Abstract
Cystic fibrosis (CF) is a lethal inherited disease that results from abnormal chloride conduction in epithelial tissues. ClC-2 chloride channels are expressed in epithelia affected by CF and may provide a key “alternative” target for pharmacotherapy of this disease. To explore this possibility, the expression level of ClC-2 channels was genetically manipulated in airway epithelial cells derived from a cystic fibrosis patient (IB3-1). Whole-cell patch-clamp analysis of cells overexpressing ClC-2 identified hyperpolarization-activated Cl− currents (HACCs) that displayed time- and voltage-dependent activation, and an inwardly rectifying steady-state current–voltage relationship. Reduction of extracellular pH to 5.0 caused significant increases in HACCs in overexpressing cells, and the appearance of robust currents in parental IB3-1 cells. IB3-1 cells stably transfected with the antisense ClC-2 cDNA showed reduced expression of ClC-2 compared with parental cells by Western blotting, and a significant reduction in the magnitude of pH-dependent HACCs. To determine whether changes in extracellular pH alone could initiate chloride transport via ClC-2 channels, we performed 36Cl− efflux studies on overexpressing cells and cells with endogenous expression of ClC-2. Acidic extracellular pH increased 36Cl− efflux rates in both cell types, although the ClC-2 overexpressing cells had significantly greater chloride conduction and a longer duration of efflux than the parental cells. Compounds that exploit the pH mechanism of activating endogenous ClC-2 channels may provide a pharmacologic option for increasing chloride conductance in the airways of CF patients.
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CF transmembrane conductance regulator (CFTR). The CFTR protein functions as a cAMP-regulated chloride channel and, in airway epithelia, as a regulator of separate sodium and chloride channels (1, 2). Loss of CFTR function affects electrolyte transport across apical membranes of epithelial cells leading to altered mucous properties, recurrent episodes of infection and inflammation, and, ultimately, organ destruction (1, 2). Almost 90% of mortality in CF is due to lung disease (3). Intriguingly, mice lacking functional CFTR do not develop lung disease (4, 5). It has been proposed that a Ca2+-activated chloride channel is active in murine lungs and preserves apical membrane chloride transport in the absence of CFTR (6). Based on these observations, exploitation of alternative pathways of chloride transport in airway epithelial cells appears to be a viable treatment option for CF.
Several different chloride channels have been characterized in pulmonary epithelia. These include CFTR, an outwardly rectifying chloride channel, the Ca2+-activated chloride channel, and a volume-sensitive chloride channel (7). Molecular studies have indicated that a member of the voltage-gated family of chloride channels, ClC-2, is expressed in airway epithelia in a developmentally regulated fashion (8). Immunohistochemical analysis of rat lung indicates that this channel is located in apical membranes and its distribution overlaps with that of CFTR (9). Furthermore, expression in Xenopus oocytes revealed that the chloride channel formed by rat ClC-2 has several biophysical features in common with CFTR; a similar anionic selectivity, insensitivity to the channel inhibitor (DIDS), and possible regulation by cAMP (10). Thus, ClC-2 appears to be a reasonable candidate to provide apical membrane chloride transport in the absence of functional CFTR.
Our laboratory previously had cloned a cDNA (hClC-2) from a human intestinal epithelial cell line (T84) that displayed almost 94% amino acid sequence identity with rat ClC-2 (11). Northern analysis using a probe derived from this cDNA revealed a single transcript of 3.3 kb in most human tissues, including lung, and an airway cell line derived from a CF patient (11). The aim of this study was to define the electrophysiologic properties of hClC-2 and to elucidate methods of activating chloride transport via this alternative channel in human cells with dysfunctional CFTR. The results herein indicate that hClC-2 has a number of properties in common with those described for rat ClC-2, including activation by hyperpolarizing voltages and by cell swelling induced by hypotonic extracellular solutions. However, hClC-2 also displays a substantial response to low extracellular pH. These studies indicate that exploitation of this method of ClC-2 activation may provide an alternative pathway for chloride movement across CF epithelia.
MATERIALS AND METHODS
Creation and Characterization of Stable Cell Lines.
IB3-1 CF airway epithelial cells (12) were transfected with 2 μg of the expression plasmid pBK-RSV (Invitrogen) containing full-length human ClC-2 cDNA (11) in the sense or antisense orientation mixed with 10 μl Lipofectin Reagent (GIBCO/BRL). Individual, stably transfected clones were isolated after selection on 180 μg/ml G418 for 6 weeks. Southern blot analysis and autoradiography were performed as described (13). For RNA analysis, 30 μg total RNA was electrophoresed in a 17.5% formaldehyde gel, transferred overnight to Genescreen membrane (DuPont/NEN), UV cross-linked (0.12 J; Stratalinker, Stratagene), baked at 80° for 2 hr, then hybridized in Quick Hyb (Stratagene) for 1 hr at 65°. Washing, autoradiography, and phosphorimage analysis was performed as described (11). Western blot analysis of the antisense cell lines was performed as described (8).
Whole-Cell Patch-Clamp Recording.
Cells were seeded on Vitrogen 100 (1:15 diluted in Ca2+Mg2+-free PBS; Collagen Corp)-coated glass coverslips (Bellco Glass). The bath solution contained 145 mM Tris⋅Cl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, 60 mM sucrose, and 5 mM Hepes, pH 7.45, and 0.2 μ filtered. The pipette solution contained 145 mM Tris⋅Cl, 5 mM Hepes, 100 nM free Ca2+ and Mg2+ (chelated with 2 mM EGTA), and 5 mM Mg2+-ATP, pH 7.45. All solutions were passed through a 0.2-μ filter. NaCl, NaI, and sodium gluconate (145 mM each) replaced Tris⋅Cl in anion permselectivity recordings. Bath pH and pipette solution pH were titrated with 1 M HCl or 1 M Tris to acidify or alkalinize the solutions, respectively. Data were analyzed by using pclamp software; membrane capacitance (Cm), membrane resistance (Rm), and series resistance (Rs) were analyzed with pclamp and quatrropro software using previously described derivations (14).
36Chloride Efflux Assay.
Cells were washed three times with Ca2+Mg2+-free PBS (GIBCO/BRL) to remove serum and loaded with 5 μCi of 36Cl− in 1.5 ml of standard HCO3−-free, Hepes- and phosphate-buffered 140 mM NaCl Ringer’s with 5 mM glucose, and titrated to pH 7.45 with 1 M NaOH. Efflux runs were performed in a 37°C warm room. Each well served as its own control. At time 0, Ringer’s at pH 7.45 was added and removed immediately. A fresh aliquot of Ringer’s at pH 7.45 was added immediately and the efflux run was started. This process was repeated at 15-sec intervals for 1 min, at which time the following test conditions were applied: Ringer’s with a pH of 3.6, pH 11.2, 50% tonicity, 250 μM CPT-cAMP, and 250 μM 8-Br-cAMP, or 2 μM ionomycin. For the next 4 min, fresh solution was added and removed at 15-sec intervals and counted on a scintillation counter. At the end of the run, cells were lysed with 0.5 M NaOH to determine the amount of 36Cl− remaining in the cells to standardize results. The total amount of Cl− at the start of the run was calculated, and the rate of 36Cl− efflux for each 15-sec interval was expressed as the fraction of 36Cl− lost and multiplied by 4 to obtain a rate per minute.
RESULTS
ClC-2 Generates pH-Dependent, Hyperpolarization-Activated Chloride Currents.
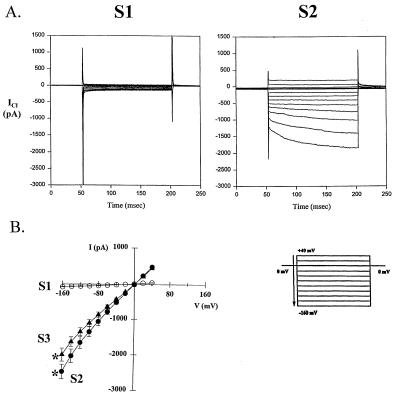
Immortalized airway epithelial cells (IB3-1) from a cystic fibrosis patient with low endogenous levels of ClC-2 RNA were used in this study (11, 12). Three IB3-1 clonal cell lines (S1, S2, and S3) transfected with a plasmid (pBK-hClC-2) containing the hClC-2 cDNA in the “sense” orientation were selected on the basis of G418 resistance. Southern blot analysis revealed that genomic DNA of cell lines S2 and S3 had multiple copies of the full-length pBK-hClC-2 plasmid (Fig. 1A). The hClC-2 cDNA was not present in cell line S1, indicating that only the portion of the pBK-hClC-2 plasmid conferring antibiotic resistance had integrated. Hybridization of total RNA from each cell line with a probe from the unique 3′ end of the hClC-2 cDNA identified abundant 3.3-kb and 3.8-kb transcripts in S2 and S3 (Fig. 1B). The size of each transcript was consistent with use of the native polyadenylation signal (3.3-kb transcript) and a synthetic polyadenylation signal in the pBK-hClC-2 plasmid (3.8-kb transcript). The intensity of each hybridizing transcript varied between 5- and 30-fold higher than the endogenous ClC-2 RNA observed in parental IB3-1 cells and S1 cells. The lack of ClC-2 overexpression in S1 is consistent with the absence of ClC-2 cDNA in the genomic DNA from this cell line. To study ClC-2 function, whole-cell patch-clamp recordings were performed under conditions where Cl− was the only permeant ion in the pipette (intracellular) and bath (extracellular) solutions. Currents were recorded in response to a specific voltage protocol; depolarization to +40 mV then voltage steps in −20-mV increments to a strong hyperpolarizing voltage of −160 mV. At physiologic pH (7.45), hyperpolarization-activated Cl− currents (HACCs) were minimal in Parental and S1 cells expressing endogenous levels of ClC-2 mRNA (S1 shown in Fig. 2A Left). However, time- and voltage-dependent currents were prominent in the cells overexpressing ClC-2 (Fig. 2A Right). Current–voltage (I–V) plots revealed a slight inward rectification of responses from the overexpressing cells (Fig. 2B).
Figure 1.
Characterization of human airway epithelial cell lines overexpressing ClC-2. (A) Autoradiograms of genomic DNA from Parental IB3-1 cells and cell lines S1, S2, and S3 digested with NheI (Left) and NheI and NotI (Right) then hybridized with a 0.5-kb SphI-HindIII fragment from the 3′ end of the ClC-2 cDNA. Clones S2 and S3 have intensely hybridizing NheI fragments of 7.5 kb that were digested by NotI to fragments of 4.8 kb and 2.7 kb, consistent with the presence of multiple copies of full-length concatenated plasmids (Lower). Open arrows indicate fragments from the endogenous ClC-2 genes present in each lane. Additional hybridizing signals in some lanes probably represent junction sequences, partial integrations, and/or incomplete digestion. In the diagram, solid boxes and arrows represent the RSV promoter and direction of transcription and hatched boxes indicate the location of the SphI-HindIII probe relative to ClC-2. (B) Autoradiogram of total RNA from Parental IB3-1 cells and clones S1, S2, and S3 (30 μg each) and T84 cells (10 μg) hybridized with a 1.45-kb probe from the 3′ end of the ClC-2 cDNA. Transcripts of 3.3 kb from the endogenous ClC-2 gene are seen in all lanes. S1 has a faint signal at 3.3 kb, comparable to the level of expression in parental cells. S2 and S3 have intense signals at 3.3 and 3.8 kb, indicating expression of stably integrated ClC-2 cDNA. Ethidium bromide staining of the gel indicated that approximately equal amounts of RNA were loaded in each lane (Lower).
Figure 2.
Cells overexpressing ClC-2 have HACCs at physiologic pH. (A) Representative whole-cell Cl− current recordings of an S1 cell expressing endogenous levels of ClC-2 and an S2 cell overexpressing ClC-2 at pH 7.45. The holding potential was 0 mV, and voltage was stepped to +40 mV for 20 msec then decreased in 20-mV increments to −160 mV. (B) Current–voltage plot of mean responses for S1 cells (n = 6) and for cells S2 (n = 17) and S3 (n = 10). Error bars indicate the standard error of the mean. Asterisks indicate a significant difference (P < 0.05) in current amplitude at −160 mV between S1 and S2 cells and between S1 and S3 cells using anova and Bonferroni ad hoc tests.
Based on the observation that acidic extracellular pH resulted in the appearance of HACCs in parental IB3-1 cells, the effect of reducing extracellular pH upon HACCs generated by cells expressing endogenous levels of ClC-2 was compared with those overexpressing ClC-2. In Parental and S1 cells, pH 6.2 had no significant effect on the observed Cl− currents. However, reduction to pH values of 5.0 and 3.79 resulted in progressively larger HACCs (Parental cells shown in Fig. 3 Left). Currents generated by Parental cells exposed to an extracellular pH of 3.79 were comparable in magnitude to HACCs from overexpressing (S2) cells at pH 7.45 (note difference in current scales in Fig. 3). Decreasing the extracellular pH of S2 cells to 6.2 reduced the magnitude of HACCs, whereas lower pH levels of 5.0 and 3.79 markedly stimulated HACCs (Fig. 3 Right). At the lowest pH, HACCs in the overexpressing (S2) cells were more than 4-fold greater than in the parental cells. This effect was restricted to extracellular changes in pH. When the pH of the intracellular solution (pipette pH) was 3.79, no significant change in HACCs was observed in Parental or S2 cells when compared with recordings at pH 7.45 (data not shown). These results suggest that HACCs generated by ClC-2 are affected by acidic extracellular pH, and the magnitude of the HACCs correlates with the level of ClC-2 expression.
Figure 3.
Hyperpolarization-activated chloride currents in parental cells and cells overexpressing ClC-2 are pH-dependent. Current–voltage plots of HACCs generated by parental and S2 cells at four different bath pH values (n = 3–10 for each pH value tested). Error bars indicate the standard error of the mean. Asterisks indicate a significant difference (P < 0.05) in current amplitude at −160 mV from pH 7.45 by anova and Bonferroni ad hoc tests.
The Anionic Selectivity and Inhibitor Sensitivity of the pH-Dependent Currents in Cells Expressing Endogenous Levels of ClC-2 and Overexpressing ClC-2 Are the Same.
The relative magnitude of hyperpolarization-activated currents in bath solutions containing different anions was examined at pH 3.8 (Fig. 4). For S1 cells expressing endogenous levels, HACCs were robust in symmetrical Cl− with a reversal potential (Erev) of −2.7 ± 2.2 mV (n = 3), less robust in I− with an Erev of −24.2 ± 6.7 mV (n = 3), and minimal in Glu− with an estimated Erev of −45 to −50 mV (n = 3; Fig. 4). Parental cells demonstrated the same selectivity series and reversal potentials for each anion as the S1 cells (data not shown). For overexpressing S2 cells, HACCs were highest in symmetrical Cl− with an Erev of −1.6 ± 0.8 mV (n = 3), less robust in I− with an Erev of −26.7 ± 9.9 mV (n = 3), and least robust in Glu− with an estimated Erev of −43 to −48 mV (n = 3; Fig. 4). These results indicate that HACCs in cells expressing low and high levels of ClC-2 have the same relative anion selectivity; Cl− > I− > Glu.
Figure 4.
pH-dependent hyperpolarization-activated currents in cells expressing endogenous levels of ClC-2 (S1) and cells overexpressing ClC-2 (S2) are chloride-selective. Current–voltage (I–V) plots of HACCs from S1 and S2 cells in Cl−, I−, and Glu− with an extracellular pH of 3.8 (n = 3 for each). Error bars indicate the standard error of the mean. Asterisks indicate a significant difference (P < 0.05) from the Cl− I–V plot by anova and Bonferroni tests.
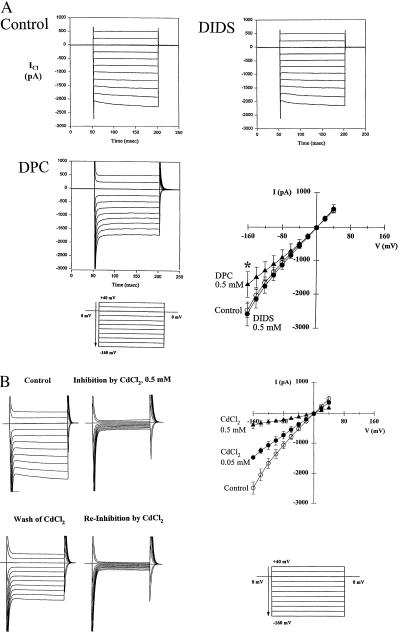
The pH-dependent currents of Parental and S2 cells showed similar responses to a variety of inhibitors. In overexpressing S2 cells at physiological pH (7.45), DIDS (0.5 mM) had no significant effect on HACCs, whereas DPC (0.5 mM) partially inhibited the HACCs at hyperpolarizing voltages in a manner that eliminated the inward rectification of the currents as illustrated in the I–V plot (Fig. 5A). HACCs recorded from Parental cells stimulated with extracellular acidic pH (3.8) were also insensitive to DIDS and partially inhibited at hyperpolarizing voltages by DPC (data not shown). It has been reported that chloride currents activated by hyperpolarization in human colon carcinoma (T84) cells are inhibited by Cd2+ (15). Because T84 cells express high levels of ClC-2 RNA (Fig. 1B), we decided to test whether HACC currents were affected by divalent metal cations. Chloride currents were recorded from S2 cells at physiological pH (7.45) in the absence and presence of a panel of cations including CdCl2, ZnCl2, NiCl2, LaCl2, and BaCl2. CdCl2 blocked the ClC-2-like HACCs in a dose-dependent manner with 0.5 mM CdCl2 abolishing the currents in a reversible fashion (Fig. 5B). CdCl2 was the most potent inhibitor, whereas LaCl3, ZnCl2, and NiCl2 produced only a partial block and Ba2Cl was ineffective (data not shown). HACCs generated by Parental cells at an extracellular pH of 3.8 were also blocked fully by 0.5 mM CdCl2 (data not shown).
Figure 5.
Inhibition by DPC and cadmium of HACCs generated in cells overexpressing ClC-2. (A) Representative current records illustrating the effect of DIDS (0.5 mM) and DPC (0.5 mM) and I–V plot of mean values ± SEM of S2 cells to DIDS (solid circles; n = 5) and DPC (solid triangles; n = 6). Currents were insensitive to DIDS whereas DPC inhibited the HACCs at hyperpolarizing voltages only, eliminating the rectification of the I–V plot. Asterisk indicates significant difference (P < 0.05) from control (open circles; n = 5) by paired Student’s t test. (B) Representative current recordings illustrating the reversible inhibitory effect of CdCl2 (0.5 mM), and I–V plots summarizing HACCs generated in the presence of 0.5 mM CdCl2 (solid triangles; n = 6) or 0.05 mM CdCl2 (solid circles; n = 6) in the bath solution. Asterisks indicate a significant difference (P < 0.05) from control (open circles; n = 6) by paired Student’s t test.
Expression of Antisense ClC-2 Reduces pH-Dependent Cl− Currents in Parental IB3-1 Cells.
To confirm that the currents potentiated by acidic bath pH in Parental cells were generated by ClC-2 channels, IB3-1 cells were created that had stably integrated plasmid containing the ClC-2 cDNA in an antisense orientation. Southern blot analysis of genomic DNA indicated that cell lines AS1, AS2, and AS3 each had multiple copies of the intact vector (Fig. 6A). Lysates of Parental and AS3 cells were analyzed for ClC-2 expression by Western blotting by using chicken anti-rat ClC-2 polyclonal antisera (8). Consistent with previous studies using this antisera, three bands corresponding to protein of molecular mass 80–89 kDa were detected in Parental cells (Fig. 6B, first lane). Prior studies have shown that the two larger bands (85 and 89 kDa) correspond to ClC-2 protein fragments whereas the smallest is nonspecific (8). Western blot analysis of an equal amount of AS3 cell lysate identified the smaller nonspecific 80-kDa band but ClC-2-specific bands of 85 and 89 kDa were not detected (Fig. 6B, second lane). The same result was obtained on two separate occasions with the investigator (C.J.B.) blinded to the identity of the cell lines on each occasion. The magnitude of HACCs in AS-3 cells was significantly lower than in Parental cells (Fig. 6C). ClC-2 Cl− currents were observed in all Parental cells with a mean current of 2,468 ± 234 pA whereas AS3 cells had a significantly lower mean current of 1,175 ± 226 pA. In 8 of 11 AS3 cells, less current was observed than the mean current observed in parental cells, and in two recordings no current was detected. One AS3 cell had currents that were greater than the mean of parental cells. Taken together, these data show that reduction in ClC-2 expression in IB3-1 cells resulted in lower pH-augmented hyperpolarization-activated whole-cell Cl− currents.
Figure 6.
Characterization of CF airway epithelial cells with antisense-mediated reduction of ClC-2. (A) Autoradiogram of genomic DNA from parental IB3-1 cells, and cell lines AS1, AS2, and AS3 that were transfected with the pBK antisense hClC-2 vector, digested with NsiI, and hybridized with a 0.5-kb ClC-2 cDNA probe. Clones AS1, AS2, and AS3 had intensely hybridizing fragments of 4.3 kb (arrow), consistent with the presence of multiple intact copies of the RSV promoter and antisense ClC-2 cDNA as shown in the map (Lower). Open arrow indicates endogenous ClC-2 genes. Solid boxes and arrows represent the RSV promoter and direction of transcription, and shaded boxes indicate the location of the cDNA probe relative to ClC-2. (B) Western blot of equal quantities (40 μg) of lysates from Parental and AS3 cells immunoblotted with chicken anti-rat ClC-2 antisera. Three bands were detected in the Parental cell lysate (first lane); two bands correspond to ClC-2 (85 and 89 kDa) and the third (80 kDa) is nonspecific (8). Both the 85 and 89 kDa forms of ClC-2 were severely reduced in the AS-3 cell line. Only the nonspecific band (80 kDa) was detected in the lysate of the AS3 cells (right lane). (C) I–V plots of mean currents ± SEM generated by Parental (open circles; n = 11) and AS-3 (open triangles; n = 11) cells in an acidic bath (pH 3.8) (Left). Right shows current values of individual cells at −160 mV (parental, open circles; AS3, solid circles) and mean current (circles with vertical bars, indicating SEM). The mean currents of the Parental and antisense cells differ significantly (P < 0.05) by anova and Bonferroni tests.
Acidic Extracellular pH and Hypotonicity Elicit Significant Cl− Efflux via ClC-2 Chloride Channels.
To determine whether changes in extracellular pH alone could initiate chloride transport via ClC-2 channels, we performed 36Cl− efflux studies on overexpressing cells (S2 and S3) and cells with endogenous expression of ClC-2 (Parental and S1). Acidic pH (3.6) stimulated 36Cl− efflux rates in all cells lines (Table 1). However, acidic pH-stimulated rates of 36Cl− efflux of overexpressing (S2 and S3) cells were significantly greater than those of Parental and S1 cells, and the duration of the responses was longer (Table 1). Alkaline pH (11.2) did not stimulate or reduce 36Cl− efflux in any of the cell lines (data not shown). A variety of other stimuli were tested, including hypotonic cell swelling (50% dilution of the bath osmolality with distilled water), cyclic AMP agonists (CPT-cAMP with forskolin), and the Ca2+ ionophore ionomycin (to increase [Ca2+]i). The response to hypotonic swelling was significantly longer in duration in cells overexpressing ClC-2 (S2 and S3) when compared with Parental and S1 cells (Table 1). This prolonged response to hypotonicity suggested a role for ClC-2 Cl− channels in swelling-induced 36Cl− efflux because the responses correlated with the level of hClC-2 expression. In contrast, cyclic AMP did not stimulate the rate of 36Cl− efflux in Parental cells, or in either of the overexpressing clones (data not shown). Ionomycin-mediated 36Cl− efflux responses were similar in magnitude and duration in overexpressing and endogenously expressing cells, consistent with the activity of an independent Ca2+-activated chloride channel (data not shown).
Table 1.
36Cl− efflux rates of human CF airway apithelial cells expressing endogenous levels (Parental and S1) or high levels (S2 and S3) of CIC-2 in response to acidic or hypotonic extracellular solutions
| Cell type | Before agonist | After agonist
|
|||
|---|---|---|---|---|---|
| 15 sec | 30 sec | 45 sec | 60 sec | ||
| Acidic | |||||
| Parental (15) | 28.36 ± 1.68 | 36.69 ± 5.37 | 32.18 ± 3.62 | 26.37 ± 3.71 | 24.85 ± 3.90 |
| S1 (21) | 24.55 ± 2.35 | 29.11 ± 1.98 | 28.07 ± 2.62 | 28.29 ± 2.51 | 28.61 ± 6.66 |
| S2 (18) | 25.71 ± 1.30 | 40.34 ± 2.15* | 35.00 ± 1.46* | 32.93 ± 1.79* | 22.47 ± 1.64 |
| S3 (15) | 28.79 ± 1.79 | 38.38 ± 2.1* | 37.92 ± 2.17* | 29.61 ± 4.17 | 22.57 ± 2.38 |
| Hypotonic | |||||
| Parental (12) | 28.29 ± 1.79 | 49.90 ± 2.66 | 85.71 ± 9.44 | 33.23 ± 4.94 | 19.64 ± 1.81 |
| S1 (12) | 19.93 ± 5.21 | 53.85 ± 5.77 | 72.66 ± 5.91 | 17.94 ± 3.30 | 15.31 ± 4.75 |
| S2 (12) | 21.22 ± 1.62 | 50.09 ± 4.07 | 73.35 ± 6.18 | 37.08 ± 4.69* | 28.75 ± 3.72* |
| S3 (12) | 21.89 ± 4.17 | 40.4 ± 4.12 | 83.21 ± 3.69 | 64.88 ± 2.17* | 23.05 ± 1.67 |
Data shown are the mean rates of 36Cl− efflux per minute ± standard error of the means for (n) experiments. Boldface type indicates 36Cl− efflux rates that were significantly greater (P ≤ 0.05) than the rate before application of agonist. An asterisk indicates time points at which the 36Cl− efflux rates of ClC-2 overexpressing cells (S2 and S3) were significantly greater (P < 0.05) than cells expressing endogenous levels of ClC-2 (Parental and S1) using the paired Student’s t-test.
DISCUSSION
Dysfunction of a cAMP-activated apical membrane chloride channel in epithelia produces the CF phenotype (1, 2). In this study, we explored the feasibility of restoring a path for chloride transport in CF epithelia by using a different channel. The ClC-2 channel was selected because its localization (9) and a number of functional characteristics are similar to those of CFTR (10). However, ClC-2 is expressed ubiquitously (10) and its mRNA transcript was present in every human cell line tested. We therefore selected the CF airway epithelial cell line IB3-1 because it lacked functional CFTR and displayed a low level of endogenous ClC-2 expression. IB3-1 cells overexpressing human ClC-2 generated robust HACCs at physiologic extracellular pH (7.4). Both parental and overexpressing cells generated HACCs, and we believe that these currents can be attributed to ClC-2 because of the following reasons. First, HACCS in both cell types had the same I–V relationship, anionic selectivity, and inhibitor profile. Second, the magnitude of HACCs correlated with increased expression of ClC-2. Third, and perhaps most convincingly, IB3-1 cell lines stably transfected with an antisense ClC-2 construct had reduced ClC-2 protein and significantly reduced HACCs. Together, these observations indicate that ClC-2 channels are the molecular basis for pH-dependent hyperpolarization-activated chloride currents in IB3-1 cells.
The properties of the ClC-2 currents in IB3-1 cells matched those of HACCs characterized in the T84 colonic tumor cell line (15). These cells have been used extensively for study of CFTR function and express ClC-2 at high levels (Fig. 1B). ClC-2 channels have also been identified by immunocytochemistry in pig pancreatic acinar cells (16) and by RNA in situ hybridization in pyramidal cells of the hippocampus (17). Both cell types displayed inwardly rectified HACCs (16–18). In the pancreatic cells, HACCs could be activated by extracellular hypotonicity and displayed a comparable anionic selectivity to human ClC-2. Thus, ClC-2 channels in mammalian cells are associated with chloride currents that have a consistent profile; activation by hyperpolarizing voltage and by cell swelling, inward rectification, Cl− ≥ Br− > I− selectivity, and inhibition by divalent cations.
Expression of ClC-2 channels in Xenopus oocytes has been associated with two different profiles. Using two-microelectrode recordings of intact oocytes, Jentsch and colleagues have shown that currents attributed to rat ClC-2 exhibit properties that are quite similar to those reported for ClC-2 channels in mammalian cells (10, 19, 20). Cuppoletti and coworkers isolated a cDNA encoding a protein termed ClC-2G from a rabbit gastric library that displays a high degree (>90%) of amino acid similarity to human ClC-2 and to rat ClC-2 (21). Injection of Xenopus oocytes with in vitro-transcribed RNA from a truncated version of this cDNA missing the canonical methionine was associated with anionic currents in reconstituted oocyte membrane vesicles (21). The characteristics of these channels were different from the properties of rat ClC-2 studied by two-microelectrode recordings of intact oocytes. ClC-2 had a linear rather than inwardly rectifying I–V relationship and a different anionic selectivity, with iodide being more permeable than chloride (21). The same investigators have recently cloned a human cDNA from lung that they have named ClC-2G (22). Analysis of reconstituted membrane vesicles from oocytes injected with RNA transcribed from the human ClC-2G cDNA had similar properties to those reported for rabbit ClC-2G. It has been suggested that ClC-2G is a novel member of the ClC-2 family. However, human ClC-2G is identical at the nucleotide level to human ClC-2 (22). Probes derived from the human ClC-2 cDNA detect a single mRNA transcript in human tissues (11) and a single DNA fragment after restriction digestion of genomic DNA (Figs. 1A and 6A), indicating that a single gene encoding ClC-2 exists in humans (11). The different biophysical profile reported for ClC-2G may be a result of the expression system utilized. Reconstituted membrane vesicles from oocytes may contain intracellular membranes with incompletely processed forms of ClC-2 or endogenous chloride channels that have properties distinct from mature ClC-2 inserted into the cell membrane.
Can ClC-2 channels provide an alternative pathway for chloride conduction in CF patients? Immunocytochemical studies indicate that ClC-2 is present the apical membranes of epithelial cells lining the small airways, the predominant site of CF lung disease (8, 9). Studies of ClC-2 expression reveal that it is expressed at high levels in the airway cells in utero and is down-regulated after birth, remaining at a low level of expression in the adult airways (8, 22). This pattern of expression suggests that ClC-2 may be involved in fluid secretion in the developing fetal lung. However, it is not clear whether the channel is expressed at sufficient levels postnatally to provide substantial chloride transport in the airways. We have demonstrated that hypotonicity and low pH can elicit a significant chloride efflux via ClC-2 in parental IB3-1 cells that express low mRNA levels of this channel. Determining whether primary airway epithelial cells produce a similar response should be the subject of future study. Activation of ClC-2 in airway cells in vivo presents a second obstacle. Although drastic changes in extracellular pH are not feasible, it may be possible to select pharmacologic agents that can activate ClC-2 channels by using cell lines created here. Elucidation of the mechanism of pH activation of ClC-2 channels may aid the search for such agents. In summary, this study illustrates that chloride conduction in airway cells devoid of functional CFTR can be increased by manipulation of an endogenous channel.
Acknowledgments
This work was supported by National Institutes of Health Specialized Center for Organized Research Grant DK48977 (G.R.C. and W.B.G.), HL48274 (P.L.Z.), and DK44003 (G.R.C.) and grants from the CF Foundation (P.L.Z., W.B.G., and G.R.C.).
ABBREVIATIONS
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- I–V
current–voltage
- HACC
hyperpolarization-activated Cl− current
References
- 1.Welsh M J, Tsui L, Boat T F, Beaudet A L. In: Cystic Fibrosis. 7th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. Vol. 127. New York: McGraw–Hill; 1995. pp. 3799–3876. [Google Scholar]
- 2.Cutting G R. In: Cystic Fibrosis. Rimoin D L, Connor J M, Pyeritz R D, editors. Vol. 131. New York: Churchill Livingstone; 1997. pp. 2685–2717. [Google Scholar]
- 3.Fitzsimmons S C. Cystic Fibrosis Foundation Patient Registry 1996 Annual Report. Bethesda, MD: Cystic Fibrosis Foundation; 1997. , 18 pp. [Google Scholar]
- 4.Snouwaert J N, Brigman K K, Latour A M, Malouf N N, Boucher R C, Smithies O, Koller B H. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 5.Colledge W H, Abella B S, Southern K W, Ratcliff R, Jiang C, Cheng S H, MacVinish L J, Anderson J R, Cuthbert A W, Evans M J. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 6.Clarke L B, Grubb B R, Yankaskas J R, Cotton C U, McKenzie A, Boucher R C. Proc Natl Acad Sci USA. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson M P, Sheppard D N, Berger H A, Welsh M J. Am J Physiol. 1992;263:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- 8.Murray C B, Morales M M, Flotte T R, McGrath-Morrow S A, Guggino W B, Zeitlin P L. Am J Resp Cell Mol Biol. 1995;12:597–604. doi: 10.1165/ajrcmb.12.6.7766424. [DOI] [PubMed] [Google Scholar]
- 9.Murray C B, Chu S, Zeitlin P L. Am J Physiol. 1996;15:L829–L837. doi: 10.1152/ajplung.1996.271.5.L829. [DOI] [PubMed] [Google Scholar]
- 10.Thiemann A, Grunder S, Pusch M, Jentsch T J. Nature (London) 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 11.Cid L P, Montrose-Rafizadeh C, Smith D I, Guggino W B, Cutting G R. Hum Mol Genet. 1995;4:407–413. doi: 10.1093/hmg/4.3.407. [DOI] [PubMed] [Google Scholar]
- 12.Zeitlin P L, Lu L, Hwang T C, Rhim J, Craig R, Cutting G R, Stetton G, Kieffer K A, Guggino W B. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 13.Cutting G R, Antonarakis S E, Buetow K H, Kasch L M, Rosenstein B J, Kazazian H H., Jr Am J Med Genet. 1989;44:307–318. [PMC free article] [PubMed] [Google Scholar]
- 14.Schwiebert E M, Flotte T R, Cutting G R, Guggino W B. Am J Physiol (Cell) 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- 15.Fritsch J, Edelman A. J Physiol. 1996;490:115–128. doi: 10.1113/jphysiol.1996.sp021130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carew M A, Thorn P. Pflügers Arch. 1996;433:84–90. doi: 10.1007/s004240050252. [DOI] [PubMed] [Google Scholar]
- 17.Smith R L, Clayton G H, Wicox C L, Escudero K W, Staley K J. J Neurosci. 1995;15:4057–4067. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staley K. J Neurophysiol. 1994;72:273–284. doi: 10.1152/jn.1994.72.1.273. [DOI] [PubMed] [Google Scholar]
- 19.Gründer S, Thiemann A, Pusch M, Jentsch T J. Nature (London) 1992;360:24–31. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- 20.Jordt S, Jentsch T J. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinowska D H, Kupert E Y, Bahinski A, Sherry A M, Cuppoletti J. Am J Physiol (Cell) 1995;268:C191–C200. doi: 10.1152/ajpcell.1995.268.1.C191. [DOI] [PubMed] [Google Scholar]
- 22.Sherry A M, Stroffekova K, Knapp L M, Kupert E Y, Cuppoletti J, Malinowska D H. Am J Physiol. 1997;273:C384–C393. doi: 10.1152/ajpcell.1997.273.2.C384. [DOI] [PubMed] [Google Scholar]