980 and 1985 [before the availability of the blood test (11) and virus inactivation procedures], each patient received multiple infusions ranging from 3,000 to 990,000 units (median 105,000 units) of commercially available contaminated concentrates. Following the very first infusions with contaminated concentrates of all 128 patients, including the 14 highly exposed and still uninfected hemophiliacs (HEH), were hepatitis C virus-positive patients as described (12, 13), but only three became infected by HIV, as determined by both serum Abs (ELISA and Western blot analysis) and genomic PCR. However, the number of HIV-1-infected subjects progressively increased with additional contaminated infusions and reached 59 in 1982, 84 in 1983, 103 in 1984, and 114 in 1985. Remarkably, the 14 HEH escaped HIV-1 infection despite receiving the multiple infusions (20–300) of 52,000–990,000 units (median 166,000 units) of contaminated concentrates.
In preliminary and sporadic experiments, the levels of chemokine production were markedly higher in PBMC collected from uninfected HEH than in those from miscellaneous seronegative control subjects. To confirm this result and to eliminate any bias due to cell culture fluctuations (see above), the production of MMR by activated fresh PBMC from 14 uninfected HEH and 14 seronegative controls was investigated at the same time and under identical culture conditions. A control group consisted of 14 seronegative volunteer donors from the blood bank of Hôpital Saint-Antoine (Paris). The mean and median levels of MMR production by PBMC from the uninfected exposed hemophiliacs were higher than those from the control. These differences were statistically significant (Student’s t test, t = 2.975 with P = 0.0063 for MIP-1α, t = 2.430 with P = 0.0223 for MIP-1β, and t = 2.210 with P = 0.0361 for RANTES) (Table 1A). It is important to note that the higher production found in the uninfected HEH did not appear to be the consequence of higher levels of activation/proliferation because similar levels of cell replication were found in the two groups. The mean and median of 3H-thymidine incorporation of 48-h PHA-activated PBMC were, respectively, 17,240 and 16,300 cpm for the uninfected hemophiliacs and 19,080 and 17,450 cpm for the control group.
Table 1.
Production of C-C chemokines in activated cells from HEH
| A | HEH, pg/ml culture
|
|||
|---|---|---|---|---|
| MIP-1α | MIP-1β | RANTES | 3H-T | |
| Mean | 33,100 | 31,900 | 39,000 | 17,200 |
| Median | 30,300 | 23,100 | 42,800 | 16,300 |
| n | 14 | 14 | 14 | 14 |
| Seronegative controls | ||||
| Mean | 15,000 | 16,500 | 24,700 | 19,000 |
| Median | 11,400 | 14,200 | 19,000 | 17,500 |
| n | 14 | 14 | 14 | 14 |
| Student’s t test | t = 2.975 | t = 2.430 | t = 2.210 | Not significant |
| P < 0.01 | P < 0.05 | P < 0.05 | ||
| B | HEH
|
Seronegative controls
|
|||
|---|---|---|---|---|---|
| MIP-1α | MIP-1β | MIP-1α | MIP-1β | ||
| Mean | 38,800 | 41,300 | 16,800 | 16,600 | |
| Median | 39,800 | 39,100 | 15,900 | 14,800 | |
| n | 8 | 8 | 8 | 8 | |
| Unexposed mild hemophiliacs
|
Unexposed severe hemophiliacs
|
||||
|---|---|---|---|---|---|
| MIP-1α | MIP-1β | MIP-1α | MIP-1β | ||
| Mean | 18,300 | 12,000 | 16,900 | 16,300 | |
| Median | 17,900 | 10,500 | 16,900 | 9,400 | |
| n | 8 | 8 | 8 | 8 | |
In a second series of experiments also performed under identical culture conditions, chemokine production by PBMC from 8 of the 14 HEH subjects randomly selected was compared with that from PBMC of various seronegative controls. As shown in Table 1B, this experiment confirmed that MIP-1α production from HEH was over twofold higher than that from a new group of seronegative controls from Hôpital Saint Antoine, seronegatives with mild hemophilia who exhibit milder factor VIII defect (and consequently did not need clotting concentrate infusion), or uninfected severe hemophiliacs who received only virus-inactivated or recombinant clotting factor concentrates. Levels of MIP-1β production in the different groups paralleled MIP-1α pattern (Table 1B). The data of these two successive experiments (Table 1), which are represented statistically in a box plot (Fig. 1c), clearly show that patients who resisted infection despite multiple exposures to HIV-1 expressed higher C-C chemokine production than unexposed controls whether or not they were hemophiliacs.
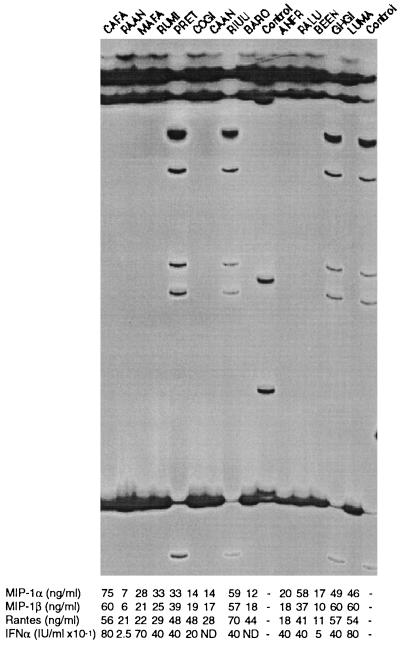
Recent discoveries of natural mutations in the CCR5 coreceptor gene that affect both HIV-1 infection and progression to AIDS-defining illness (8, 14) prompted us to examine the coding genes for three chemokine receptors in these patients. A common 32-bp deficiency was discovered within the CCR5 locus in addition to several other point mutations at lower allele frequencies (8). The CCR5 Δ32 genotypes are illustrated in Fig. 2, including 3 +/Δ32 heterozygotes (PRET, RIUU, and GHGI). All other patients were homozygous for the common “normal” allele. A screen of the entire coding region using SSCP (15, 16) did not reveal any other nucleotide sequence polymorphism among these patients. The other eight rare CCR5 alleles (<1% in Caucasians) reported by Dean et al. (8) were not observed in these patients. A similar heterozygous Δ32 deletion also was present in two individuals of the 12 tested controls. It is noteworthy that the three CCR5 +/Δ32 heterozygous hemophiliacs each produced high quantities of C-C chemokines for MIP-1α equal to 33, 49, and 59 × 103 pg/ml. Whether heterozygous Δ32 deletion determined the high level of chemokine secretion is presently unknown because elevated levels also were observed in multiply exposed but uninfected individuals homozygous for the normal CCR5 allele.
Figure 2.
SSCP/HA analysis of CCR5 (formerly CKR5) among genotypes of 14 exposed uninfected individuals. The central portion (347–753 bp) of CCR5 was amplified and run on an SSCP/HA gel. The gel resolves the single-stranded molecules (ss) and heteroduplexes (Het), and double-stranded DNA is at the bottom of the gel. The heteroduplexes are formed in heterozygous individuals (PRET, RIUU, GHGI, and rt. control) by annealing of a strand of the wild-type allele with the complementary strand of the deletion allele. Lane 10 is a +/m control with a point mutation; all others are homozygous CCR5 +/+.
Primers were designed also to detect polymorphisms within 80% of the coding region of the CXCR4 locus. Polymorphism screens of several hundred individuals revealed a rare SSCP variant but no variation among the patients here screened. Finally, screens for mutations among CCR2B, a second coreceptor for macrophage-tropic HIV strains revealed a common variant that altered amino acid number 64 from valine to isoleucine. One patient (CAFA) was heterozygous for the CCR2B-1/2 genotype, and the rest were homozygous for the common “1” allele. The significance of this genotype on chemokine activity, HIV pathogenesis, or AIDS outcome presently is not understood.
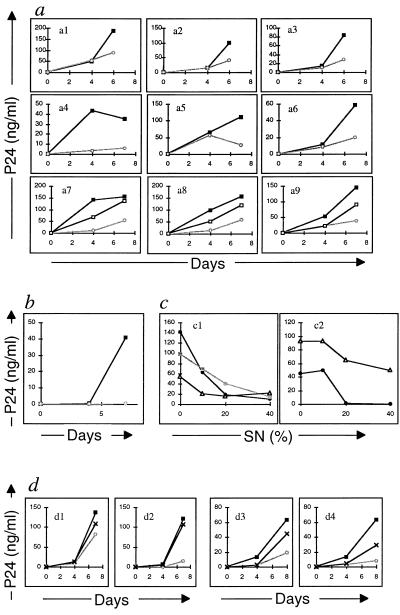
To further confirm whether antiviral C-C chemokines or genetic heterozygote Δ32 abnormality play a role in HIV infection of immune cells, we infected activated PBMC from the three HEH with a (+/Δ32) CCR5 heterozygotic abnormality, three HEH without the mutation, and three seronegative controls. PBMC from all of the individuals could be infected either by the laboratory NSI strain (Fig. 3) or by a primary isolate (AUD) of HIV-1 as detected by p24 ELISA in culture SN (Fig. 3 b–d). However, when these PBMC were cultured in the presence of SN from 48-h PHA-activated autologous cells (which contain the β chemokines) or with a mixture of recombinant MMR, viral infection was inhibited markedly (Fig. 3 a and b). The inhibition of viral infection was dose-dependent (Fig. 3c). Furthermore, when the MMR containing SN or MMR recombinant “cocktail” were preincubated with neutralizing anti-MMR Abs, their inhibitory effect was suppressed either markedly or totally (Fig. 3d).
Figure 3.
HIV-1 infection of activated PBMC from HEH. HIV-1 infection of PBMC in the presence of recombinant C-C chemokines (MMR) was assessed by p24 ELISA test. (a) Kinetics of HIV-1 infection of seronegative cells by HIV-1 NSI, in the absence (—▪—) or presence (—○—) of 40% of SN or of MMR (—□—). The assay included cells from three control seronegative (a1–a3); 3 HEH without Δ32 abnormality (+/+) (a4–a6); and 3 HEH with Δ32 abnormality (Δ32/+) (a7–a9) (a7 = RIUU; a8 = PRET; and a9 = GHGIO). (b) Kinetics of HIV-1 infection by primary isolate (AUD) in control cells in the absence (—▪—) or presence (—○—) of SN. (c) Dose-dependent inhibition of HIV-1 infection by autologous cell SN. This assay was performed for 7 days on cells from three HEH with Δ32 abnormality (—•—) RIUU; (—▪—) PRET and (—▵—) GHGIO. Cells were infected with either HIV-1 Z96 (c1) or HIV-1 AUD (c2). (d) Effect of a mixture of neutralizing Abs to MMR on HIV-1 infection of PBMC from one HEH subject with Δ32 abnormality (d1 and d2) and one control donor (d3 and d4). Cells were infected with either HIV-1 Z96 (d1 and d3) or HIV-1 AUD (d2 and d4). Absence of SN (—▪—); presence of SN (—○—); and presence of SN preincubated with neutralizing Abs (—x—).
The discovery of the anti-HIV-1 effect of some proinflammatory chemokines produced by immune cells (1) and the subsequent discovery of chemokine receptors as the second HIV-1 receptor (4, 5, 17) initiated a series of studies to determine whether these anti-HIV-1 cytokines or their receptor play a role during HIV-1 infection. Clarification of the specificity of the anti-HIV C-C chemokine effect has come from recent understanding of their mode of action. As indicated above, it recently has been shown that the HIV-1 second receptors are the chemokine receptors and belong to a family of seven transmembrane receptors (5, 18). Results in the original and subsequent reports (6) on the magnitude of the antiviral effects by these chemokines already implied a dependency on the gp120 V3 loop, believed to be involved in steps leading to the fusion of the virus and cell membranes after binding of the viral envelope to CD4. According to Wu et al. (19), a high affinity binding of gp120 for CCR5, leading to membrane fusion and virus entry, involves the gp120 V3 loop and follows HIV-1 attachment to CD4. The involvement of the V3 loop was established further by Trkola et al. (20), who showed that mAbs to the V3 loop inhibited the interaction of gp120 with the CCR5 second receptor, and by Cocchi et al. (6), who showed with HIV-1 chimeras that the chemokine inhibitory effect was at least in part dependent on the V3 sequence.
Most hemophiliacs of the Milan cohort who repeatedly received infusions of contaminated factor VIII and IX concentrates exhibited a natural resistance, though transient, to the inoculated HIV-1 but not to hepatitis C virus. The resistance to HIV-1 initially observed varied from one individual to another because infection occurred for each subject of the cohort after a variable number (6–200) of infusions. Remarkably, the HEH who received over a median of 80 contaminated infusions (20–300) remained uninfected to the present time. The immune cells of these seronegative HEH produced over twice the levels (mean and median) of MMR than other seronegative individuals whether hemophiliacs or not, although there was no detectable difference in the activation (T cell proliferation) of HEH and control cells. Others have shown elevated production of these molecules by CD4+ T cells from two frequently sexually exposed but uninfected homosexual subjects (21). Furthermore, because recent reports correlated protection with a variant form of chemokine receptor corresponding to mutation(s) or deletion(s) of the CCR5 structural gene (8, 22), this gene was analyzed in the uninfected HIV-1-exposed cohort. Genetic abnormalities were found in 3 of the 14 subjects (see Fig. 2). Of interest, these three individuals produced high levels of C-C chemokines after cellular activation: 59,000, 33,000, and 49,000 pg/ml, respectively. Whether the chemokine response was related to the CCR5 genotype alteration or was coincidental remains to be shown. However, because five other subjects in the cohort without CCR5 genetic abnormalities were also high producers of C-C chemokines, we can infer that other factors, either genetic, including other chemokine receptors (3, 5, 17), or nongenetic, also may be involved in the increase of the chemokine secretion induced by immune cell activation. The protective direct effect of C-C chemokine on HIV-1 infection was verified by the last series of experiments that showed that SN from cultured PBMC obtained from the HEH cells (and known to contain the C-C chemokines, MMR, or a cocktail of recombinant C-C chemokines) inhibited HIV-1 infection. This inhibitory effect was dose-dependent and was abolished by anti-MMR neutralizing Abs. The anti-HIV protective effect of C-C chemokine containing SN is also supported by experiments showing that in vitro PBMC from seronegative individuals become more resistant to HIV-1 infection when these cells release large quantities of C-C chemokines, i.e., at the initiation of activation rather than when they secrete basal levels, i.e., 3–6 days after activation. In a representative experiment using cells from a HEH subject (PRET), PBMC infection performed at the initiation of activation (with MIP-1α release = 47 ng/ml) was 11-fold less effective than when viral treatment followed activation (with MIP-1α release = 1.1 ng/ml).
The clinical data related to natural resistance to HIV contamination, including those reported in this study, corroborate the experimental results. It is well known that there is a small percentage of persons who remain uninfected in populations at high risk of HIV infection. This includes hemophiliacs who received contaminated blood products and individuals exposed to “unsafe” sexual activity with HIV-1-positive partners (23). Resistance to infection was correlated in exposed but uninfected Gambians with a higher specific activity of cytotoxic T lymphocytes (CTL) (23), whereas in the present study resistance in uninfected hemophiliacs was correlated with a higher production of MMR. It seems that C-C chemokine secretion and differentiation of CTL are two effector processes of the same cellular arm of the immune reaction, the former acting as early as 12 ± 6 h after initiation of the immune activation and before cell proliferation (Fig. 1) and the latter being effective 5–8 days after differentiation of effective anti-HIV-1 CTL from naïve or memory precursor cells through a series of cell divisions. Confirmation of this assertion came from a set of experiments carried out on PBMC from five subjects of the HEH cohort and five normal individuals. These cells, and not cells from seronegative controls, exhibited a memory cytotoxic activity directed against HIV-1 gp160 antigens, which was reactivated by mixed lymphocyte cultures carried out in the presence of effector PBMC and irradiated autologous blasts infected by HIV-1. CTL activity was measured by lysis of target autologous B cell lines infected with gp160 recombinant vaccinia and was HLA-restricted and dependent on the effector-to-target cell ratio in the range of 40:1–0.3:1 (not shown).
Exposure to HIV-1 may or may not result in infection. The outcome depends on viral (number of infectious particles; viral strain) but also host factors, including the cellular immune response to the infection. In this study, we showed that anti-HIV-1 chemokines, released as an initial effector component of the cellular response, play a major role in determining resistance to HIV-1 infection, at least in vitro (Fig. 3). We infer from this that resistance is due to their greater capacity to develop a stronger cellular immune response, including the early release of C-C chemokines. The few resistant individuals could be defined as “high responders” of the cellular arm of the immune response by analogy to the known high responder mice producing elevated levels of Abs in response to antigen stimulation (humoral arm) as described by Biozzi et al. (24).
To combat HIV-1, the host cellular immune defense possesses two specific weapons both resulting from the immune cell response to activation. In the initial line of defense, the C-C chemokines are produced very early, and in the second line of defense, CTL are strongly effective after several days of differentiation of naïve or memory precursor cells. In uninfected individuals, these anti-HIV-1 effector components of the cellular response can prevent infection, particularly in the few “high cellular responders,” such as the HEH of the Milan cohort. These effectors may be capable of containing HIV-1 until immune incompetence, triggered by HIV-1 through escape mutants and resultant higher production of pathogenic viral proteins, leads to uncontrolled viral growth and immune suppression (25). The escape mutants may arise by the development of SI variants from the earlier NSI variants, the chief virus type transmitted from person-to-person (26). The NSI variants are β chemokine-sensitive, whereas the emerging SI variants are not sensitive (27). Increase of acute infection processes with highly replicating viruses (28) resulting from uncontrolled viral growth is a source of HIV-1 proteins, including gp120 and extracellular Tat (29). These pathogenic proteins (30) should contribute to the dramatic immune suppression of uninfected T cells characterizing AIDS.
ABBREVIATIONS
- Abs
antibodies
- CTL
cytotoxic T lymphocytes
- HEH
highly exposed hemophiliacs
- HIV-1
HIV type 1
- MIP
macrophage inflammatory protein
- MMR
MIP-1α, MIP-1β, and RANTES
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- NSI
nonsyncitial inducing
- SN
supernatant
References
- 1.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 2.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:563–566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 4.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmom S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 7.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 8.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 9.Biti R, French R, Young J, Bennetts B, Stewart G, Liang T. Science. 1996;272:1955–1958. [Google Scholar]
- 10.Ritter L M, Bryans M, Abdo O, Sharma V, Wilkie N M. Mol Cell Biol. 1995;15:3110–3118. doi: 10.1128/mcb.15.6.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarngadharan M G, Popovic M, Bruch L, Schupbach J, Gallo R C. Science. 1984;224:506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher M L, Trowell J M, Craske J, Pavier K, Rizza C R. Br Med J. 1983;287:1754–1757. doi: 10.1136/bmj.287.6407.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernoff P B, Lee C A, Karayiannis P, Thomas H C. Br J Haematol. 1985;60:469–479. doi: 10.1111/j.1365-2141.1985.tb07444.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, et al. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 15.White M B, Carvalho M, Derse D, O’Brien S J, Dean M. Genomics. 1992;12:301–306. doi: 10.1016/0888-7543(92)90377-5. [DOI] [PubMed] [Google Scholar]
- 16.Ravnik-Glavac M, Glavac D, Dean M. Hum Mol Genet. 1994;3:801–807. doi: 10.1093/hmg/3.5.801. [DOI] [PubMed] [Google Scholar]
- 17.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 18.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, et al. Nature (London) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 20.Trkola A, Draagic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. Nature (London) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 21.Paxton W A, Martin S R, Tse D, O’Briend T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, et al. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stohlman H, Koup R A, Landau N. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 23.Rowland-Jones S L, McMichael A. Curr Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 24.Biozzi G, Stiffel C, Mouton D, Bouthillier Y, Decreusefond C. J Exp Med. 1972;135:1071–1094. doi: 10.1084/jem.135.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg S, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, et al. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 26.Balotta C, Vigano A, Riva C, Colombo M C, Salvaggio A, de Pasquale M P, Crupi L, Papagno L, Galli M, Moroni M, et al. AIDS Res Hum Retroviruses. 1996;12:1247–1253. doi: 10.1089/aid.1996.12.1247. [DOI] [PubMed] [Google Scholar]
- 27.Jansson M, Popovic M, Karlsson A, Cocchi F, Rossi P, Albert J, Wigzell H. Proc Natl Acad Sci USA. 1996;93:15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffin J M. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 29.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H K, Brady J N, Gallo R C. Nature (London) 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 30.Zagury D. Nat Med. 1997;3:156–157. doi: 10.1038/nm0297-156. [DOI] [PubMed] [Google Scholar]