Abstract
Neuronal death leading to gross brain atrophy is commonly seen in Alzheimer’s disease (AD) patients. Yet, it is becoming increasingly apparent that the pathogenesis of AD involves early and more discrete synaptic changes in affected brain areas. However, the molecular mechanisms that underlie such synaptic dysfunction remain largely unknown. Recently, we have identified dynamin 1, a protein that plays a critical role in synaptic vesicle endocytosis, and hence, in the signaling properties of the synapse, as a potential molecular determinant of such dysfunction in AD. In the present study, we analyzed beta-amyloid (Aβ)-induced changes in synaptic vesicle recycling in cultured hippocampal neurons. Our results showed that Aβ, the main component of senile plaques, caused ultrastructural changes indicative of impaired synaptic vesicle endocytosis in cultured hippocampal neurons that have been stimulated by depolarization with high potassium. In addition, Aβ led to the accumulation of amphiphysin in membrane fractions from stimulated hippocampal neurons. Moreover, experiments using FM1-43 showed reduced dye uptake in stimulated hippocampal neurons treated with Aβ when compared to untreated stimulated controls. Similar results were obtained using a dynamin 1 inhibitory peptide suggesting that dynamin 1 depletion caused deficiency in synaptic vesicle recycling not only in Drosophila but also in mammalian neurons. Collectively, these results showed that Aβ caused a disruption of synaptic vesicle endocytosis in cultured hippocampal neurons. Furthermore, we provided evidence suggesting that Aβ-induced dynamin 1 depletion might play an important role in this process.
Keywords: synaptic dysfunction, amphiphysin, beta-amyloid, endocytosis, FM1-43
Alzheimer disease (AD) is a debilitating disorder that leads to significant cognitive deficits. Pathologically, AD is characterized by the presence of extracellular senile plaques, composed of the amyloid-beta protein (Aβ), and intraneuronal neurofibrillary tangles, composed of the tau protein (Glenner and Wong 1984; Grundke-Iqbal et al. 1986). In addition, significant atrophy due to the loss of neurons in the cholinergic and glutamatergic areas of the brain have been detected in this disease (Teipel et al. 2005; van de Pol et al. 2006). While neuronal death is important and could contribute to the decline in cognition, synapse loss is the best correlate with the severity of dementia in AD (Davies et al. 1987; Terry et al. 1991). However, this loss of synapses seems to lag behind the emergence of cognitive impairment (Tiraboschi et al. 2000). Thus, it is likely that a stage of synaptic dysfunction precedes synapse loss and neuronal death in AD (reviewed by Small et al. 2001; Yao 2004).
The basic function of a synapse is to transmit signals from the presynaptic terminal to the postsynaptic element through neurotransmitter release. In order for synaptic signaling to be efficient, a series of protein-protein interactions must occur at the presynaptic terminal, often in rapid succession during sustained stimulation. Complex neuronal networks are dependent on these protein-protein interactions, and the loss or malfunction of any of these synaptic proteins could cause disruptions in neuronal circuitry. In the past few years, a convincing case has been made for the particular toxicity of soluble, oligomeric forms of Aβ at the synaptic level (Walsh et al. 2002; Lacor et al. 2004; Kelly et al. 2005; Kokubo et al. 2005; Snyder et al. 2005; Walsh et al. 2005; Kelly and Ferreira 2006). Indeed, a collection of synaptic proteins have been shown to be decreased in the brains of AD patients (Honer 2003). One of these proteins, dynamin 1, a GTPase protein which pinches off synaptic vesicles from the plasma membrane following fusion and exocytotic release of neurotransmitter, is of particular importance and interest (Yao et al. 2003). Recently, a connection has been made between the presence of Aβ and the reduction of dynamin 1 both in AD animal models and in cultured hippocampal neurons (Kelly et al. 2005; Kelly and Ferreira 2006). Unquestionably, dynamin 1 function is necessary for normal vesicular endocytosis in a number of models (van der Bliek and Meyerowitz 1991; van der Bliek et al. 1993; Damke et al. 1994; Damke et al. 2001). This process is in turn essential for synaptic vesicle recycling in neurons (Yamashita et al. 2005). Thus, dynamin 1 loss-of-function studies have shown that synapses lose their ability to successively release neurotransmitter as synaptic vesicles become trapped at the plasma membrane and the synaptic vesicle pool is depleted in Drosophila neurons (Koenig and Ikeda 1989). Collectively, these data suggest that Aβ-induced dynamin 1 depletion might contribute to synaptic dysfunction in AD.
In the present study, we analyzed the effects of Aβ on synaptic vesicle recycling in cultured hippocamapal neurons. Our results showed that Aβ induced ultrastuctural signs of incomplete synaptic vesicle endocytosis, including fused synaptic vesicles and depleted synaptic vesicle pools in stimulated neurons. In addition, our findings showed that stimulated neurons abnormally accumulated amphiphysin, at the membrane during Aβ treatment. Furthermore, Aβ-treated/stimulated neurons had decreased endocytic uptake of the extracellular styryl dye FM1-43. Similar results were obtained when dynamin 1 function was blocked using a specific inhibitory peptide. These results provided evidence suggesting that dynamin 1 is involved in synaptic vesicle endocytosis not only in Drosophila neurons but also in mammalian ones. Together, these data suggest that Aβ-induced dynamin 1 depletion might play an important role in disruption of synaptic vesicle endocytosis leading to synaptic dysfunction in AD.
EXPERIMENTAL PROCEDURES
Preparation of hippocampal cultures
Embryonic day (E) 18 rat embryos were used to prepare primary hippocampal cultures as previously described (Goslin and Banker 1991). Briefly, hippocampi were dissected and freed of meninges. The cells were dissociated by trypsinization followed by trituration with a fire-polished Pasteur pipette. For electron microscopy and subcellular fractionation experiments, hippocampal neurons were plated at high density (500,000 cells/60-mm dish) in MEM with 10% horse serum (MEM10). After 2 hours, the medium was changed to glia-conditioned MEM containing N2 supplements plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM) (N2 medium) (Bottenstein and Sato 1979). For FM1-43 imaging, hippocampal neurons were plated onto poly-L-lysine-coated coverslips in MEM10. After 2 hours, the coverslips were transferred to dishes containing an astroglial monolayer and maintained in MEM containing N2 supplements plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM). These cultures contain approximately 95% pyramidal neurons and 5% glial cells.
Aβ aggregation and treatment
Synthetic Aβ1-40 (Bachem, Torrance, CA) was dissolved in N2 medium (0.1 mg/ml) and incubated for 3 days at 37° C to preaggregate the peptide (Rapoport et al. 2002). Within this preparation exists a mixture of unsoluble fibrilar and soluble oligomeric Aβ. We have previously shown that the soluble oligomeric Aβ aggregates into a ∼50-75 kDa Aβ species and is the toxic form of the peptide (Kelly et al. 2005; Kelly and Ferreira 2006). This preaggregated Aβ was added to the culture medium at a final concentration of 2 μM. Although this concentration is lower than the one usually added to cultured neurons, it might be still higher than the Aβ levels detected in AD brains. However, the concentration of this peptide in affected areas might be much higher than the one detected in the whole brain. With this considerations in mind, we have chosen a concentration that cause early events of neurotoxicity without causing overt neuronal death in cultured hippocampal neurons (Kelly et al. 2005). It is worth noting that Aβ1-42 has similar effects on dynamin 1 in this model system (Kelly et al. 2005). For these experiments, Aβ final concentrations were calculated using the initial concentration of the monomeric form of the peptide. Fourteen-days in culture hippocampal neurons were grown in the presence of the preaggregated Aβ for 24 hours.
Dynamin 1 inhibitor treatment
Hippocampal neurons kept in culture for 14 days were incubated with the membrane-permeable dynamin 1 inhibitory peptide (P4, QVPSRPNRAP) (50 μM and 100 μM; Tocris, Ellisville, MO) or dynamin 1 scrambled control peptide (P4C, PRAPNSRQPV) (100 μM; Tocris) for 30 minutes. P4 blocks the binding of dynamin 1 to amphiphysin and prevents the recruitment of dynamin 1 to the endocytic complex. Biochemical assays showed that this peptide is highly specific and binds with high affinity to a unique site in the dynamin-proline rich domain (Grabs et al. 1997; Kittler et al. 2000). Treated neurons were then stimulated and used for subcellular fractionation or FM1-43 imaging as described below.
Stimulation of synaptic vesicle recycling
For these experiments, control hippocampal neurons kept in culture for 14 days, were incubated in a modified Tyrode buffer containing 150 mM NaCl, 4 mM KCl, 2 mM MgCl2, 10 mM glucose, 10 mM HEPES, and 2 mM CaCl2, pH 7.4 for 10 minutes. Sister cultures were incubated in a high-potassium (90 mM) Tyrode buffer also for 10 minutes (stimulated neurons). This high-potassium buffer has been shown to stimulate sustained synaptic vesicle recycling in cultured hippocampal neurons (Sara et al. 2002). Neurons were then washed in a Tyrode buffer containing nominal CaCl2 to reduce spontaneous synaptic activity. Control and stimulated hippocampal neurons were used for electron microscopy, subcellular fractionation, or FM1-43 imaging as described below.
Subcellular fractionation and determination of protein distribution
Subcellular fractionation was performed as previously described (van der Bliek et al. 1993; Damke et al. 1994). Briefly, neurons were scraped in phosphate buffered saline (PBS) plus 5 mM ethylenediaminetetraacetic acid (EDTA) and gently centrifuged at 3,000 × g for 5 minutes. Cells were then resuspended in 100 μl subcellular fractionation buffer containing 0.25 M sucrose, 1 mM MgCl2, 2 mM EGTA, 25 mM HEPES, pH 7.4. Hippocampal neurons were then lysed by two cycles of alternating freezing in liquid nitrogen and thawing in a room temperature water bath. Membrane and cytosol fractions were obtained by centrifuging the samples at 100,000 × g for 30 minutes in a Beckman Airfuge (Beckman Coulter, Fullerton, CA). Supernatants (cytosol fraction) were then taken off, added to 100 μl Laemmli Buffer, and boiled for 10 minutes (Laemmli 1970). Pellets (membrane fraction) were resuspended in 200 μl Laemmli Buffer and boiled for 10 minutes. Total protein levels in membrane and cytosol fractions were determined using the modified Lowry assay (Bensadoun and Weinstein 1976). Equal protein amounts were loaded and separated in 7.5% sodium-dodecyl sulfate-polyacrylamide (SDS-PAGE) gels as described below. The protein distribution between membrane and cytosol fractions was assayed by Western blot as described below.
Electrophoresis and Immunoblotting
Subcellular fractions were prepared from hippocampal neurons that developed in culture for 14 days as previously described and samples were run on 7.5% SDS-PAGE gels. Transfer of protein to Immobilon membranes (Millipore, Billerica, MA) and immunodetection were performed according to Towbin et al. (Towbin et al. 1979) as modified by Ferreira et al. (Ferreira et al. 1989). Immunodetection was performed using the following antibodies: anti-amphiphysin (1:800; Santa Cruz Biotechnology, Santa Cruz, CA), anti-AP2 (1:600; Sigma, St. Louis, MO), anti-GluR2/3 (1:200; Chemicon, Temecula, CA), anti-Erk2 (1:5,000; Santa Cruz). Secondary antibodies conjugated to horseradish peroxidase (Promega, Madison, WI) followed by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ) were used for the detection of proteins. Immunoreactive bands were imaged using a ChemiDoc XRS system (Bio-Rad, Hercules, CA). Densitometry of these bands was performed using Quantity One software (Bio-Rad). At least 3 independent experiments for each experimental condition were used for the quantitative Western blot analysis. Data were expressed as means ± S.E.M. Statistical significance was analyzed by ANOVA followed by Fisher’s LSD post-hoc test.
Electron microscopy
Untreated control, stimulated, and Aβ-treated/stimulated hippocampal neurons were fixed in 3.5% gluteraldehyde at room temperature for 1 hour. Following fixation, neurons were rinsed 5 × 2 minutes with 0.125M phosphate buffer and post-fixed with 1% osmium tetroxide for 1 hour at room temperature. Neurons were then rinsed, dehydrated in increasing concentrations of ethanol, and embedded in Epon. After polymerization, the dish was peeled off, the cells were punched out of the block, and remounted. Samples were sectioned using a Leica Ultra cut ultramicrotone (Leica Microsystems, Wetzlar, Germany) to 70nm and placed on 200 mesh copper grids. Thin sections were counterstained with uranyl acetate and lead citrate and examined using a Jeol 1220 electron microscope (Jeol USA, Peabody, MA) at 80kV. Digital images were taken using Gatan Digital Micrograph 2.5 (Gatan, Inc., Pleasanton, CA).
Flourescence imaging
Two weeks in culture hippocampal neurons were exposed to 3 μM of the styryl dye FM1-43 (Molecular Probes, Eugine, OR) in either the 90 mM K+ buffer to stimulate synaptic vesicle recycling or the 4 mM K+ control buffer for 10 minutes at room temperature. Following the dye loading phase, neurons were washed 2 × 5 minutes in the low Ca2+ buffer to prevent any additional synaptic activity. Representative images were then taken and quantification of FM1-43 positive puncta (immunoreactive spots ranging from 1 to 10 μm, the size of synaptic vesicle clusters at presynaptic terminals) was performed using Metamorph Image analysis software (Universal Imaging Corporation, Fryer Company Inc., Huntley, IL). At least 7 fields from 3 independent experiments for each experimental condition were used for the quantitative analysis. Data were expressed as means ± S.E.M. Statistical significance was analyzed by ANOVA followed by Fisher’s LSD post-hoc test.
RESULTS
Aβ caused accumulation of amphiphysin at the membrane of stimulated cultured hippocampal neurons
We have previously shown that Aβ induced dynamin 1 depletion in cultured hippocampal neurons. An expected consequence of dynamin 1 depletion is the disruption of synaptic vesicle endocytosis during synaptic activity. To obtain evidence of such Aβ potential effects, we used Western blot analysis to detect membranous accumulations of proteins involved in synaptic vesicle endocytosis in control hippocampal neurons and in neurons stimulated in the presence of high potassium stimulation for 10 minutes. This particular method and length of stimulation has previously been shown to initiate synaptic vesicle recycling without significantly changing synaptic morphology in cultured hippocampal neurons (Sara et al. 2002). For these experiments, membrane and cytosol fractions of untreated control, stimulated, and Aβ-treated/stimulated neurons were used to determine the distribution of amphiphysin. Amphiphysin is part of a collection of proteins that are recruited from the cytosol to the plasma membrane during synaptic vesicle recycling (reviewed by Wigge and McMahon 1998). A shift in distribution of this type of proteins from the cytosol to the membrane fraction, as endocytic structures become trapped at the plasma membrane, has been shown to be a consequence of dynamin 1 loss-of-function in HeLa cells (Damke et al. 1994).
First, we confirmed the proper separation of membrane and cytosol fractions. For these experiments, we analyzed the distribution of the extracellular signaling-related kinase 2 (Erk 2) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR 2/3, normally enriched in the cytosol and membrane fractions, respectively (Bennett and Dingledine 1995; Rubinfeld et al. 1999). Western blot analysis showed that the majority of Erk 2 immunoreactivity was contained in the cytosol fraction obtained from two weeks in culture hippocampal neurons. Faint Erk2 immunoreactivity was detected in the membrane fraction (Fig. 1A). When the same membrane was stripped and blotted using an anti-GluR 2/3 antibody, a strong immunoreactive band was detected in the membrane fraction. In contrast, no GluR 2/3 immunoreactive band was detected in the cytosol fraction (Fig. 1A). These results indicated that cytosol proteins and membrane proteins were highly enriched in the cytosol and the membrane fractions, respectively.
Figure 1. Aβ caused accumulation of amphiphysin at the membrane of stimulated cultured hippocampal neurons.
(A) Western blot analysis showing the enrichment of Erk 2 (Erk 2) in the supernatant (S) (cytosol fraction) and GluR 2/3 (GluR 2/3) in the pellet (P) (membrane fraction) following subcellular fractionation of cultured hippocampal neurons. (B) Western blot analysis of amphiphysin (Amp) protein levels in the pellet and supernatant fractions from untreated control (C(U)), stimulated (C(S)), Aβ-treated (Aβ(U)), and Aβ-treated and stimulated (Aβ(S)) cultured hippocampal neurons. Amphiphysin distribution was assayed by determining the P/S ratio. (C) Quantitative analysis of amphiphysin distribution (P/S ratio) in cultured hippocampal neurons treated as described above. The values obtained in untreated control neurons were considered 100%. Values represent the mean ± S.E.M. Differences in the P/S ratio were assessed using ANOVA followed by Fisher’s LSD post-hoc test. The table shows the P-values of the statistical analysis.
Using these fractions, we determined the distribution of amphiphysin in untreated control, stimulated, Aβ-treated/unstimulated, and Aβ-treated/stimulated hippocampal neurons. For these experiments, equal volumes of membrane and cytosol fractions were loaded, and a membrane-to-cytosol ratio of protein levels was quantified. Any increase in the ratio relative to untreated control neurons indicated an accumulation of the protein at the membrane. Conversely, any decrease in this ratio indicated a build-up of the protein in the cytosol. Quantitative Western blot analysis showed more amphiphysin in the cytosolic fraction than in the membrane fraction of untreated control neurons (Fig. 1B). This subcellular amphiphysin localization indicated some degree of synaptic vesicle recycling, likely due to spontaneous synaptic activity in these neurons. We then determined whether sustained stimulation in the 90 mM K+ buffer for 10 minutes altered amphiphysin distribution in cultured hippocampal neurons. Stimulated neurons showed increased amphipysin immunoreactivity in the membrane fraction without an increase in the cytosol fraction when compared to controls. These results indicated a redistribution of amphiphysin from the cytoplasm to the membrane in stimulated neurons when compared to untreated controls (Fig. 1B and 1C). This redistribution is consistent with heightened synaptic activity and the need for increased synaptic vesicle recycling under stimulated conditions. Next, we tested whether Aβ (2 μM) exposure for 24 hours had any affect on amphiphysin distribution in unstimulated neurons (Fig.1B and 1C). Quantitative analysis showed that Aβ-treated/unstimulated neurons had the same amphiphysin distribution as untreated control neurons. Finally, we examined whether Aβ altered amphiphysin distribution in stimulated hippocampal neurons. Quantitative analysis showed that Aβ-treated/stimulated neurons had increased amphiphysin in the membrane fraction and decreased amphiphysin in the cytosol fraction compared to stimulated neurons. This shift in amphiphysin localization significantly increased the membrane-to-cytosol ratio compared to stimulated neurons and suggested an abnormal build-up of amphyphysin at the membrane (Fig. 1B and 1C).
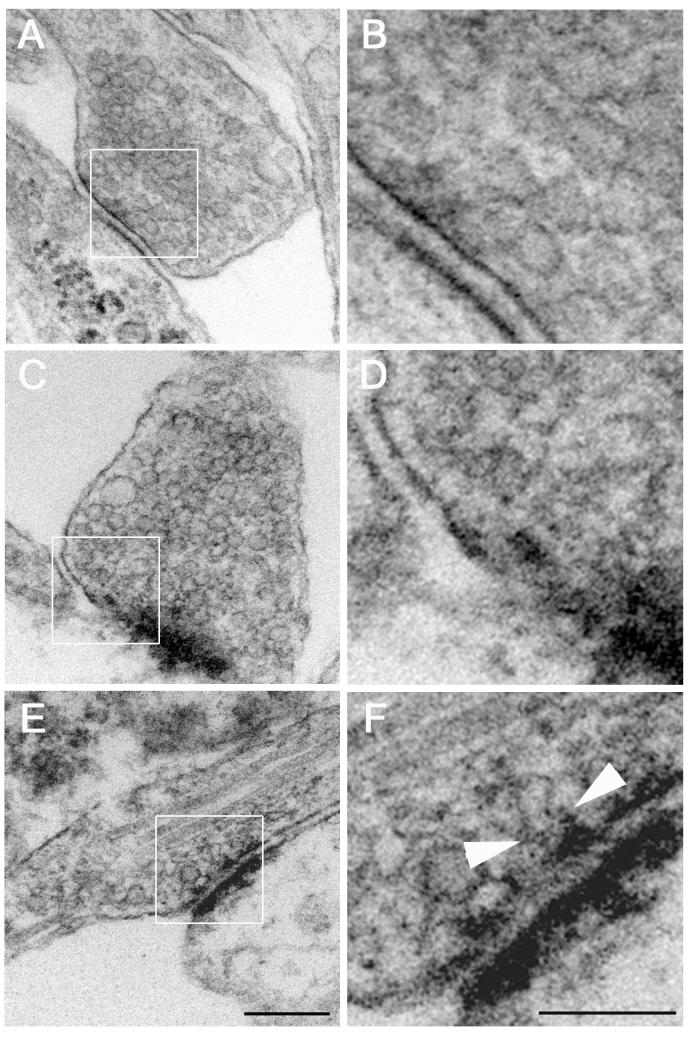
We determined next whether this Aβ-induced impaired synaptic vesicle endocytosis in stimulated cultured hippocampal neurons was accompanied by morphological changes in the synaptic terminals. Ultrastructural studies of synapses from Drosophila (shibire model) have shown that dynamin 1 loss-of-function led to depletion of synaptic vesicles from the synaptic vesicle pool and invaginated pits at the plasma membrane under conditions of synaptic activity (Koenig and Ikeda 1989; Poodry 1990; van der Bliek and Meyerowitz 1991). However, no comparable information from mammalian central neurons is available. To obtain such data, we first examined synapses of untreated control hippocampal neurons. Ultrastructural analysis showed that these neurons had a collection of uniformly shaped synaptic vesicles located in the presynaptic terminal (Fig. 2A). In addition, these vesicles appeared free from, and showed no signs of continuity with, the plasma membrane (Fig. 2B). We next analyzed the effect of 10-minute high potassium stimulation on the ultrastructural appearance of synapses in these hippocampal neurons. When cultured hippocampal neurons were stimulated to induce synaptic vesicle recycling, there was no significant change in synaptic morphology (Fig. 2C and 2D). Stimulated synapses maintained their synaptic vesicle pool and vesicles that were free from the plasma membrane, suggesting efficient vesicle endocytosis and membrane retrieval during the recycling process. On the other hand, synapses of Aβ-treated neurons displayed shibire-like morphology following stimulation. Although the synaptic contact between the presynaptic terminal and the postsynaptic element was still intact, the synaptic vesicle pool in these neurons was largely depleted when compared to the one observed in untreated controls (Fig. 2E). In addition, there were signs of incomplete vesicle endocytosis in the form of invaginated, or elongated membranous structures that stretched from the presynaptic plasma membrane in these Aβ-treated hippocampal neurons (Fig. 2F).
Figure 2. Aβ caused morphological signs of impaired synaptic vesicle endocytosis in stimulated cultured hippocampal neurons.
Electronmicrographs of synapses from untreated control (A-B), stimulated (C-D), and Aβ-treated and stimulated (E-F) cultured hippocampal neurons. (B, D, F) Higher magnification images of the boxed areas to their left. Notice the signs synaptic vesicle pool depletion and incomplete synaptic vesicle endocytosis (arrows) in the Aβ-treated/stimulated neurons. Scale Bars: 0.20 μm (A, C, E); 0.10 μm (B, D, F)
Inhibition of dynamin 1 caused accumulation of amphiphysin at the membrane of stimulated cultured hippocampal neurons
The results described above suggested that Aβ led to a failure of synaptic vesicle endocytosis. In addition, we have previously shown that Aβ caused a significant reduction in dynamin 1 levels (Kelly et al. 2005; Kelly and Ferreira 2006). Together, these data suggest that dynamin 1 could be a key mediator in this process. However, the addition of Aβ to cultured hippocampal neurons could affect a number of pathways and/or proteins involved in synaptic vesicle recycling leading to impaired vesicle endocytosis. To obtain more direct evidence of the role of dynamin 1 in the altered synaptic vesicle endocytosis in Aβ-treated neurons, we examined whether inhibition of dynamin 1 would cause a similar membranous build-up of amphiphysinin in cultured hippocampal neurons. To inhibit dynamin 1 activity, we used a dynamin 1 inhibitory peptide (P4) which blocks the binding of dynamin 1 to amphiphysin and prevents the recruitment of dynamin 1 to the endocytic complex (Grabs et al. 1997; Kittler et al. 2000). When these neurons were treated with 50 μM P4 for 30 minutes prior to stimulation they did not show any change in the amphiphysin membrane-to-cytosol ratio when compared to untreated stimulated neurons (Fig. 3A and 3B). However, when these neurons were treated with 100 μM of the P4 peptide for 30 minutes prior to stimulation, there was a significant shift in amphiphysin immunoreactivity from the cytosol fraction to the membrane fraction. This shift resulted in a significant increase in the membrane-to-cytosol ratio compared to untreated stimulated neurons (Fig. 3A and 3B). These data suggested that a build-up of amphiphysin at the membrane of stimulated neurons was a consequence of dynamin 1 inhibition. We next tested whether this was a dynamin 1 specific response or the addition of this type of peptide altered the membrane-to-cytosol ratio of amphiphysin in a dynamin 1 independent manner. For these experiments, we used a dynamin 1 control peptide (P4C), which was a scrambled version of the amino acids in the P4 peptide. Treatment with 100 μM P4C for 30 minutes prior to stimulation caused no significant alteration in amphiphysin distribution between the membrane and the cytosol fractions when compared to untreated stimulated neurons (Fig. 3A and 3B).
Figure 3. Dynamin 1 inhibition caused accumulation of amphiphysin at the membrane of stimulated cultured hippocampal neurons.
(A) Western blot analysis of amphiphysin (Amp) protein levels in the pellet (P) and supernatant (S) fractions from untreated control (C(U)), stimulated (C(S)), or dynamin 1 inhibitor (P4)-treated and stimulated cultured hippocampal neurons. The P4 peptide was used at 50μ M (50P4(S)) and 100 μM (100P4(S)). A control P4 peptide (P4C(S)) was also used at 100 μM. (B) Quantitative analysis of amphiphysin distribution (P/S ratio) in cultured hippocampal neurons treated as described above. The values obtained in unstimulated control neurons were considered 100%. Values represent the mean ± S.E.M. Differences in the P/S ratio were assessed using ANOVA followed by Fisher’s LSD post-hoc test. The table shows the P-values of the statistical analysis.
Aβ caused a decreased uptake of FM1-43 dye in stimulated cultured hippocampal neurons
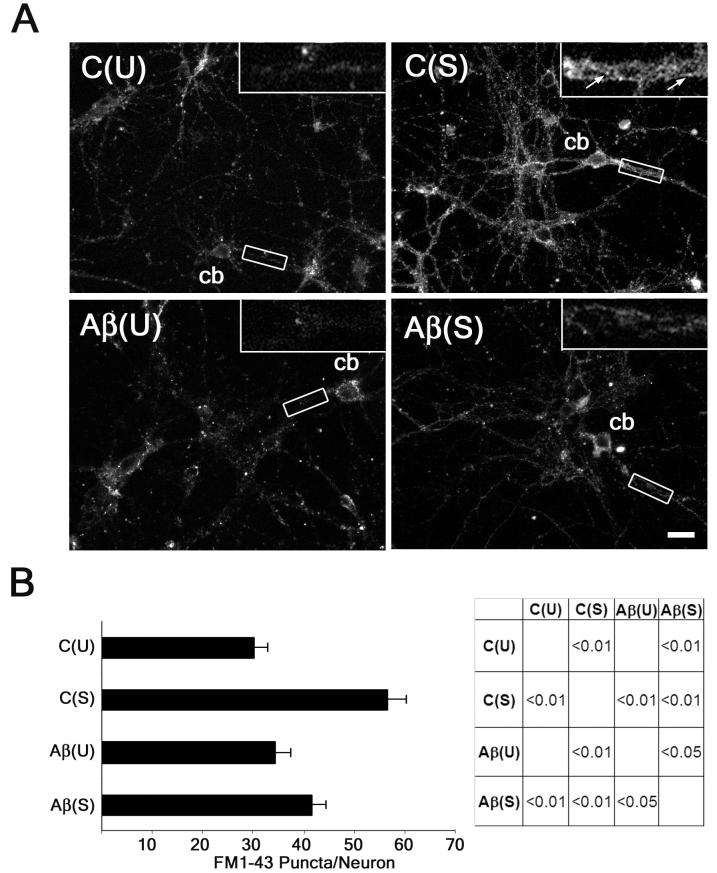
Collectively, the data described above suggested that Aβ caused a disturbance in the endocytic step of synaptic vesicle recycling in cultured hippocampal neurons. We next used FM1-43 dye to measure the ability to these neurons to endocytose the dye under stimulation. FM1-43 dye is taken up in synaptic vesicles after they fuse with the plasma membrane for neurotransmitter release and is entrapped in the presynaptic terminal when the synaptic vesicles are endocytosed from the plasma membrane (Ryan et al. 1993). To analyze the baseline dye uptake in untreated control neurons, we first incubated cultured hippocampal neurons with the 4 mM K+ buffer in the presence of 3 μM FM1-43 for 10 minutes (Fig. 4A). These neurons showed diffuse punctate staining along their neuritic processes which was accompanied by staining in the soma. When puncta were counted on a per neuron basis, there was dye uptake at ∼30 sites. These results indicated some level of endocytic activity at rest in these neurons (Fig. 4B). In order to determine dye uptake during synaptic activity, neurons were stimulated with the 90 mM K+ buffer in the presence of 3 μM FM1-43 for 10 minutes. These neurons showed remarkably increased dye uptake that was distributed throughout the neuronal network in a punctate fashion when compared to untreated control neurons (Fig. 4A). When quantified, puncta staining in these stimulated neurons was increased ∼2-fold when compared to untreated controls (Fig. 4B). In addition, somatic staining in stimulated neurons was also more robust compared to untreated controls. To test whether Aβ had any affect on dye uptake, we incubated cultured hippocampal neurons in Aβ prior to incubation with the 4 mM K+ buffer in the presence of 3 μM FM1-43 for 10 minutes. Aβ-treatment did not cause any change in dye uptake as FM1-43 staining along the processes and in the somatic region was similar to untreated controls (Fig. 4A and 4B). We then tested whether Aβ caused any disruption in dye uptake during stimulation. For these experiments, we incubated hippocampal neurons in the presence of Aβ prior to their incubation with the 90 mM K+ buffer in the presence of 3 μM FM1-43 for 10 minutes. These Aβ-treated/stimulated neurons displayed significantly reduced staining throughout the neuronal network and in the somatic region compared to stimulated neurons (Fig. 4A). Staining was still punctate in nature in these Aβ-treated neurons, but puncta per neuron decreased significantly when compared to stimulated neurons (Fig. 4B). Additionally, stimulation for a shorter period of time (2 min) in Aβ-treated neurons also showed reduced FM1-43 dye uptake when compared to stimulated controls (Sup. Fig.1).
Figure 4. Aβ caused decreased uptake of FM1-43 dye in stimulated cultured hippocampal neurons.
(A) Cultured hippocampal neurons were incubated with FM1-43 dye (3 μM) for 10 minutes under the following conditions: untreated controls (C(U)), stimulated (C(S)), Aβ-treated (Aβ(U)), and Aβ-treated and stimulated (Aβ(S)). Notice the increased dye uptake in the processes (shown in the inserts, arrows point to FM1-43 puncta) of the C(S) neurons when compared to the C(U) neurons and the decreased dye uptake in the processes of the Aβ(S) neurons when compared to the C(S) neurons. (B) Quantitative analysis of FM1-43 dye uptake in cultured hippocampal neurons treated as described above. Analysis was performed by quantifying FM1-43 puncta/neuron. Values represent the mean ± S.E.M. Differences in FM1-43 uptake were assessed using ANOVA followed by Fisher’s LSD post-hoc test. The table shows the P-values of the statistical analysis. cb: cell body. Scale Bar: 20 μm
The data described above indicated that both the inhibition of dynamin 1 and the treatment with Aβ resulted in a similar impairment of synaptic vesicle endocytosis. To further validate this correlation, we analyzed whether dynamin 1 inhibition resulted in a change of FM1-43 uptake that was similar to pretreatment with Aβ in cultured hippocampal neurons. For these experiments, we incubated cultured hippocampal neurons with the P4 dynamin 1 inhibitory peptide for 30 minutes followed by stimulation in the presence of 3 μM FM1-43 for 10 minutes. Treatment with 50 μM P4 peptide caused a slight qualitative and quantitative reduction in FM1-43 staining along the processes of hippocampal neurons compared to untreated stimulated neurons (Fig. 5C and 5G). Incubation with 100 μM of the P4 peptide further reduced the FM1-43 staining in these neurons. Neurons treated with 100 μM P4 had qualitatively decreased staining throughout the neuronal network and reduced somatic staining compared to untreated stimulated neurons (Fig. 5D). Stained puncta per neuron in 100 μM P4-treated/stimulated hippocampal neurons was significantly reduced compared to both 50 μM P4-treated/stimulated and untreated stimulated neurons (Fig. 5G). To confirm that the decrease in dye uptake was specific to dynamin 1 and not an unspecific response, we incubated the neurons in 100 μM P4C peptide followed by stimulation. FM1-43 dye uptake was still robust in the processes and the somas of these 100 μM P4C-treated/stimulated neurons, resulting in similar qualitative and quantitative uptake compared to untreated stimulated neurons (Fig. 5E and 5G). Finally, to rule out any unspecific stimulation caused by the P4C peptide we exposed the neurons to 100 μM P4C and then incubated the neurons with the 4 mM K+ buffer in the presence of FM1-43. In these P4C-treated neurons there were no significant qualitative or quantitative differences in dye uptake when compared to untreated controls (Fig. 5F and 5G). Similar results where obtained when P4-treated neurons were compared to untreated controls (Sup. Fig.2).
Figure 5. Dynamin 1 inhibition caused a decreased uptake of FM1-43 dye in stimulated cultured hippocampal neurons.
Cultured hippocampal neurons were incubated with FM1-43 dye (3 μM) for 10 minutes under the following conditions: untreated control (A), stimulated (B), 50 μM P4-treated/stimulated (C), 100 μM P4-treated/stimulated (D), 100 μM P4C-treated/stimulated (E), and 100 μM P4C-treated (F). Notice the decreased dye uptake in the processes (shown in the inserts, arrows point to FM1-43 puncta) of the P4-treated/stimulated (C, D) neurons when compared to the stimulated (B) neurons. (G) Quantitative analysis of FM1-43 dye uptake in cultured hippocampal neurons treated as described above. Analysis was performed by quantifying FM1-43 puncta/neuron. Values represent the mean ± S.E.M. Differences in FM1-43 uptake were assessed using ANOVA followed by Fisher’s LSD post-hoc test. The table shows the P-values of the statistical analysis. cb: cell body. Scale Bar: 20 μm
DISCUSSION
The results presented herein indicated that Aβ impaired synaptic vesicle endocytosis, and hence the normal recycling of these organelles, during sustained synaptic activity in cultured hippocampal neurons. In addition, our data provided further evidence suggesting that these Aβ effects could be mediated, at least in part, by the significant depletion of dynamin 1 induced by this peptide. Together, these findings identify potential mechanisms underlying synaptic dysfunction in early stages of AD.
Synapse loss correlates with the severity of cognitive decline in AD (Davies et al. 1987; Terry et al. 1991). However, it has become increasingly clear that synaptic dysfunction might also play an important role in the earliest stages of this disease (Davies et al. 1987; Terry et al. 1991). This hypothesis has been further supported by studies using animal models of AD. These transgenic mice developed cognitive impairments that preceded any significant neuronal loss in affected brain areas (Hsiao et al. 1996; Giacchino et al. 2000; Takeuchi et al. 2000; Jacobsen et al. 2006). In addition, data obtained recently indicated that the intracellular injection of Aβ disrupted cognitive function in rodents (Clearly et al., 2005). Conversely, immunization with antibodies raised against this peptide reverse memory defects in AD animal models (Billings et al., 2005; Dodart et al., 2002; Kotilinek et al., 2002). Research on the potential molecular mechanisms that could lead to synaptic dysfunction in the context of this disease has focused in several synaptic proteins. As a result of this work, it has been shown that dynamin 1 was significantly reduced not only in the hippocampus of an AD mouse model and in cultured hippocampal neurons treated with Aβ but also in the brain of AD patients (Yao et al. 2003; Kelly et al. 2005; Kelly and Ferreira 2006). The mechanisms leading to this dynamin 1 depletion seem to involve calpain, a protease system that is active in AD (Tsuji et al. 1998; Lee et al. 2000; Battaglia et al. 2003; Tomizawa et al. 2003; Chen and Fernandez 2005; Demuro et al. 2005; Gong et al. 2005; Kelly et al. 2005; Fifre et al. 2006; Kelly and Ferreira 2006). Furthermore, we have reported that this Aβ-mediated dynamin 1 degradation was the result of calpain activation induced by the sustained calcium influx mediated by N-methyl-D-aspartate (NMDA) receptors in hippocampal neurons (Kelly and Ferreira 2006). However, the mechanisms by which Aβ increases NMDA receptor-mediated calcium influx are not known. Aβ could induce the expression of these receptors or their membrane localization. Results obtained recently showing an Aβ-induced decrease in the levels of NMDA receptor availability in hippocampal neurons seem to argue against this possibility (Snyder et al., 2005). This mechanism does not seem to mediate the effects of Aβ in our model system either since we did not detect changes in the levels or localization of these receptors in Aβ-treated cultured hippocampal neurons (Kelly and Ferreira, unpublished observations). Alternatively, the aggregated peptide could interact directly or indirectly with these receptors modifying different aspects of their function. Further analysis would provide additional insights into the role of NMDA receptors in the increase in calcium influx triggered by Aβ.
Little is known also about the functional consequences of Aβ-induced dynamin 1 depletion in central neurons. The role of dynamin 1 in synaptic vesicle endocytosis has been well documented in Drosophila. In the absence of dynamin 1, synapses lose their ability to successively transmit signals under stimulation due to incomplete endocytosis of the synaptic vesicles (Delgado et al. 2000). Our results provide evidence suggesting that Aβ-induced dynamin 1 depletion caused similar deficiency in synaptic vesicle recycling in mammalian hippocampal neurons. When placed in culture, hippocampal neurons form functional synapses that are structurally and molecularly identical to synapses formed in vivo (Bartlett and Banker 1984b, 1984a; Ferreira et al. 1997). Synaptic vesicle recycling appeared to be functional and efficient in these neurons, as there were no signs of synaptic vesicle depletion or incomplete endocytosis in the presynaptic terminals of stimulated cells. The treatment of hippocampal neurons with Aβ prior to stimulation did not seem to cause synaptic retraction or synapse loss. The integrity of the synaptic contacts between the presynaptic terminal and the postsynaptic element were well preserved and no apparent severance between these two structures was observed. However, the ultrastructural analysis of synapses from these treated neurons showed signs of impaired synaptic vesicle endocytosis following driven synaptic activity including depleted pools of synaptic vesicles and invaginated pits at the plasma membrane. It is worth mentioning that similar morphological abnormalities have been described in the Drosophila dynamin 1 loss-of-function model (Koenig and Ikeda 1989). Interestingly, several studies have shown that presynaptic terminals are enlarged both in human AD brains and in cultured neurons from AD animal models (Bertoni-Freddari et al. 1988; DeKosky and Scheff 1990; Games et al. 1995; Snyder et al. 2005). The enlargement of the presynapse in these cases might be the consequence of incomplete synaptic vesicle endocytosis and integration of synaptic vesicle membrane into the plasma membrane.
The results obtained from the biochemical analysis performed in this study provided further evidence consistent with functional abnormalities due to dynamin 1 depletion in hippocampal neurons cultured in the presence of Aβ. Synaptic vesicle endocytosis involves a number of proteins that translocate from the cytoplasm to the plasma membrane to form the proper endocytic complex (reviewed by Murthy and De Camilli 2003). One of these proteins, amphiphysin, specifically serves as a dynamin 1 binding partner to recruit it to the membrane. Our results showed that a normal redistribution of amphiphysin from the cytosol to the membrane occured during stimulation in cultured hippocampal neurons. This redistribution is likely the result of amphiphysin translocating to the membrane to support the need for increased synaptic vesicle endocytosis (David et al. 1996). However, we showed here that pretreatment with Aβ caused an abnormal accumulation of amphiphysin in membrane fractions and a depletion from cytosol fractions upon stimulation of these neurons. There are two possible explanations for the excessive membrane association of amphiphysin. First, the synaptic vesicles could be endocytosed, but the amphiphysin may not be released from the synaptic vesicles. Alternatively, the synaptic vesicles might not be endocytosed normally causing amphiphysin to become trapped and accumulate at the plasma membrane. The abnormal morphology detected in the synapses of these Aβ-treated hippocampal neurons seems to support the notion that endocytosis is impaired under these experimental conditions. Our results showing that Aβ caused decreased FM1-43 dye uptake during stimulated synaptic activity in cultured hippocampal neurons further support this view. This dye is normally taken up as synaptic vesicles are endocytosed after exposure to the extracellular environment. Low levels of dye uptake were detected even under control conditions. These results suggested the presence of basal spontaneous synaptic activity in control unstimulated cultured hippocampal neurons. These data were in agreement with a previous report showing spontaneous release of neurotransmitter and synaptic vesicle endocytosis under these cultured conditions (Sara et al. 2005). This basal level of endocytosis was also reflected by the appearance of amphiphysin in the membrane fraction of control neurons as detected by quantitative Western blot analysis. Addition of Aβ prior to potassium stimulation did not reduce the amount of dye uptake attributed to spontaneous synaptic activity under control conditions, suggesting that Aβ did not cause any deficit in synaptic vesicle endocytosis when these hippocampal neurons were in a resting, basal state. On the other hand, after these neurons were pretreated with Aβ and stimulated to drive sustained synaptic activity, a significant inability to take up the dye emerged. Taken collectively, these data suggested that this deficiency emerges when the level or speed of synaptic vesicle recycling is increased under sustained synaptic activity. In contrast, no decrease in dye uptake was observed when hippocampal neurons overexpressing APP were cultured as microislands (Ting et al., 2007). Possible explanations of this discrepancy are the different age (embryonic vs. postnatal), species (rats vs. mice), and culture conditions (dissociated vs. microisland cultures) used in these two studies. More importantly, while we used tightly control amounts of Aβ and aggregation times, Ting and coworkers used cells that overexpressed APP and were kept in culture for 10 to 17 days (Ting et al., 2007). Under these experimental conditions, the undertermined amounts of Aβ could accumulate in the culture medium for long periods of time and could be aggregated beyond the oligomeric forms into the less toxic fibrils, and hence, fail to elicit changes in synaptic vesicle recycling similar to the ones reported in this study.
Finally, it is worth noting that similar ultrastructural and functional results were obtained when dynamin 1 depletion was achieved using a specific dynamin 1 inhibitor. The parallel effects of these two treatments further support the hypothesis that Aβ-induced depletion of dynamin 1 could play a key role in synaptic dysfunction in AD. However, because Aβ can affect a number of proteins involved in synaptic vesicle recycling, we cannot completely rule out that changes in other proteins may contribute to altered synaptic vesicle endocytosis in this disease. Aβ could also induced changes in postsynaptic elements that might contribute to synaptic dysfunction. Thus, it has been recently shown that APP over expression led to a selective reduction of α-amino-3-hydroxy-5methyl-4-isoxazole-propionic acid (AMPA) receptors, and hence, disruption in excitatory transmission in hippocampal neurons (Chang et al., 2006; Ting et al., 2007). However, it is unlikely that these defects are mediated by dynamin 1 depletion since these proteins are localized in different subcellular compartments.
Collectively, our results showed that Aβ caused a disruption of synaptic vesicle endocytosis in stimulated hippocampal neurons. They also suggested that this disruption of synaptic vesicle endocytosis was dependent, at least in part, on dynamin 1 loss-of-function in these neurons. Even discrete disruptions of synaptic vesicle endocytosis could be significant in the context of early-stage AD, resulting in a wide-ranging decline of cognition at the behavioral level. Thus, the process of Aβ-induced dynamin 1 depletion could be a viable therapeutic target to treat synaptic dysfunction in the earliest stages of AD.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grant NIH/NS39080 to A.F. B.L.K was supported in part by NIA/AG20506 training grant and an American Foundation for Aging Research Fellowship. We thank Rachel Bergstrom and Roxanna Sinjoanu for excellent technical support. We also thank Linda Juarez (RRC EM facility at University of Illinois-Chicago) for her technical support with the electron microscopy.
Footnotes
Section Editor: Dr. Menahem Segal
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci. 1984a;4:1944–1953. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. II. Synaptic relationships. J Neurosci. 1984b;4:1954–1965. doi: 10.1523/JNEUROSCI.04-08-01954.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia F, Trinchese F, Liu S, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for Alzheimer’s disease: position paper. J Mol Neurosci. 2003;20:357–362. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Meier-Ruge W, Ulrich J. Quantitative morphology of synaptic plasticity in the aging brain. Scanning Microsc. 1988;2:1027–1034. [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-protein specifically disrupt cognitive function. Nat. Neurosci. 2006;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proc Natl Acad Sci U S A. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Fernandez HL. Mu-calpain is functionally required for alpha-processing of Alzheimer’s beta-amyloid precursor protein. Biochem Biophys Res Commun. 2005;330:714–721. doi: 10.1016/j.bbrc.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Binns DD, Ueda H, Schmid SL, Baba T. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell. 2001;12:2578–2589. doi: 10.1091/mbc.12.9.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, McPherson PS, Mundigl O, de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci U S A. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain A burden in Alzheimer’s disease model. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Busciglio J, Caceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Res Dev Brain Res. 1989;49:215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Lu Q, Orecchio L, Kosik KS. Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A beta. Mol Cell Neurosci. 1997;9:220–234. doi: 10.1006/mcne.1997.0615. [DOI] [PubMed] [Google Scholar]
- Fifre A, Sponne I, Koziel V, Kriem B, Yen Potin FT, Bihain BE, Olivier JL, Oster T, Pillot T. Microtubule-associated protein MAP1A, MAP1B, and MAP2 proteolysis during soluble amyloid beta-peptide-induced neuronal apoptosis. Synergistic involvement of calpain and caspase-3. J Biol Chem. 2006;281:229–240. doi: 10.1074/jbc.M507378200. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Giacchino J, Criado JR, Games D, Henriksen S. In vivo synaptic transmission in young and aged amyloid precursor protein transgenic mice. Brain Res. 2000;876:185–190. doi: 10.1016/s0006-8993(00)02615-9. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- Goslin, Banker . In: Rat Hippocampal Neurons in Low-Density Culture, in Culturing Nerve Cells. Banker G, Goslin K, editors. Vol. 1. MIT Press; Cambridge: 1991. pp. 251–281. [Google Scholar]
- Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- Honer WG. Pathology of presynaptic proteins in Alzheimer’s disease: more than simple loss of terminals. Neurobiol Aging. 2003;24:1047–1062. doi: 10.1016/j.neurobiolaging.2003.04.005. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Vassar R, Ferreira A. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J Biol Chem. 2005;280:31746–31753. doi: 10.1074/jbc.M503259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Kayed R, Glabe CG, Yamaguchi H. Soluble Abeta oligomers ultrastructurally localize to cell processes and might be related to synaptic dysfunction in Alzheimer’s disease brain. Brain Res. 2005;1031:222–228. doi: 10.1016/j.brainres.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J. Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Poodry CA. shibire, a neurogenic mutant of Drosophila. Dev Biol. 1990;138:464–472. doi: 10.1016/0012-1606(90)90212-2. [DOI] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld H, Hanoch T, Seger R. Identification of a cytoplasmic-retention sequence in ERK2. J Biol Chem. 1999;274:30349–30352. doi: 10.1074/jbc.274.43.30349. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Sara Y, Mozhayeva MG, Liu X, Kavalali ET. Fast vesicle recycling supports neurotransmission during sustained stimulation at hippocampal synapses. J Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Abeta toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Irizarry MC, Duff K, Saido TC, Hsiao Ashe K, Hasegawa M, Mann DM, Hyman BT, Iwatsubo T. Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss. Am J Pathol. 2000;157:331–339. doi: 10.1016/s0002-9440(10)64544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Flatz WH, Heinsen H, Bokde AL, Schoenberg SO, Stockel S, Dietrich O, Reiser MF, Moller HJ, Hampel H. Measurement of basal forebrain atrophy in Alzheimer’s disease using MRI. Brain. 2005;128:2626–2644. doi: 10.1093/brain/awh589. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Ting JT, Kelley BG, Lambert TJ, Cook DG, Sullivan JM. Amyloid precursor protein overexpression depresses excitatory transmisssion through both presynaptic and postsynaptic mechanisms. Proc Natl Acad Sci U S A. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Alford M, Masliah E, Thal LJ, Corey-Bloom J. The decline in synapses and cholinergic activity is asynchronous in Alzheimer’s disease. Neurology. 2000;55:1278–1283. doi: 10.1212/wnl.55.9.1278. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Sunada S, Lu YF, Oda Y, Kinuta M, Ohshima T, Saito T, Wei FY, Matsushita M, Li ST, Tsutsui K, Hisanaga S, Mikoshiba K, Takei K, Matsui H. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol. 2003;163:813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer’s disease brains. Neurosci Lett. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- van de Pol LA, Hensel A, Barkhof F, Gertz HJ, Scheltens P, van der Flier WM. Hippocampal atrophy in Alzheimer disease: age matters. Neurology. 2006;66:236–238. doi: 10.1212/01.wnl.0000194240.47892.4d. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, Agnaf OE, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P, McMahon HT. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci. 1998;21:339–344. doi: 10.1016/s0166-2236(98)01264-8. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- Yao PJ. Synaptic frailty and clathrin-mediated synaptic vesicle trafficking in Alzheimer’s disease. Trends Neurosci. 2004;27:24–29. doi: 10.1016/j.tins.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Zhu M, Pyun EI, Brooks AI, Therianos S, Meyers VE, Coleman PD. Defects in expression of genes related to synaptic vesicle trafficking in frontal cortex of Alzheimer’s disease. Neurobiol Dis. 2003;12:97–109. doi: 10.1016/s0969-9961(02)00009-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.