Abstract
Neurotrophic signaling pathways have been implicated in the maintenance of the mesolimbic dopa-mine system, as well as in changes in this system induced by chronic morphine exposure. We found that many of these signaling pathway proteins are expressed at appreciable levels within the ventral tegmental area (VTA) and related regions, although with substantial regional variation. Moreover, phospholipase Cγ1 (PLCγ1) was significantly and specifically up-regulated within the VTA by 30% following chronic exposure to morphine. PLCγ1 mRNA expression is enriched in dopaminergic neurons within the VTA; however, the up-regulation of PLCγ1 in this region was not seen at the mRNA level. In contrast to PLCγ1, insulin receptor substrate (IRS)-2, a protein involved in phosphatidylinositol 3-kinase signaling, and another putative IRS-like protein were significantly down-regulated within the VTA by 49 and 45%, respectively. Levels of several proteins within the Ras-ERK pathway were not altered. Regulation of neurotrophic factor signaling proteins may play a role in morphine-induced plasticity within the mesolimbic dopamine system.
Keywords: Opiates, Phospholipase Cγ, Neurotrophins, Tyrosine phosphorylation, Ventral tegmental area, Insulin receptor substrate
Neurotrophic factors (NTFs), best understood for their critical roles in the early development of the nervous system, have more recently been found to play important roles in the adult nervous system as well. NTFs can induce or maintain the growth, survival, and differentiation of neurons (Barde, 1989; Korsching, 1993; Lindsay et al., 1994). These biological activities give them the potential to mediate dynamic plasticity or persistent functional alterations of specific neuronal populations within the CNS. This has been demonstrated in models of neuronal stress and injury (Sauer et al., 1995), as well as activity-dependent synaptic plasticity (Levine et al., 1995; Rutherford et al., 1997).
Most, if not all, NTFs bind to receptors that activate protein tyrosine kinases, which in turn use a relatively small set of conserved signaling pathways. These receptors, through tyrosine autophosphorylation or phosphorylation of adapter proteins, create “docking sites” for proteins containing SH2 domains. This binding leads to the phosphorylation and activation of a set of intracellular signaling proteins. The best-characterized of these signaling pathways include one that contains phospholipase C (PLC) γ, which subsequently regulates the phosphatidylinositol pathway; another, termed the mitogen-activated protein (MAP) kinase pathway, which involves Ras, Raf, MAP–extracellular signal-regulated kinase (ERK) kinase (MEK), and ERK; and a third that leads to the activation of phosphatidylinositol 3-kinase (PI-3-K) and AKT (also known as protein kinase B) (Russell, 1995; Kaplan and Miller, 1997). PLCγ, in contrast to the other known forms of PLC, β andδ, is the only form known to be modulated by tyrosine phosphorylation. The PLCγ1 isoform has been shown to be widely expressed in brain (Ross et al., 1989), whereas PLCγ2, primarily found in hematopoietic cells, is restricted in the brain to the cerebellar vermis and the anterior pituitary (Tanaka and Kondo, 1994). PLCβ is regulated by G protein-coupled receptors, and the regulation of PLCδ remains uncertain (Rhee and Bae, 1997).
Two lines of evidence implicate NTFs and their signaling pathways in adaptive changes to drugs of abuse in the mesolimbic dopamine system. This system comprises the dopaminergic neurons of the ventral tegmental area (VTA) and their forebrain targets, including the nucleus accumbens (NAc). First, alterations observed in VTA neurons following chronic morphine exposure are suggestive of the changes seen in cultured neurons and in vivo following reduced NTF support. These changes include reductions in the size of cell body and neurites of VTA dopamine neurons (Sklair-Tavron et al., 1996), reduced tyrosine hydroxylase (TH) mRNA expression (Boundy et al., 1998), reduced levels of neurofilaments and axoplasmic transport from the VTA, and accumulation of TH protein within the VTA cell bodies (Nestler et al., 1996). Brain-derived NTF and neurotrophin-4, NTFs that have been shown both to support the survival of midbrain dopaminergic neurons in culture and to protect their dopaminergic phenotype in vivo following toxic insults, are able to oppose these effects of chronic morphine, whereas nerve growth factor and ciliary NTF, which do not support the dopaminergic phenotype, have no such effects (Berhow et al., 1995). Given these suggestive findings, our hypothesis is that some of the effects of chronic morphine on the VTA–NAc pathway are mediated via the down-regulation of endogenous NTF signaling in these brain regions. This down-regulation need not occur at the level of the NTFs themselves or their receptors, but may instead result from changes in the intracellular signaling elements downstream of receptor activation.
In a recent study, we failed to detect regulation of BDNF or neurotrophin-3 expression in the VTA after chronic morphine administration (Numan et al., 1998). Therefore, the goal of the present study was to explore this hypothesis further by examining the effect of chronic morphine administration on levels of NTF-associated signal transduction proteins in the mesolimbic dopamine system. We show that chronic exposure to morphine up-regulates levels of PLCγ in the VTA, an effect that was not observed in several other brain regions studied. In contrast, chronic morphine regulation was not observed for most of the other NTF signaling proteins examined. It is interesting that changes in the opposite direction were observed among putative elements of another, parallel, NTF signaling pathway.
MATERIALS AND METHODS
Morphine treatment
Rats used for protein isolation in this study were male Sprague–Dawley animals weighing 150–170 g obtained from CAMM (Wayne, NJ, U.S.A.). Regional expression data were obtained with drug-naive rats weighing 180–190 g. Morphine was administered chronically to rats with initial weights of 170–195 g. This treatment consisted of five daily subcutaneous implantations of single controlled-release pellets (75 mg of morphine base; National Institute on Drug Abuse) with the animal under light halothane anesthesia. Control rats received sham surgery. This morphine treatment paradigm has been shown to produce profound states of tolerance and dependence and to result in characteristic biochemical adaptations within the VTA and NAc. Rats were generally prepared in groups of 12 (six control and six morphine-treated). On day 6, the rats were killed by decapitation, and the brain regions of interest collected by rapid dissection in ice-cold artificial CSF and frozen on dry ice. The VTA and substantia nigra (SN) were dissected from 1-mm-thick coronal brain slices using a punch method with a 15-gauge needle, the NAc and dorsal striatum were dissected with a 12-gauge needle, and the hippocampus (HC) and frontal cortex (Cx) were obtained by gross dissection. To test the specificity of the opiate response, naltrexone (50 mg/kg; 50 mg/ml in an emulsion of light mineral oil/mannide oleate/saline) was administered subcutaneously before each morphine or sham treatment. This regimen has been shown to be required to block the development of morphine dependence as well as several morphine-induced biochemical adaptations in the mesolimbic dopamine system (Beitner-Johnson et al., 1993). To test the effect of acute morphine exposure, morphine sulfate (10 mg/kg) was administered subcutaneously, and animals were killed 30 min later, at which time many of the acute biochemical and behavioral effects of the drug are manifest (see Guitart and Nestler, 1989).
The opiate treatment paradigm used for the in situ hybridization experiments followed protocols previously described by Rasmussen et al. (1990). Sprague–Dawley rats (n = 8) weighing between 265 and 285 g were used for this study. For 5 days, once a day, rats (n = 4) were lightly anesthetized with halothane and implanted with a morphine pellet (75 mg of morphine base; National Institute on Drug Abuse) subcutaneously. Control rats (n = 4) were lightly anesthetized but not implanted with pellets. On day 6 all rats were anesthetized with an overdose of pentobarbital and decapitated. The brains were removed, rapidly frozen with dry ice, and processed as described below.
All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by our Institutional Animal Care and Use Committee.
Western blotting
Frozen brain samples were solubilized by probe sonication in a buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris (pH 7.4), 150 mM NaCl, 5 mM each EDTA and EGTA, 10 μg/ml each aprotinin and leupeptin, and 1 μg/ml pepstatin A. Protein concentration was determined in triplicate by the method of Lowry et al. (1956). From 5 to 100 μg of total protein was subjected to SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose [or polyvinylidene difluoride for insulin receptor substrate (IRS)-1] membranes for immunoblotting. Membranes were blocked in 5% milk for 1 h, incubated overnight in primary antibody, and then visualized with appropriate horseradish peroxidase-linked secondary antibodies, enhanced chemiluminescence (ECL; Amersham, Pis-cataway, NJ, U.S.A.), and Kodak (Rochester, NY, U.S.A.) XAR film. Luminescence of specific bands was quantified on an LKB Image Analyzer or on a Macintosh-based image analysis system with NIH Image software. The data, in arbitrary densitometry units, from control versus chronic morphine-treated rats were compared using an unpaired two-tailed Student’s t test.
The amount of protein loaded for each protein was determined by pilot experiments to lie within the linear range of detection (data not shown): 5 μg per lane was loaded for TH, PLCγ1, and ERK1 and 2, 20 μg per lane was used for Grb2 and Shc, and 100 μg per lane was used for PI-3-K, IRS-1, IRS-2, and Ras. These aliquots (10–40 μl) were obtained from the same sonicated punch samples, electrophoresed on identical gels, and immunoblotted at the same time under identical conditions. To assure that the morphine treatments resulted in the expected biochemical changes in each group of rats used, TH levels were measured in each set of rats, and sample groups were selected that exhibited the previously established induction of TH in the VTA. In these experiments, the average ± SEM increase in TH levels after chronic morphine treatment was 58 ± 12% ( p = 0.0064). In these same samples, levels of neuron-specific enolase and actin were unchanged.
TrkB levels were measured only in VTA. Because pilot experiments showed that reliable signals for this protein could not be obtained from western blots of whole VTA extracts, we used a method first to concentrate these proteins before western blotting. In this method, 200 μg of total protein in sonication buffer was diluted 15-fold into an identical buffer except that it contained 1% Triton X-100 instead of SDS. Thirty-five micro-liters of wheat germ agglutinin-agarose was added to each tube, and the mixture was rocked at 4°C for 1 h. The wheat germ agglutinin-agarose pellet was then washed extensively with the Triton X-100-containing buffer, and the eluted protein was subjected to SDS-polyacrylamide gel electrophoresis and immunodetection as above. In our laboratory, the yield of TrkB from brain tissues by this method approaches 100% with no detectable signal in the wheat germ agglutinin-agarose supernatant.
The following antibodies were used in these studies: TH, kindly supplied by John W. Haycock (Louisiana State University, New Orleans, LA, U.S.A.); TrkB, MEK1, and MEK2 from Transduction Laboratories (Lexington, KY, U.S.A.); ERK1, ERK2, Shc, and Grb2 from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.); PLCγ1, PI-3-K 85-kDa subunit, pIRS1 (against the baculovirus-expressed protein), IRS1-CT (anti-C terminus), IRS-2, and Raf from Upstate Biotechnology (Lake Placid, NY, U.S.A.); and Ras from Oncogene Sciences (Union-dale, NY, U.S.A.). Peroxidase-linked goat anti-rabbit, rabbit anti-mouse, and rabbit anti-rat antibodies were obtained from Vector (Burlingame, CA, U.S.A.).
Immunoprecipitations
For coimmunoprecipitation studies, pooled punches of VTA and SN were solubilized in 600 μl of the same Triton X-100-containing buffer as described above for diluting the TrkB samples. After solubilization, samples were centrifuged at 10,000 g for 5 min at 4°C, and the supernatants were collected. Samples were then assayed for protein concentration by the method of Lowry et al. (1956) and adjusted by dilution to 1 μg of protein/μl. Brain region lysate was divided into 200-μl aliquots, and immunoprecipitating antibody was added to each as indicated in the figure legends. This was incubated with rocking for 1 h at 4°C, and then the immunoprecipitate was collected on protein G-agarose beads. The beads were washed five times with 1 ml each of the Triton X-100 buffer. The immune complexes were then solubilized in 1 × protein electrophoresis reducing sample buffer and subjected to western blotting as described above.
Stereotaxic surgery
Adult male Sprague–Dawley rats (275–305 g; n = 5; CAMM) were used for the 6-hydroxydopamine (6-OHDA) lesion study. All rats received an injection of desipramine (25 mg/kg, i.p.) 30 min before surgery to protect norepinephrine terminals (Breese and Traylor, 1971). Rats (n = 4) were then anesthetized with pentobarbital (5 mg/100 g, i.p.; Abbott Laboratories, North Chicago, IL, U.S.A.) and received an injection of 6-OHDA (10 μg/μl in 0.9% NaCl and 0.2% ascorbic acid) stereotaxically placed into the right ascending medial forebrain bundle (MFB): AP −2.6, ML −2.0, DV −8.3 (1 μl/10 min), according to the coordinate system of Paxinos and Watson (1986). As a control for lesion specificity, one rat was injected with vehicle (1 μl of 0.9% NaCl and 0.2% ascorbic acid) stereotaxically placed into the MFB (same coordinates as above). Following a 2-week survival period, the rats were anesthetized with an overdose of pentobarbital and decapitated. The brains were quickly removed and frozen with dry ice.
In situ hybridization
Coronal sections (10 μm) throughout the rostrocaudal extent of the VTA were cut in a cryostat, thaw-mounted onto Super-frost Plus glass slides, and stored at −20°C until hybridization. These sections were processed for the in situ hybridization localization of PLCγ1 or TH mRNAs by using 35S-labeled cRNA probes as described previously (Numan and Seroogy, 1997; Seroogy and Herman, 1997). The VTA was identified by standard anatomical landmarks (Paxinos and Watson, 1986). In brief, the slide-mounted sections were brought to room temperature and then placed in 4% paraformaldehyde for 10 min. This was followed by washes in 0.1 M phosphate-buffered saline, 0.1 M phosphate-buffered saline containing 0.2% glycine, and 0.25% acetic anhydride in 0.1 M triethanolamine. The sections were then dehydrated with increasing concentrations of ethanol, delipidated in chloroform, and air-dried. Sections were hybridized overnight at 60°C in hybridization cocktail consisting of 1× Denhardt’s solution, 50% formamide, 0.15 mg/ml yeast tRNA, 40 mM dithiothreitol, 0.33 mg/ml denatured salmon sperm DNA, 10% dextran sulfate, 20 mM Tris-HCl, 1 mM EDTA, and the 35S-labeled cRNA probe at a concentration of 1.0 × 106 cpm/50 μl per slide. Sense and antisense cRNA probes were prepared by in vitro transcription using linearized DNA constructs in the presence of RNA polymerase (T7 or SP6) and 35S-UTP (New England Nuclear, Boston, MA, U.S.A.). The cDNA construct for PLCγ1 was prepared using PCR to published rat sequences and contains 1.0 kb from the SacI site at nucleotide 2,867 to the 3′end of the coding region. The rat TH cDNA construct (kindly provided by Esther L. Sabban, New York Medical College, Valhalla, NY, U.S.A.) results in a cRNA probe of 1.1 kb in length. For posthybridization treatment, sections were washed several times in 4× saline–sodium citrate (SSC; 1× SSC = 0.15 M NaCl and 0.015 M sodium citrate, pH 7.0) containing 10 mM sodium thiosulfate at 37°C. The sections were then incubated in ribonuclease A (0.05 mg/ml) for 30 min at 45°C. This was followed by several washes in decreasing concentrations of SSC (2×, 0.5×, and 0.1×) at 37°C. All but the final wash also contained 10 mM sodium thiosulfate. The sections were briefly rinsed in deionized water, dipped in 95% ethanol, and finally air-dried. To generate film autoradiograms, the sections were exposed to β-Max Hyperfilm (Amersham) for 18 h and 11–14 days for TH and PLCγ1, respectively. As controls for specificity, some sections were pretreated with ribonuclease A (0.05 mg/ml) for 30 min at 45°C before hybridization with the 35S-labeled cRNA probes. Some sections were also hybridized with PLCγ1 sense strand 35S-labeled riboprobes. No specific labeling was observed under any of these control conditions.
Film autoradiograms were analyzed by densitometry using NIH Image software to compare densities of hybridization of each probe between control- and morphine-treated VTAs. From each control and treated animal 10 measurements of hybridization density, also referred to as gray level, were taken. A paired background measurement was subtracted from each gray level, leading to a corrected gray level. Once a mean corrected gray level for VTA was attained for each animal, statistical analysis was performed. Statistical analysis included Student’s unpaired t test.
RESULTS
Regional variation in expression of PLCγ1 and other NTF signaling proteins
As a first step in studying the effect of chronic morphine administration on NTF signal transduction pathways, we examined the relative levels of several NTF-associated signaling proteins in representative brain regions. Most of the signaling proteins examined are thought to be ubiquitously expressed in neurons, but differential expression of these various proteins could reflect functionally relevant differences in NTF signaling capacity within specific brain regions. The anatomical focus of this study was on the elements of the mesolimbic dopamine system (VTA and the NAc), because of the role of these structures in drug addiction and the well-defined biochemical adaptations in this region caused by chronic exposure to drugs of abuse. We also included assessment of the SN and dorsal striatum, which make up the nigrostriatal dopamine system. These regions are morphologically and developmentally related to the mesolimbic dopamine system but are not implicated in drug addiction and do not show the same adaptations in response to chronic drug exposure. Cx and HC were selected for further comparison.
As expected, it was found that PLCγ1 (migrating at 145 kDa) and the other NTF signaling proteins examined are expressed in all brain regions studied. However, the various regions demonstrated different levels of expression of the proteins (Fig. 1). PLCγ1, PI-3-K, and IRS-2 all tended to be expressed at higher levels in more anterior structures. Relative to the level in the VTA (defined as 100%), PLCγ1 ranged from 168 ± 11% ( p < 0.05) in the NAc to 189 ± 32% ( p < 0.05) in Cx. With IRS-2, this difference was more pronounced, reaching levels of 235 ± 38% ( p < 0.05) and 263 ± 34% ( p< 0.01) the levels of VTA in HC and Cx, respectively. PI-3-K, on the other hand, demonstrated more selective enrichment in NAc [206 ± 40%, p = 0.054 (trend)] and Cx (223 ± 46%, p < 0.05). These selective regional variations in levels of the different proteins led to substantial differences in the relative ratios of these proteins, with a near inversion of the ratios of PLCγ1 and IRS-2 between SN and Cx. Although these data represent relative, not absolute, measures of protein levels within these regions, they suggest nonetheless possibly significant differences in signaling capacities among their respective pathways.
FIG. 1.
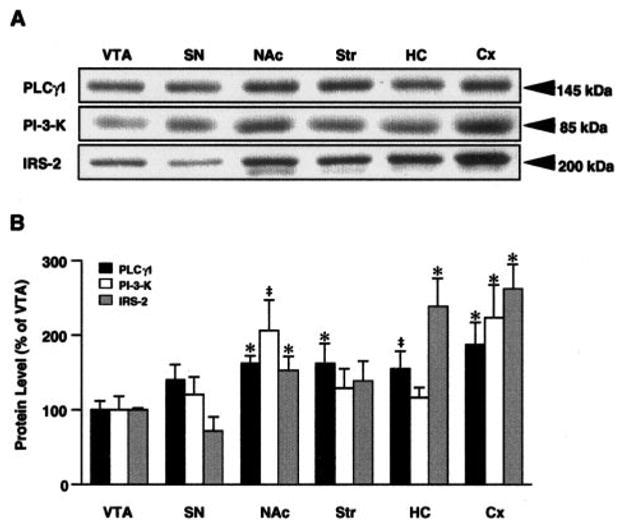
Relative expression of PLCγ1, PI-3-K, and IRS-2 in six brain regions: VTA, SN, NAc, striatum (Str), HC, and Cx. Brain regions were obtained by dissection from naive rats and extracts subjected to western blot analysis with the antibodies indicated. A: Representative lanes of autoradiograms derived from samples of individual rats. Each band shown was the dominant bandon the membrane, and the Mr is appropriate for each protein. B: Percent relative levels compared with VTA. Data are average ± SEM (bars) values from four rats in this analysis. *p ,< 0.05, ‡p = 0.05–0.07 (indicates a trend toward significance).
Levels of expression of several elements of the Ras–MAP kinase signaling pathway, including Ras, Raf, Grb2, and Shc, showed very little regional variation (data not shown). A detailed regional study of levels of ERK1 and 2 and of MEK has been published previously (Ortiz et al., 1995); the earlier finding that the ratio of ERK1 to ERK2 levels varies from more anterior to posterior structures was reproduced in the present investigation (data not shown).
Regulation of PLCγ1 and IRS proteins by chronic morphine administration
We next assessed the effect of chronic exposure to morphine on levels of PLCγ1 and other NTF signaling proteins within the VTA, NAc, and other brain regions. Levels of PLCγ1 were increased by 30 ± 10% ( p< 0.05) in the VTA by this treatment (Fig. 2). Naltrexone treatment concurrent with morphine completely abolished this effect (−2 ± 8% change from control, n = 6, p = 0.82), indicating that morphine regulation of PLCγ1 is achieved via activation of opioid receptors. Also, this effect was specific to the VTA; chronic morphine treatment did not alter levels of PLCγ1 in the other brain regions examined: Cx, 2 ± 11%, n = 9; HC, 4 ± 7%, n = 9; striatum, 3 ± 7%, n = 12; NAc, 11 ± 5%, n = 9; SN, 14 ± 9%, n = 18 ( p values all > 0.20). As a control, we studied morphine regulation of PLCδ, another major form of PLC in brain, in the VTA and SN. PLCδ does not contain an SH2 domain and is not thought to be directly involved in NTF signaling. In contrast to PLCγ1, this protein was found to be not regulated by chronic morphine in the VTA (Fig. 2). As seen in previous studies, levels of TH were significantly elevated within the VTA after chronic morphine administration, with no significant changes seen in the other brain regions (see Fig. 2 and Materials and Methods). PLCγ has not been found to be rapidly regulated at the protein level in response to activation in cell culture systems, unlike the protein products of immediate-early genes such as c-fos or enzymes rapidly degraded in response to activation such as protein kinase C. This suggests that the PLCγ regulation seen after morphine exposure is likely to be a chronic process. Indeed, we have found that acute morphine exposure did not alter levels of PLCγ1 immunoreactivity in the VTA (data not shown).
FIG. 2.
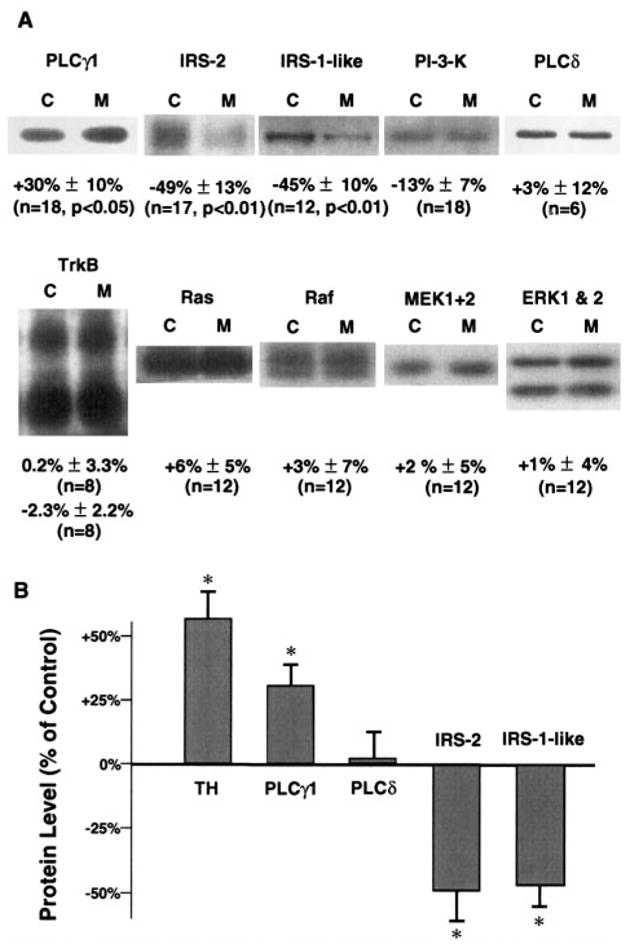
Chronic morphine selectively regulates PLCγ1 and IRS protein levels in the VTA. VTA samples from control or chronic morphine-treated rats were subjected to immunoblot analysis. A: Pairs of representative bands from autoradiograms at the known apparent molecular weights for each of the proteins listed, except for the IRS-1-like protein, which has not been previously reported. Each pair consists of a lane derived from a control (C) or morphine-treated (M) rat. Below each pair of bands is the percent change from the control value by morphine (mean± SEM) from the analyzed sets of proteins. Values of p > 0.10 are not given. Values of n indicate the number of rats in each treatment group. The apparent molecular sizes of the bands shown are as follows: PLCγ1, 145 kDa; IRS-2, 185 kDa; IRS-1-like protein, 205 kDa; PI-3-K, 85 kDa; PLCδ, 85 kDa; TrkB, 140 and 90 kDa; Ras, 21 kDa; Raf-1, 70–75 kDa; MEK1+2, 45 kDa; ERK1, 44 kDa; and ERK2, 42 kDa. B: Graphical representation of the changes in TH (see Materials and Methods), PLCγ1, PLCδ, IRS-2, and IRS-1-like protein in VTA by chronic morphine. Data are mean ± SEM (bars) values. *p < 0.05.
Chronic exposure to morphine was found to also decrease levels of IRS and IRS-like proteins in the VTA. IRS-2 was reduced by 49 ± 13% ( p < 0.01) in this brain region (Fig. 2) and not in any other brain region examined: Cx, 2 ± 11%, n = 9; HC, 4 ± 7%, n = 9; striatum, 9 ± 5%, n = 6; NAc, 12 ± 11%, n = 11; SN, −17 ± 9%, n = 11 ( p values all > 0.20). In addition, down-regulation of IRS-2 in the VTA was also blocked by concurrent naloxone treatment (−3 ± 9%, n = 5, p = 0.75) and not seen in response to acute morphine administration (data not shown).
A prominent 205-kDa band revealed by an anti-IRS-1 antiserum was also strongly down-regulated by chronic morphine treatment (−45 ± 10%, p < 0.01). This antiserum was raised against the entire IRS-1 protein expressed in baculovirus and may be expected to cross-react with conserved epitopes on related proteins. The 205-kDa band migrated at a higher Mr than expected for actual IRS-1 but did coprecipitate in PI-3-K immunoprecipitates (Fig. 3A), as would be expected of an IRS protein. A more specific anti-C-terminal IRS-1 antibody did not recognize any proteins at this molecular size in any of the brain regions examined. In addition, this 205-kDa, morphine-regulated band was seen only in VTA and SN among the regions examined (Fig. 3B). Although the identity of this IRS-like protein remains unknown, it could represent a splice variant of IRS-1 or a novel IRS protein antigenically related to IRS-1, with a restricted pattern of expression. It is interesting that the IRS-1-like protein was also significantly down-regulated in the SN to a similar degree as in VTA (−63 ±8%, n = 7, p < 0.01). Finally, there was a trend for chronic morphine to decrease levels of PI-3-K in the VTA, although this effect was small in magnitude and did not achieve statistical significance (−13 ± 7%, p = 0.34; Fig. 2).
FIG. 3.
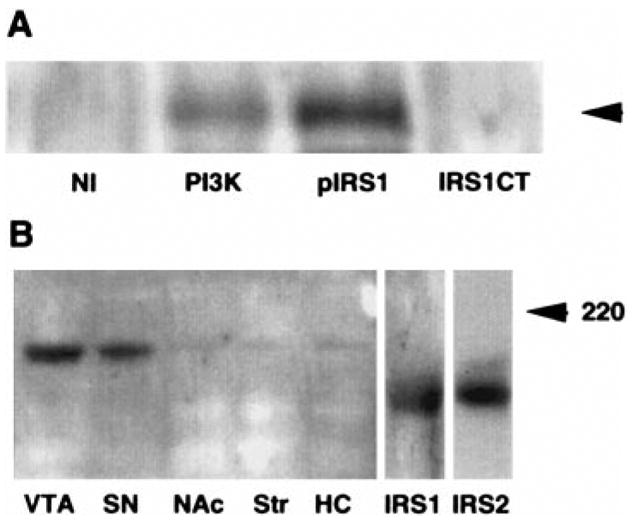
An IRS-1-like protein appears to be associated with PI-3-Ks in ventral midbrain. A: Immunoprecipitates were prepared from solubilized, pooled ventral midbrain samples as described in Materials and Methods. Equal aliquots were immuno-precipitated with 1 μg of control mouse IgG (NI), 1 μg of mouse monoclonal anti-p85 PI-3-K IgG (PI3K), 5 μg of rabbit polyclonal anti-IRS-1 IgG (pIRS1), or 5 μg of a highly specific rabbit polyclonal anti-C-terminal IRS-1 IgG (IRS1CT). Immunoprecipitates were then subjected to SDS-polyacrylamide gel electrophoresis. The membrane was probed with pIRS1. The arrow indicates a band of apparent molecular size of 205 kDa, the same apparent molecular size as the protein regulated in the VTA by chronic morphine (Fig. 2). This IRS-1-like protein was immunoprecipitated by the polyclonal anti-IRS-1 antibody, pIRS1, as well as by an anti-PI-3-K antibody. This latter finding confirms the association of this protein with PI-3-K in ventral midbrain extracts. In contrast, a highly specific anti-C-terminal IRS-1 antibody, IRS1-CT, was unable to precipitate this protein in these brain extracts, although it could readily immunoblot and immunoprecipitate IRS-1 in liver homogenates (data not shown). B: This IRS-1-like protein is highly enriched in ventral midbrain nuclei. A western blot using pIRS1 on extracts from five brain regions is shown. The only major band detected is at 205 kDa. For comparison, liver IRS-1 (Mr 180) and hippocampal IRS-2 (Mr 185) are also shown (IRS1 and IRS2, respectively). The images were aligned by the 220-kDa (indicated) and 97-kDa molecular size markers. The results shown in each panel are representative of two independent experiments.
In contrast to PLCγ1, IRS proteins, and PI-3-K, levels of expression of several proteins in the Ras–ERK pathway were not significantly changed by chronic morphine treatment in the VTA. This includes TrkB (both the full-length, catalytic form and the truncated form), Grb2, Shc, Ras, Raf, MEK1 and MEK2, and ERK1 and 2 (Fig. 2).
Distribution of PLCγ1 mRNA in ventral midbrain
As a first step toward investigating the mechanism of regulation of PLCγ1 protein in VTA in response to chronic morphine, we examined the expression of PLCγ1 mRNA within the ventral midbrain. We found that PLCγ1 mRNA was expressed at moderate levels in the VTA and SN pars compacta. Scattered cells, more lightly labeled for PLCγ1 mRNA, were seen in the pars reticulata of the SN and adjacent structures. A marked reduction in PLCγ1 message in the VTA and SN was observed following lesioning with 6-OHDA, demonstrating that a majority of this expression is in dopaminergic neurons (Fig. 4). Chronic morphine exposure did not lead to an increase in PLCγ1 mRNA expression in the VTA, and, in fact, we observed no change except a small, nonsignificant decrease (−13%, p = 0.25; Fig. 5).
FIG. 4.
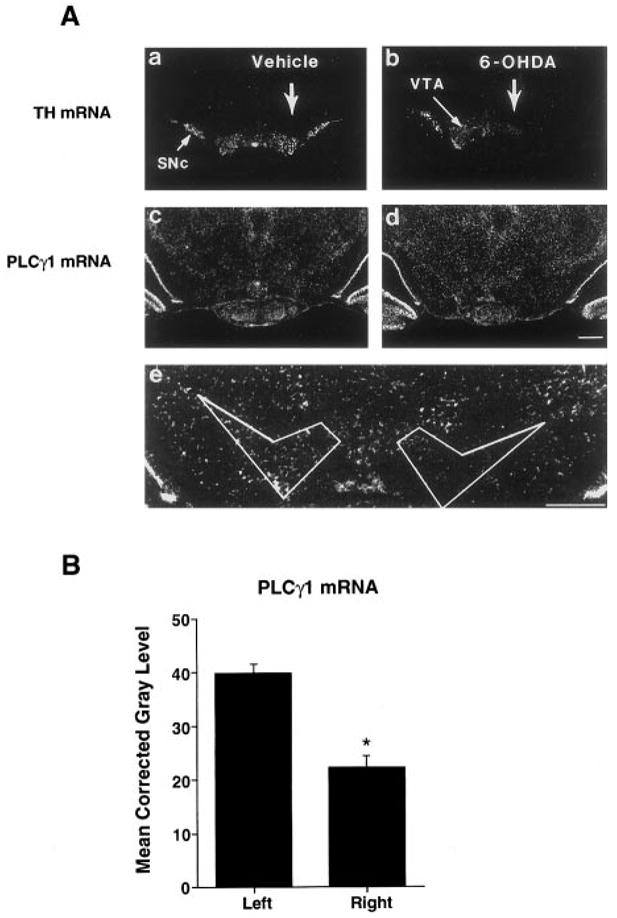
A: Modulation of TH (a and b) and PLCγ1 (c and d) mRNA expression in the ventral midbrain following unilateral injection of vehicle (a and c) or 6-OHDA (b and d) into the right MFB. Note the decrease in both TH and PLCγ1 mRNA levels on the side of the 6-OHDA lesion as compared with the unlesioned side (b and d). In contrast, there appeared to be no changes in TH or PLCγ1 mRNA expression following injection of vehicle into the right MFB (a and c), compared with the contralateral control. e: Higher magnification of the tissue section shown in (d) and the region subjected to quantitative analysis. This area was defined by TH expression and anatomic landmarks. SNc, SN pars compacta. Bar = 1,000 μm. The results shown are representative of four rats receiving unilateral 6-OHDA lesions. B: Densitometric analysis of film autoradiograms confirmed the reduction of PLCγ1 mRNA content in the VTA and SNc (−43.8 ± 5.3%, mean ± SEM, p < 0.001, n = 4).
FIG. 5.
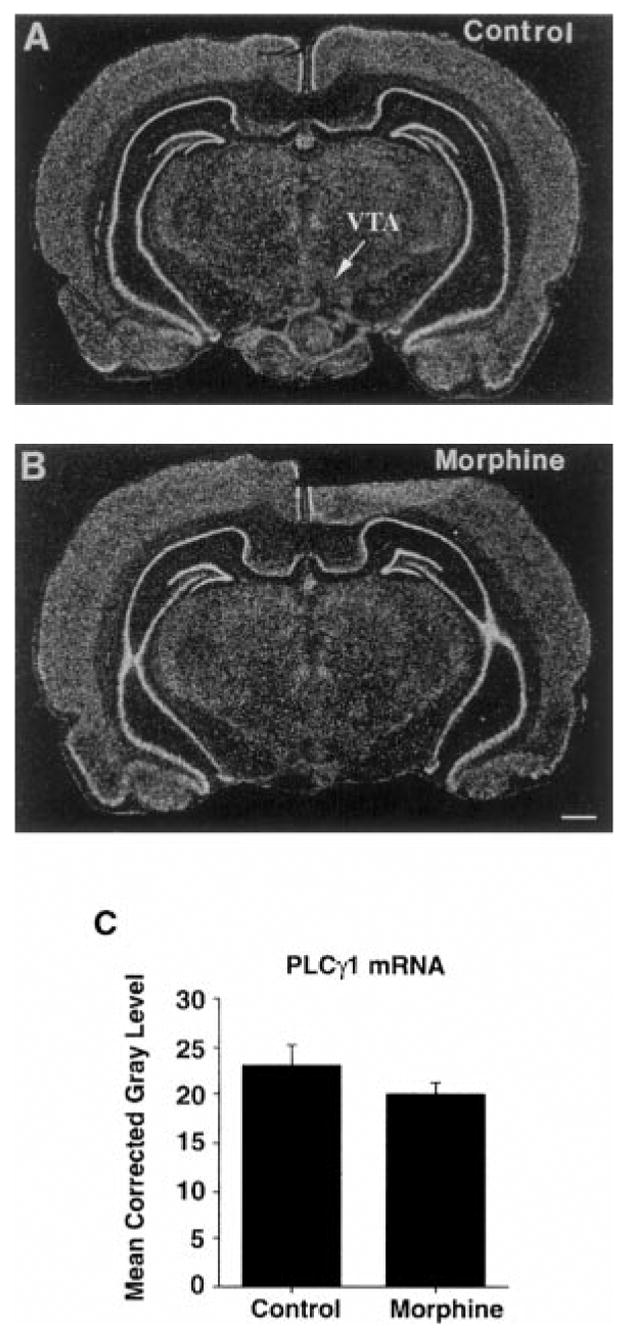
Effect of chronic morphine treatment on PLCγ1 mRNA expression in the VTA shown in film autoradiograms demonstrating expression of PLCγ1 mRNA in the VTAs of (A) control and (B) morphine-treated rats. C: Densitometric analysis of film autoradiograms revealed no significant alterations in PLCγ1 mRNA levels. Data are mean ± SEM (bars) values (n = 4). Bar = 1,000 μm.
DISCUSSION
The present study demonstrates that several components of NTF signaling pathways are present within the VTA and that there is regional specificity to the relative abundance of the various proteins. Much previous research has characterized the expression of NTFs and their tyrosine kinase-linked receptors in the ventral midbrain, but surprisingly little attention has focused on the intracellular signaling proteins activated by NTFs within this brain region. Because these proteins transduce the extracellular NTF signals to cell nuclei, cytoskeleton, and other intracellular compartments, their levels may exert as much influence on biochemical composition and functional activity as levels of the neurotrophins and their receptors themselves. Although alterations in neurotrophins or their receptors would alter the total amplitude of the neurotrophic signal, changes in the levels of specific intracellular signaling proteins would be expected to affect the balance of specific biochemical end points and alter the basic phenotypic characteristics of the neurons. Furthermore, because many CNS neurons receive redundant support from multiple NTFs (Snider, 1994), altered levels of a common downstream signaling protein might in some cases have a far greater functional impact on the neurons than changes in levels of individual NTFs or receptors.
PLCγ1 is an example of such a potentially critical intracellular signaling protein. It is part of the proximal signal transduction mechanism activated by several tyrosine kinase receptors (Rhee and Choi, 1992), including those with potent trophic effects on midbrain dopaminergic neurons, such as brain-derived NTF, neurotrophin-4 (Zirrgiebel et al., 1995), and presumably glial cell line-derived NTF. The embryonic lethality of the PLCγ1 knockout mouse (Ji et al., 1997) indicates that at least some of the enzyme’s roles cannot be fulfilled by other PLC family members or other proteins. PLCγ catalyzes the production of diacylglycerol and inositol trisphosphate, which are known to serve as potent second messengers in the regulation of cell function (Berridge, 1993). PLCγ1 is the only known tyrosine kinase-regulated PLC in brain and as such probably serves a substantially different functional role than other PLCs. Our demonstration that PLCγ1 is significantly expressed and regulated in the ventral midbrain suggests that PLCγ1 may play a particularly important role in the survival and phenotypic regulation of neurons in this region.
The results of this study, in conjunction with the findings of several recent investigations, establish the ways in which the three major signaling pathways for NTFs are altered in the VTA by chronic morphine administration. First, levels of PLCγ1 are up-regulated in the VTA, with no change in another form of PLC, PLCδ. Second, there appears to be a down-regulation of the PI-3-K pathway, with trends toward lower levels of PI-3-K itself and more dramatic reductions in levels of IRS-2 and of a putative IRS-like protein that is enriched within the VTA. This reduction in IRS linker proteins could partially uncouple PI-3-K from receptor tyrosine kinase activation. Third, there is no change in the Ras–Raf–ERK pathway, at least in terms of total levels of the individual proteins. A previous study did find higher levels of activated ERK (sustained via higher levels of ERK phosphorylation) in the VTA but no change in total ERK immunoreactivity (Berhow et al., 1996). Finally, results of the present study and a recent report (Numan et al., 1998) indicate the lack of alterations in levels of expression of certain NTFs themselves or in their Trk receptors in the VTA after chronic morphine administration. It is interesting that most of these morphine-induced changes in the VTA are specific to this brain region, although it remains unknown whether they reflect direct effects of morphine on VTA neurons or effects mediated indirectly via altered afferent inputs. In any event, these findings suggest that alterations in the levels of intracellular signaling proteins like PLCγ1 may be a major mechanism for regulating the actions of NTFs in the VTA in response to morphine. The results also document what appear to be complex changes in the relative strengths of the various arms of NTF signaling pathways.
It is noteworthy that the chronic morphine-induced up-regulation of PLCγ1 immunoreactivity in the VTA was not associated with an equivalent change in levels of PLCγ1 mRNA. One possibility is that regulation of the enzyme occurs at a posttranscriptional level. Supporting this possibility is the observation that the 5′ untranslated region of the gene contains features associated with translational control (Kozak, 1992). Another possibility is that the higher level of PLCγ1 immunoreactivity seen within the VTA is derived from other neurons that innervate this brain region. Indeed, although immunoblotting procedures provide a sensitive method for reliably detecting relatively small changes in protein abundance within a particular anatomic location, it does not permit the identification of specific cellular populations or sub-cellular compartments in which the regulation may occur. Similarly, the lack of morphine regulation of PLCγ1 and IRS proteins in the other brain regions studied does not rule out the possibility that such regulation could occur in a select subpopulation of neurons in those regions, effects that are not detectable by western blotting. For example, adaptations in certain signal transduction proteins have been observed in some of these other regions, e.g., Cx, in laboratory animals as well as in human opiate addicts (see Ozaita et al., 1998).
The functional significance of the observed chronic morphine-induced up-regulation of PLCγ1 in VTA, in relation to other biochemical and behavioral adaptations produced by chronic morphine administration, is unknown. However, increased PLCγ1 levels could be plausibly related to these adaptations in several ways. PLCγ1 can act as an important mediator of some actions of NTFs such as release of intracellular Ca2+ stores and activation of protein kinase C (Berridge, 1993). Given that elevation of intracellular Ca2+ levels via glutamate receptors in the VTA has been implicated in sensitization to the rewarding and locomotor-activating effects of morphine, it is possible that up-regulation of PLCγ1 contributes to these phenomena (Carlezon et al., 1997). It has also been shown that PLCγ1 can interact with NMDA receptors (Gurd and Bissoon, 1997), perhaps influencing their activity as well.
PLCγ1 up-regulation would also be expected to increase protein kinase C activation. Protein kinase C-α itself has been reported to be regulated in rat Cx by opiate exposure (Ventayol et al., 1997). Protein kinase C phosphorylates a large number of intracellular targets, and overexpression of activated protein kinase C increases neuronal firing activity both in cultured neurons and in vivo (Song et al., 1998). Protein kinase C can also lead to activation of ERK (Kolch et al., 1993), and ERK activity (as opposed to total levels) has been shown to be increased in the VTA by chronic morphine treatment (Berhow et al., 1996). However, protein kinase C can also act as a negative feedback signal on NTF pathways, by phosphorylating and inactivating NTF receptors (Chen et al., 1996) and likely their proximal signaling elements such as IRS proteins and PLCs themselves. Unfortunately, in situ protein kinase C activity in specific brain regions is difficult to ascertain; however, it has been shown that by artificially overexpressing a constitutively active PKC in SN neurons, the functional activity of these neurons is altered (Song et al., 1998). Chronic morphine regulation of PLCγ1 levels in the VTA may thus have a complex effect on NTF function, increasing the strength of some downstream pathways while decreasing others and possibly contributing to a reduction in trophic support to VTA neurons. In this context, it is possible that up-regulation of PLCγ1 contributes to the morphological changes that chronic morphine exerts on VTA dopamine neurons (Sklair-Tavron et al., 1996). Alternatively, it may represent a counterregulatory mechanism, to offset partially the effects of chronic morphine.
Clearly, additional studies are needed to study the validity of these various hypotheses, as well as to elucidate the functional consequences of down-regulation of the PI-3-K–IRS pathway in the VTA by chronic morphine. Nevertheless, results of the present study provide a more complete understanding of the effects of chronic morphine administration on NTF signal transduction pathways in the VTA. These studies also highlight the complex types of adaptations caused by chronic drug exposure within this brain region. In addition, drug regulation of specific NTF-associated signaling proteins further supports the notion that perturbation of NTF pathways contributes to the drug-addicted state and raises the possibility that specific signaling proteins could even be targeted for the development of novel treatments for addictive disorders.
Acknowledgments
We thank Harold Schultz for expert technical assistance. This work was supported by grants from the National Institute on Drug Abuse and by the Abraham Ribicoff Research Facilities of the State of Connecticut Department of Mental Health and Addiction Services.
Abbreviations used
- Cx
frontal cortex
- ERK
extracellular signal-regulated kinase
- HC
hippocampus
- IRS
insulin receptor substrate
- MAP
mitogen-activated protein
- MEK
MAP–ERK kinase
- MFB
medial forebrain bundle
- NAc
nucleus accumbens
- NTF
neurotrophic factor
- 6-OHDA
6-hydroxydopamine
- PI-3-K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- SDS
sodium dodecyl sulfate
- SN
substantia nigra
- SSC
saline–sodium citrate
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
References
- Barde Y. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis–Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–1773. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–979. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Gold SJ, Messer CJ, Chen J, Son JH, Joh TH, Nestler EJ. Regulation of tyrosine hydroxylase promoter activity by chronic morphine in TH9.0–LacZ transgenic mice. J Neurosci. 1998;18:9989–9995. doi: 10.1523/JNEUROSCI.18-23-09989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Traylor TD. Depletion of brain noradrenaline and dopamine by 6-hydroxydopamine. Br J Pharmacol. 1971;42:88–99. doi: 10.1111/j.1476-5381.1971.tb07089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon W, Boundy V, Haile C, Lane S, Kalb R, Neve R, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Chen P, Xie H, Wells A. Mitogenic sigaling from the EGF receptor is attenuated by a phospholipase C-gamma/protein kinase C feedback mechanism. Mol Biol Cell. 1996;7:871–881. doi: 10.1091/mbc.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart X, Nestler EJ. Identification of morphine- and cyclic AMP-regulated phosphoproteins (MARPPs) in the locus coeruleus and other regions of rat brain: regulation by acute and chronic morphine. J Neurosci. 1989;9:4371–4387. doi: 10.1523/JNEUROSCI.09-12-04371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd JW, Bissoon N. The N-methyl-D-aspartate receptor subunits NR2A and NR2B bind to the SH2 domains of phospholipase C-γ. J Neurochem. 1997;69:623–630. doi: 10.1046/j.1471-4159.1997.69020623.x. [DOI] [PubMed] [Google Scholar]
- Ji Q, Winnier G, Niswender K, Horstman D, Wisdom R, Magnuson M, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development. Proc Natl Acad Sci USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- Kolch W, Heldecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marmé D, Rapp UF. Protein kinase Cα activates Raf-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- Levine E, Dreyfus C, Black I, Plummer M. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;17:927–933. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Weigand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecules to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Nestler EJ, Berhow MT, Brodkin ES. Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol Psychiatry. 1996;1:190–199. [PubMed] [Google Scholar]
- Numan S, Seroogy KB. Increased expression of trkB mRNA in rat caudate-putamen following 6-OHDA lesions of the nigrostriatal pathway. Eur J Neurosci. 1997;9:489–495. doi: 10.1111/j.1460-9568.1997.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Escribá PV, Ventayol P, Murga C, Mayor F, Jr, García-Sevilla JA. Regulation of G protein–coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. J Neurochem. 1998;70:1249–1257. doi: 10.1046/j.1471-4159.1998.70031249.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; Sydney: 1986. [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae SB. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Choi KD. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992;267:12393–12396. [PubMed] [Google Scholar]
- Ross CA, MacCumber MW, Glatt CE, Snyder SH. Brain phospholipase C isozymes: differential mRNA localizations by in situ hybridization. Proc Natl Acad Sci USA. 1989;86:2923–2927. doi: 10.1073/pnas.86.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DS. Neurotrophins: mechanisms of action. Neuroscientist. 1995;1:3–6. [Google Scholar]
- Rutherford L, DeWan A, Lauer H, Turrigiano G. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Wong V, Bjorklund A. Brain-derived neurotrophic factor and neurotrophin-4/5 modify neurotransmitter-related gene expression in the 6-hydroxydopamine-lesioned rat striatum. Neuroscience. 1995;65:927–933. doi: 10.1016/0306-4522(95)00019-f. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Herman JP. In situ hybridization approaches to the study of the nervous system. In: Turner AJ, Bachelard HS, editors. Neurochemistry: A Practical Approach. Oxford University Press; Oxford: 1997. pp. 121–150. [Google Scholar]
- Sklair-Tavron L, Shi W, Lane S, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak S, During MJ, Bryan J, Ashe O, Ullrey DB, Trask LE, Grant FD, O’Malley KL, Riedel H, Goldstein DS, Neve KA, LaHoste GJ, Marshall JF, Haycock JW, Neve RL, Geller AI. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J Neurosci. 1998;18:4119–4132. doi: 10.1523/JNEUROSCI.18-11-04119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka O, Kondo H. Localization of mRNAs for three novel members (β3, β4 and γ2) of phospholipase C family in mature rat brain. Neurosci Lett. 1994;182:17–20. doi: 10.1016/0304-3940(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Ventayol P, Busquets X, Garcia-Sevilla JA. Modulation of immunoreactive protein kinase C-alpha and beta isoforms and G proteins by acute and chronic treatments with morphine and other opiate drugs in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:491–500. doi: 10.1007/pl00004974. [DOI] [PubMed] [Google Scholar]
- Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D. Characterization of TrkB receptor–mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]


