Abstract
Endothelial dysfunction is a well established response to cardiovascular risk factors and precedes the development of atherosclerosis. Endothelial dysfunction is involved in lesion formation by the promotion of both the early and late mechanisms of atherosclerosis including up-regulation of adhesion molecules, increased chemokine secretion and leukocyte adherence, increased cell permeability, enhanced low-density lipoprotein oxidation, platelet activation, cytokine elaboration, and vascular smooth muscle cell proliferation and migration. Endothelial dysfunction is a term that covers diminished production/availability of nitric oxide and/or an imbalance in the relative contribution of endothelium-derived relaxing and contracting factors. Also, when cardiovascular risk factors are treated the endothelial dysfunction is reversed and it is an independent predictor of cardiac events. We review the literature concerning endothelial dysfunction in regard to its pathogenesis, treatment, and outcome.
Keywords: endothelial dysfunction, coronary atherosclerosis, coronary artery disease
Introduction
During the last 2 decades, it has been shown that the vascular endothelium is an active paracrine, endocrine, and autocrine organ that is indispensable for the regulation of vascular tone and the maintenance of vascular homeostasis. The basic mechanisms involved in atherogenesis indicate that deleterious alterations of endothelial physiology, otherwise known as endothelial dysfunction, represent a key early step in the development of atherosclerosis and are also involved in plaque progression and the occurrence of atherosclerotic complications (Anderson, Gerhard, et al 1995; Kinlay and Ganz 1997). Endothelial dysfunction is characterized by reduction of the bioavailability of vasodilators, particularly nitric oxide (NO), and/or an increase in endothelium-derived contracting factors (Lerman and Burnett 1992). The resulting imbalance leads to an impairment of endothelium-dependent vasodilation, which is the functional characteristic of endothelial dysfunction. In addition to impaired endothelium-dependent vasodilation, endothelial dysfunction also comprises a specific state of endothelial activation, which is characterized by a proinflammatory, proliferative, and procoagulatory states that favor all stages of atherogenesis (Anderson 1999). Considering the relationship between endothelial dysfunction and atherosclerosis, it is likely that the status of an individual endothelial function may reflect the propensity to develop atherosclerotic disease, and thus may serve as a marker of an unfavorable cardiovascular prognosis. Herein, we review the literature about endothelial dysfunction in regard to its pathogenesis, treatment, and outcome.
Pathophysiology of endothelial dysfunction
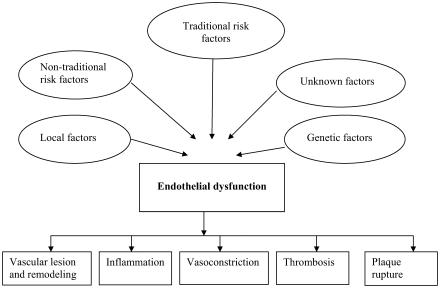
The endothelium maintains normal vascular tone and blood fluidity, with no or little expression of proinflammatory factors under normal homeostatic conditions. However, both traditional and novel cardiovascular risk factors including smoking, aging, hypercholesterolemia, hypertension, hyperglycemia, and a family history of premature atherosclerotic disease are all associated with alteration in endothelial function (Sorensen et al 1994; Gokce and Vits 2002; Libby et al 2002). This results in a chronic inflammatory process accompanied by a loss of antithrombotic factors and an increase in vasoconstrictor and prothrombotic products, in addition to abnormal vasoreactivity, therefore elevating risk of cardiovascular events (Bonetti et al 2003) (Figure 1). More recently, endothelial dysfunction has also been associated with obesity, elevated C-reactive protein, and chronic systemic infection (Celermajer et al 1992; Steinberg et al 1996; Thogersen et al 1998; Cushman et al 1999; Fichtlscherer et al 2000; Al Suwaidi et al 2001; Prasad et al 2002).
Figure 1.
The various factors that affect the endothelium and the consequences of endothelial dysfunction.
Oxidative stress and endothelial cell dysfunction
Reactive oxygen species (ROS) are generated at sites of inflammation and injury, and at low concentrations can function as signaling molecules participating in the regulation of fundamental cell activities such as cell growth and cell adaptation responses; whereas at higher concentrations, ROS can cause cellular injury and death. The vascular endothelium, which regulates the passage of macromolecules and circulating cells from blood to tissues, is a major target of oxidative stress, playing a critical role in the pathophysiology of several vascular diseases and disorders. Specifically, oxidative stress increases vascular endothelial permeability and promotes leukocyte adhesion, which is coupled with alterations in endothelial signal transduction and redox-regulated transcription factors (Lum and Roebuck 2001).
How is endothelial function assessed?
Endothelium-dependent vasodilation can be assessed in the coronary and peripheral circulations. The most relevant methodological issues in the research on endothelial function and dysfunction have recently been published (Deanfield et al 2005). We provide a summary of the available modalities of testing.
Coronary circulation
Noninvasive tests for the assessment of coronary endothelial function that have been described include Doppler echocardiography, positron emission tomography, and phase-contrast magnetic resonance imaging. However, the gold standard test for the evaluation of coronary endothelial function requires invasive quantitative coronary angiography to examine the changes in diameter in response to intracoronary infusions of endothelium-dependent vasodilators such as acetylcholine. Endothelial function of the coronary microvasculature can be assessed with intracoronary Doppler techniques to measure coronary blood flow in response to pharmacological or physiological stimuli (Anderson 1999; Al Suwaidi et al 2001; Farouque and Meredith 2001). Diagnostic coronary angiography is first performed with a standard femoral percutaneous approach, with no nitroglycerin given beforehand. Vasomotor responses to acetylcholine and adenosine are then assessed (Al Suwaidi et al 2001). After the control coronary angiogram has been obtained, a 0.014-inch Doppler guide wire is introduced through an 8-F guiding catheter into the left anterior descending coronary artery. Once baseline flow velocity data are obtained at the position, ie, once a stable Doppler signal is achieved, a bolus of intracoronary adenosine (24–36 μg, from a solution of 6 mg adenosine in 1 L of saline) is administered. Then intracoronary infusion of selective concentrations of acetylcholine (10−6, 10−5, and 10−4 mol/L) is administered for a total of 3 minutes through a 2.2-F Ultrafuse coronary infusion catheter. Symptoms, hemodynamic data, and electrocardiographic and Doppler velocities are recorded at the end of each infusion or bolus injection, followed by selective coronary angiogram. Coronary blood flow is calculated using the formula D2 × APV, where D represents the coronary diameter and APV equals the average peak velocity from Doppler tracing.
Peripheral circulation
Brachial artery ultrasound is a widely used noninvasive measure of endothelial function. Upper-arm occlusion for 5 minutes results in reactive hyperemia after the release of the cuff; the increase in shear stress results in endothelium-dependent flow-mediated vasodilation. Importantly, endothelial dysfunction assessed by this technique correlates with measures of coronary endothelial dysfunction (Anderson, Uehata, et al 1995). Peripheral vascular endothelial function can be assessed by strain-gauge venous impedance plethysmography. This technique examines the change in forearm blood flow in response to direct intraarterial (brachial artery) administration of agonists. Noninvasive measures of arterial compliance and waveform morphology provide a marker of vascular health (Deanfield et al 2005).
Role of endothelial dysfunction in acute coronary syndromes
Endothelial dysfunction may play a fundamental role in the pathogenesis of acute coronary syndromes (Libby 2001). Plaque destabilization, the process that predisposes to rupture of the plaque, results from a complex interplay of inflammatory effects that involve cellular plaque components and various proinflammatory mediators (Libby et al 2002). Endothelial dysfunction is associated with increased oxidative stress (Napoli et al 2001), an important promoter of inflammatory processes. NO may reduce endothelial expression of several inflammatory mediators and adhesion molecules that increase plaque vulnerability (Kubes et al 1991; De Catarina et al 1995; Peng et al 1995; Barnes and Karin 1997). Precipitation of acute coronary syndrome may also involve physical factors related to endothelial dysfunction. Increased vasoreactivity results in local vasoconstriction in response to metabolic and sympathetic stimuli in the area of culprit lesions in patients with unstable angina (Bogaty et al 1994). All of these processes may contribute to plaque rupture and hence the development of acute coronary syndrome.
Endothelial dysfunction and cardiovascular risk factors
Diabetes and endothelial dysfunction
Molecular and cellular basis of endothelial dysfunction in diabetes
Hyperglycemia may lead to intracellular changes in the redox state resulting in depletion of the cellular NADPH pool. Overexpression of growth factors has also been implicated in diabetes with proliferation of both endothelial cells and vascular smooth muscle, possibly promoting neovascularization. Chronic hyperglycemia leads to non-enzymatic glycation of proteins and macromolecules (Calles-Escandon and Cipolla 2001).
The diabetic state is typified by an increased tendency for oxidative stress and high levels of oxidized lipoproteins, especially the so-called small, dense low-density lipoprotein. The high levels of fatty acids and hyperglycemia have also both been shown to induce an increased level of oxidation of phospholipids as well as proteins. In humans it is associated with a prothrombotic tendency as well as increased platelet aggregation, with tumor necrosis factor implicated as a link between insulin resistance, diabetes, and endothelial dysfunction; a hypothesis has been advanced that insulin and/or insulin precursors may be atherogenic (Calles-Escandon and Cipolla 2001).
We have reviewed 22 experimental and clinical studies from 1991 to 2004 that evaluated endothelial dysfunction in diabetic patients; most of these were prospective studies. Diminished capacity of NO synthase to generate NO has been demonstrated experimentally when endothelial cells were exposed either in vitro or in vivo to a diabetic environment (Arbogast et al 1982; Aanderud et al 1985; Koh et al 1985; Lorenzi et al 1986; Hattori et al 1991; Nordt et al 1993; Avogaro et al 1999; Cipolla 1999; Salvolini et al 1999). Most of these studies in humans indicate that endothelial dysfunction is closely associated with microangiopathy and atherosclerosis in diabetic patients.
Endothelial dysfunction in insulin-dependent diabetes mellitus
The association between diabetes and endothelial dysfunction is particularly true in patients with type 1 diabetes who have either early (microalbuminuria) or late (macroalbuminuria) nephropathy. A variety of markers indicate endothelial dysfunction: poor endothelial cell-dependent vasodilation and increased blood levels of von Willebrand factor (vWF), thrombomodulin, selectin, plasminogen activator inhibitor, type IV collagen, and tissue plasminogen activator (t-PA) have been demonstrated in this patient population (Yaqoob et al 1993; Dosquet et al 1994; Myrup et al 1994; Makimattila et al 1996; Huszka et al 1997; Cosentino and Luscher 1998; Elhadd et al 1998; Malamitsi-Puchner et al 1998; Huvers et al 1999). While the dysfunction of endothelial cells is considered to be an early marker of vascular disease in type 2 diabetes, it does not seem to fully manifest itself until later in the course of type 1 diabetes (Clarkson et al 1996). Furthermore, it has been shown that the levels of vascular cell adhesion molecule-1 were more markedly elevated in type 1 diabetes patients with diabetic retinopathy than in those with micro- or macroalbuminuria, whereas no difference in intracellular adhesion molecule-1 and endothelial leukocyte adhesion molecule-1 levels was apparent regarding the clinical status of diabetic microangiopathy (Fasching et al 1996). In diabetic subjects, endothelium-dependent vasodilation correlated inversely with serum insulin concentration but not with glucose concentration, glycosylated hemoglobin, or duration of diabetes (Johnstone et al 1993).
In another study, a significantly raised mean concentration of a free N-terminal fibronectin 30-kDa domain (a marker of endothelial dysfunction) was found in plasma of diabetic patients with proliferative retinopathy as compared with healthy individuals, and a positive correlation was observed between free N-terminal fibronectin and vWF and the degree of albuminuria, suggesting an association between endothelial cell dysfunction and proliferative retinopathy (Skrha et al 1990).
The general consensus is that the occurrence of endothelial cell dysfunction in type 1 diabetes signifies a very high risk of micro- and macroangiopathy, and although the diabetic state predisposes to endothelial cell dysfunction in this disease, it is not sufficient to cause it. It is more likely that other agents (genes, environment) have a role in determining which patients will develop aggressive angiopathy and hence endothelial cell dysfunction. Irrespective of whether endothelial cell dysfunction is a cause or a consequence of vascular injury in type 1 diabetes, it is hoped that therapeutic efforts aimed at restoring endothelial cell function to normal will affect the natural history of vasculopathy in type 1 diabetes (Calles-Escandon and Cipolla 2001).
Endothelial dysfunction in non-insulin-dependent diabetes mellitus
The role of endothelial dysfunction in type 2 diabetes is more complicated than that for type 1. The effects of aging, hyperlipidemia, hypertension, and other factors add to the complexity of the problem. In contrast to the situation with type 1 diabetes, endothelial dysfunction can occur in type 2 diabetes even when patients have normal urinary albumin excretion, a marker of endothelial dysfunction often elevated years before any evidence of microangiopathy becomes evident (Janka 1985; Hsueh and Anderson 1992; Bloomgarden 1998; De Mattia et al 1998; Neri et al 1998; Watts and Playford 1998; Gazis et al 1999).
There is growing evidence suggesting the coexistence of insulin resistance and endothelial dysfunction. Insulin-induced vasodilation, which is partially mediated by NO release, is impaired in obese individuals who do not have type 2 diabetes but who display insulin resistance (Steinberg et al 1994; Ferri et al 1997). Moreover, the obese state, a model of human insulin resistance, is associated with high levels of endothelin in plasma. Plasminogen activator inhibitor concentrations in blood also are high in patients with otherwise uncomplicated obesity (Calles-Escandon et al 1996).
The insulin resistance syndrome encompasses more than a subnormal response to insulin-mediated glucose disposal; patients with this syndrome also frequently display elevated blood pressure, hyperlipidemia, and dysfibinolysis even without any clinically demonstrable alteration in plasma glucose concentrations (Steinberg et al 1994).
The vasodilatory responses to acetylcholine have been shown to be reduced in healthy normoglycemic individuals with a history of type 2 diabetes in one or both parents (relatives), individuals with impaired glucose tolerance, and patients with type 2 diabetes without vascular complications compared with healthy normoglycemic individuals with no history of type 2 diabetes in a first-degree relative (as control). The plasma levels of endothelin-1 were significantly higher in these three groups. These results suggest that abnormalities in vascular reactivity and biochemical markers of endothelial cell activation are present early in individuals at risk of developing type 2 diabetes (Caballero et al 1999).
Low and high physiological hyperinsulinemia have been shown to abolish endothelium-dependent vasodilation, whereas endothelium-independent vasodilation was unaffected. Vitamin C fully restored insulin-impaired endothelial function without affecting endothelium-independent vasodilation (Arcaro et al 2002). Other investigators concluded that insulin therapy partly restores insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease (Rask-Madsen et al 2001). In other studies in which the long-term effects of insulin glargine on vascular function in patients with type 2 diabetes were investigated, the results seem to support the idea that long-term insulin therapy has beneficial rather than harmful effects on vascular function (Paolisso and Giugliano 1996; Vehkavaara and Yki-Jarvinen 2001). A recently performed double-blind, crossover trial of 12 patients with recently diagnosed type 2 diabetes concluded that insulin resistance is a major contributor to endothelial dysfunction in type 2 diabetes, with both endothelial dysfunction and insulin resistance amenable to treatment by rosiglitazone (Pistrosch et al 2004). In a more recent study in which the relationship between the angiotensin-converting enzyme (ACE) gene and endothelial dysfunction was investigated, it was concluded that ACE DD genotype is related to endothelium-dependent arterial dilation in the early stage of type 2 diabetes mellitus and in healthy individuals (Xiang et al 2004).
Several therapeutic interventions have been tested in clinical trials with the aim of improving endothelial function in patients with diabetes. Insulin sensitizers may have a beneficial effect in the short and the long term, but the virtual absence of trials with cardiovascular end points precludes any definitive conclusion. Several trials offer optimism that treatment with ACE inhibitors may have a positive effect on the progression of atherosclerosis (O'Driscoll, Green, Rankin, et al 1997; Mullen et al 1998; O'Driscoll et al 1999; Prasad et al 2000; Hornig et al 2001). Although hypolipidemic agents are widely used, their effect on endothelial function in diabetes is not clear (Evans et al 2000). The role of antioxidant therapy remains controversial (Table 1).
Table 1.
Modes of therapy of endothelial dysfunction
| References | Treatment | Patient condition | Result on endothelial function |
|---|---|---|---|
| Creager et al 1992; Quyyumi et al 1997; Boger et al 1998 | L-arginine intravenous, oral intracoronary | Hypercholesterolemia, CAD, heart failure | +EDVD |
| −EIDVD | |||
| Creager et al 1992; Boger et al 1998 | D-arginine | Hypercholesterolemia, CAD, heart failure | No effect |
| Thorne et al 1998; Wang et al 1999 | Oral arginine | Hypercholesterolemia, CAD, heart failure | Debated |
| Andrews et al 2001 | N-acetylcysteine | CAD | +EDVD |
| O'Driscoll, Green, Rankin, et al 1997; Hernandez-Perera et al 1998; Dupuis et al 1999; Lefer et al 1999; Yokoyama et al 1999; Vita et al 2000; Masumoto et al 2001; Stein et al 2001 | Statins | DM type 1, DM type 2, CAD, regardless of lipid level | +EDVD |
| Evans et al 2000 | Fibrates | DM | +EDVD |
| Ryan et al 2000; Fuentes et al 2001 | Mediterranean diet | CAD | +EDVD |
| DeSouza et al 2000 | Regular aerobic exercise | Young, old sedentary | +EDVD |
| Ziccardi et al 2002 | Weight loss | Premenopausal obese women | +EDVD |
| Ting et al 1997; Verhaar et al 1999; Chambers et al 2000; Kaufmann et al 2000; Stuhlinger et al 2001 | Antioxidant, vitamin C, folate, tetrahydrofolate | Coronary microcirculation | +EDVD |
| Skyrme-Jones, O'Brien, Berry, et al 2000; Skyrme-Jones, O'Brien, Luo, et al 2000 | Vitamin E | DM | +EDVD |
| Sesso 1999; Chou et al 2001; Duffy et al 2001 | Flavonoids | CAD | +EDVD |
| Guthikonda et al 2003 | Allopurinol | Smokers | +EDVD |
| Husain et al 1998 | Aspirin | CAD | +EDVD |
| Gilligan, Quyyumi, et al 1994; Venkov et al 1996; Thompson et al 2000; Webb et al 2000 | Estrogen | CAD, hypercholesterolemia | +EDVD |
| Wakatsuki et al 2001 | Progesterone | CAD | Offset estrogen effect |
| Clarke et al 2001; Wassmann et al 2002 | Tamoxifen, raloxifene | CAD | +EDVD |
| Lissin and Cooke 2000; Walker et al 2001 | Phytoestrogen | Normal | +EDVD |
| Webb et al 1999 | Testosterone | CAD | +EDVD |
| Bijlstra et al 1995; Mancini et al 1996; O'Driscoll, Green, Rankin, et al 1997; Mullen et al 1998; O'Driscoll et al 1999; McFarlane et al 1999; Anderson et al 2000; Prasad et al 2000; Hornig et al 2001 | ACEI (most studies) | DM, CAD | +EDVD |
| Cheetham et al 2000; Hornig et al 2001 | ARB | DM, CAD | Debated results |
| Berger et al 2001; Cardillo et al 2002 | Endothiline receptor blockers | Hypertension, heart failure | +EDVD |
| ENCORE I Study 2003 | Nifedipine | PCI | +EDVD |
| Mather et al 2001 | Metformin | DM | +EDVD |
| Tack et al 1998; Pistrosch et al 2004 | Rosiglitazone | DM | +EDVD |
| Goodfellow et al 2000 | Omega 3 fatty acid | Hypercholesterolemia | +EDVD |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CAD, coronary artery disease; DM, diabetes mellitus; EDVD, endothelium-dependent vasodilation; EIDVD, endothelium-independent vasodilation; PCI, percutaneous coronary intervention.
Endothelial dysfunction in hypertension
Endothelial dysfunction has been documented in both the forearm and coronary beds of patients with essential hypertension. We evaluated 111 patients with normal or mild coronary artery disease by intracoronary Doppler and intravascular ultrasound examination of the left anterior descending coronary artery. Patients were divided into three groups: hypertensive with left ventricular hypertrophy (LVH) (n = 13), hypertensive without LVH (n = 30), and normotensive (n = 68). We found that vessel area and coronary blood flow in patients with LVH were significantly greater than in patients in the other two groups (p < 0.01, p < 0.05); furthermore, the response to both acetylcholine and adenosine was significantly impaired in patients with LVH (Hamasaki et al 2000), indicating that endothelium-dependent and -independent vasodilation were impaired in patients with hypertension and LVH.
We have reviewed several other prospective studies that evaluated different aspects of endothelial dysfunction in patients with systemic hypertension; salt-sensitive hypertension is also associated with endothelial dysfunction characterized by defective endothelium-dependent vasodilation. Impairment of the L-arginine-NO pathway may be responsible for this abnormal endothelial response (Bragulat et al 2001). Another study found that potassium increases endothelium-dependent vasodilation in essential hypertensive patients (Taddei et al 1994); the same investigator demonstrated impairment of endothelium-dependent vasodilation in renovascular and primary aldosteronism hypertensive patients and indicated that a cyclooxygenase-dependent vasoconstrictor mechanism participated in the blunting of endothelium-dependent vasodilation in essential hypertensive patients (Taddei et al 1993). Other investigators studying the vascular effect of the arginine analog NG-monomethyl-L-arginine have found that patients with essential hypertension have a defect in the endothelium-derived NO system that may at least partly account for both the increased vascular resistance under basal conditions and the impaired response to endothelium-dependent vasodilators (Panza, Casino, et al 1993). Evidence from the same group has led to the conclusion that the endothelial abnormality of patients with essential hypertension is not restricted to the muscarinic receptors (Panza et al 1994) and clinically effective antihypertensive therapy does not restore the impaired endothelium-dependent vasorelaxation in patients with essential hypertension. This indicates that such endothelial dysfunction is either primary, or becomes irreversible once the hypertensive process has become established (Panza, Quyyumi, et al 1993). Another study has shown that the endothelium-dependent vasodilatory response to acetylcholine in the forearm resistance arteries is impaired in patients with essential hypertension (Yoshida et al 1991). In contrast, it has also been argued that selective impairment of the responsiveness of the forearm vasculature to muscarinic agonists is not universal in patients with essential hypertension (Cockcroft et al 1994).
Endothelial dysfunction and aging
Vascular cells have a finite lifespan when cultured in vitro and eventually enter an irreversible growth arrest called “cellular senescence”. Recently, senescent vascular cells have been demonstrated in human atherosclerotic lesions but not in non-atherosclerotic lesions. Moreover, these cells express increased levels of proinflammatory molecules and a decreased level of endothelial NO synthase, suggesting that cellular senescence in vivo contributes to the pathogenesis of human atherosclerosis (Minamino et al 2004).
One widely discussed hypothesis of senescence is the telomere hypothesis. Introduction of telomere malfunction has been shown to lead to vascular dysfunction that promotes atherogenesis, whereas telomere lengthening extends cell lifespan and protects against vascular dysfunction associated with senescence. More recent evidence suggests that telomere-independent mechanisms are implicated in vascular cell senescence. Activation of Ras, an important signaling molecule involved in atherogenic stimuli, induces vascular cell senescence and thereby promotes vascular inflammation in vitro and in vivo. A large body of data is consistent with cellular senescence contributing to age-associated vascular disorders (Minamino et al 2004).
We reviewed published experimental and clinical studies about aging and endothelial function, most of which were small prospective studies. The hypothesis that oxidative stress, particularly oxidation of tetrahydrobiopterin, may contribute to attenuation of endothelium-dependent relaxation was tested in aged mice using the vasomotor function of isolated carotid arteries by video dimension analyzer, while vascular levels of tetrahydrobiopterin and its oxidation products were measured by high performance liquid chromatography (Blackwell et al 2004). Evidence presented seems to suggest that aging is associated with endothelial dysfunction and reduced arterial elasticity. In addition, reduced arterial elasticity parallels changes in impaired endothelium-dependent vasodilation. It appears that reduced arterial elasticity may be used as a noninvasive measure for the determination of endothelial function (Tao et al 2004). Another study concluded that there is a blunted response to acetylcholine with advancing age in both normotensive controls and essential hypertensive patients, suggesting that aging is associated with reduced endothelium-dependent vasodilation in humans (Taddei et al 1995). In normotensive individuals, an earlier primary dysfunction of the NO system and a later production of oxidative stress cause age-related reduction in endothelium-dependent vasodilation. These alterations are similar but anticipated in hypertensive patients compared with normotensive individuals (Taddei et al 2001).
High cholesterol and endothelial dysfunction
Cholesterol is one of the most well established risk factors for premature coronary artery disease (Multiple Risk Factor Intervention Trial Group 1982). Cholesterol levels and coronary artery disease risk show a strong and linear relationship; hypercholesterolemia and high levels of total cholesterol and low-density lipoprotein (LDL) cholesterol result in impaired endothelial function in both peripheral and coronary circulation (Creager et al 1990; Casino et al 1993). Studies concluded that cholesterol levels even in the normal range may be inversely related to endothelium-dependent vasodilation, and this finding has important clinical implications. This suggests that lowering cholesterol levels even when it is within the normal range may improve the production and release of endothelium-dependent NO and hence improve endothelial function (Masumoto et al 2001) (Table 1). This idea is supported by recent reports that lowering cholesterol levels enhances endothelium-dependent vasodilation not only in subjects with massively elevated cholesterol levels but also in those with normal cholesterol levels. It is worth noting that lowering of average cholesterol levels in patients with documented coronary artery disease leads to decreased rates of myocardial infarction, and this protective effect may in part be due to improvement in endothelial function (Gilligan, Guetta, et al 1994; Casino et al 1995). In addition to lipid-lowering therapy, administration of tetrahydrobiopterin, an essential cofactor for NO production, could restore NO activity in familial hypercholesterolemia (Verhaar et al 1999).
Obesity and endothelial dysfunction
The pathogenesis of vascular disease in obesity remains unclear but probably relates to the effect of the metabolic syndrome (insulin resistance, dyslipidemia, hyperoxidative stress, and hypertension) on the biology of endothelium-derived NO. The notion of increased oxidative stress in central obesity is based on an expansion of the cytosolic triglyceride storage pool in non-adipose tissue (Bakker et al 2000). The accumulation of long-chain fatty acyl-coenzyme A esters is hypothesized to inhibit mitochondrial adenosine translocation, with subsequent overproduction of oxygen free radicals such as superoxide. Strong evidence is accumulating that antioxidants may improve insulin resistance and endothelial dysfunction (Paolisso and Giugliano 1996).
We evaluated the association between endothelial function and obesity by angiography in patients (n = 379) with normal or mildly diseased coronary arteries who underwent coronary vascular activity evaluation using intracoronary infusion of acetylcholine. Patients were divided into three groups based on body mass index (BMI): group 1, BMI < 25 (n = 117; normal weight); group 2, BMI 25–30 (n = 149; overweight); and group 3, BMI > 30 (n = 131; obese). Although there were no significant differences between the three groups in regard to other cardiovascular risk factors, the percentage change of coronary blood flow to acetylcholine was significantly lower in the obese patients than in the normal-weight group (85.2% ± 12% in group 1, 63.7% ± 10% in group 2, and 38.1% ± 9.6% in group 3; p = 0.009). Furthermore, by multivariate analysis, overweight and obesity status were independently associated with endothelial dysfunction (Al Suwaidi et al 2001).
Weight loss may improve endothelial function indirectly by improving blood pressure and lipid profile. In a double-blind, placebo-controlled study using orlistat, a lipase inhibitor that prevents fat absorption, and weight loss in 23 patients, Bergholm et al (2003) demonstrated that lowering of LDL cholesterol rather than moderate weight loss improved endothelial function. However, further studies are required to confirm these observations.
There are relatively few comparable studies in the literature. Direct measures of the effects of weight loss on endothelial function have been reported to improve endothelium-dependent vasodilation with the use of a very low calorie diet for 2 weeks in obese hypertensive subjects (Sasaki et al 2002). Circulating markers of endothelial activation have been reported to be improved in obese subjects after 12 weeks of caloric restriction (800 kcal/day), achieving 9% weight loss (Ferri et al 1999), and circulating levels of inflammatory cytokines were similarly reduced in obese women after a 1-year multidisciplinary weight loss program which achieved at least 10% reduction in weight (Ziccardi et al 2002). In summary, the available literature suggests beneficial effects of weight loss on a number of axes related to vascular function. Further studies exploring the salient features of the interventions, including the degree of weight loss achieved and the timing of vascular measures in relation to the restoration of a eucaloric diet, are needed to better understand the interactions of obesity and endothelial function.
Smoking and endothelial dysfunction
Cigarette smoking is strongly associated with atherosclerosis and ischemic heart disease but also is a major risk factor for acute coronary thrombosis (Chen et al 1995; Njolstad et al 1996). Indeed, 75% of sudden cardiac deaths due to acute thrombosis are in cigarette smokers (Burke et al 1997). Smoking causes endothelial dysfunction, and passive smoking is associated with dose-related impairment of endothelium-dependent dilation in healthy young adults, suggesting early arterial damage (Hung et al 1995).
Flow-mediated dilation is significantly impaired in both passive and active smokers when compared with non-smokers. In passive smokers, there is an inverse relationship between the intensity of exposure to tobacco smoke and flow-mediated dilation.
Cigarette smoking is associated with increased platelet thrombus formation. Small areas of denudation and thrombus deposition are a common finding on the surface of atheromatous plaques and are usually subclinical. In the presence of an imbalance in the coagulation or fibrinolytic systems, such microthrombi may propagate, ultimately leading to arterial occlusion (Davies et al 1988; Bürrig 1991; Celermajer et al 1996).
Endothelial dysfunction in hyperhomocysteinemia
The nature of the link between homocysteine and cardiovascular disease has not yet been clearly established. Epidemiological studies suggested that even mild elevations of plasma homocysteine are associated with an increased risk of atherosclerosis, including coronary artery disease (Boers et al 1985; Clarke et al 1991; Stampfer et al 1992). Abnormal metabolism and handling of homocysteine have been demonstrated after a methionine challenge in individuals with premature atherosclerosis, most of whom were heterozygous for cystathionine β-synthase deficiency (Clarke et al 1991). Importantly, hyperhomocysteinemia may be a modifiable risk factor for atherosclerosis, as plasma homocysteine levels may be lowered by dietary supplementation with folate and pyridoxine (Clarke et al 1991; Selhub et al 1995).
Homocysteine influences multiple vascular responses, including coagulation, platelet function, vascular smooth muscle responses, and endothelial function. Acute infusion of homocysteine has been shown to induce frank endothelial cell damage in primates (Harker et al 1983). Homocysteine alters the production and/or bioactivity of vasoregulatory mediators (Quere et al 1997; Zhang et al 1998), including NO, by cultured endothelial cells. Furthermore, homocysteine has been shown to impair endothelium-dependent vasodilation and regulation of blood flow in primates and humans (Lentz et al 1996; Woo et al 1997; Bellamy et al 1998). The exact mechanisms underlying the beneficial effects of folates on the endothelium remain to be elucidated. Thus far, most studies have focused on the homocysteine-lowering effects of folates; however, more recently the beneficial effects of folate treatment independent of homocysteine lowering have been reported. Potential mechanisms of action include antioxidant actions, effects on cofactor availability, or direct interactions with the enzyme endothelial NO synthase (Verhaar et al 2002).
Endothelial dysfunction as a prominent feature of end-stage renal disease
Recently, several studies demonstrated impairment of (coronary or peripheral, or both) endothelium-dependent vasodilation in patients with moderate renal impairment, as well as in patients with advanced renal impairment treated by hemodialysis (Annuk et al 2001) or peritoneal dialysis (Miyazaki et al 2000). The reasons for end-stage renal disease patients having signs of endothelial dysfunction are not fully understood but are probably multifactorial. Increased oxidative stress, hyperhomocysteinemia, dyslipidemia, hyperglycemia, hypertension, and retention of L-arginine inhibitors may all be important contributors.
Endothelial dysfunction associated with other diseases
A few scattered articles have described endothelial dysfunction in other diseases, such as pulmonary hypertension (Cella et al 2001), hypertrophic cardiomyopathy (Dimitrow 2002), multiple organ dysfunction syndrome (Aird 2003), acute renal failure (Ruschitzka et al 1999), HIV infection (Nolan et al 2003), antiphospholipid syndrome (Blum and Simsolo 2004), and hyperprolactinemia (Yavuz et al 2003).
Therapy
Endothelial dysfunction is a reversible disorder, and strategies aimed at reducing cardiovascular risk factors, such as cholesterol lowering, antihypertensive therapy, smoking cessation, ACE inhibitor therapy, estrogen replacement therapy in postmenopausal women, supplementation with folic acid, and physical exercise, also translate into an improvement in endothelial health, further supporting the association between risk factors and endothelial dysfunction. Moreover, the observation that several pharmacological interventions that improve endothelial function are associated with a decrease in cardiovascular events independent of risk factor modification supports the concept that cardiovascular risk factors share a common pathway that leads to endothelial dysfunction.
Various forms of therapy have been investigated in medical studies (Table 1). The potential benefits associated with L-arginine therapy are presumably mediated by increased NO activity (Creager et al 1992; Boger et al 1998). In addition to improved endothelial function, other changes that have been described include lower plasma endothelin concentrations, increased apoptosis of vascular cells in intimal lesions (leading to regression of atherosclerosis and decreased symptoms), and prevention of the progression of atherosclerotic plaques (Rector et al 1996; Quyyumi et al 1997). The endothelium-dependent vasorelaxation with statins results in part from endothelial NO activation. This is independent of the cholesterol-lowering effect of these drugs, although a reduction in oxidized LDL contributes to this response, since oxidized LDL, but not native LDL, down-regulates endothelial NO synthase activity; statins increase the activity of this enzyme independently of lipid lowering. In addition to their effect on NO, statins affect the vasoconstrictor endothelin, further shifting the balance toward vasodilation (O'Driscoll, Green, and Taylor 1997; Vita et al 2000).
Fibrate therapy also improves fasting and postprandial endothelial function in patients with type 2 diabetes. The mechanism for this may be an increase in high-density lipoprotein and an attenuation of postprandial lipemia and the associated oxidative stress (Evans et al 2000).
Folate improves endothelial dysfunction by reducing the serum levels of homocysteine. Elevated levels of homocysteine promote endothelial dysfunction by their toxic effects on the endothelium, probably mediated by an increase in oxidative stress and inhibition of NO production (Verhaar et al 1999).
More recently, the HOPE (Heart Outcome Prevention Evaluation) trial assessed the role of an ACE inhibitor, ramipril, in patients who were at high risk for cardiovascular events but who did not have left ventricular dysfunction or heart failure. Ramipril significantly reduced the rate of death, myocardial infarction, and stroke in a broad range of high-risk patients who did not have a low ejection fraction or heart failure, thus suggesting that the use of ACE inhibitors may prevent the progression of initially clinically silent atherosclerosis (Dagenais et al 2001).
Even more recently, the insulin sensitizer thiazolidinediones were found to improve endothelial function in diabetic patients (Tack et al 1998; Pistrosch et al 2004).
Although various interventions have been shown to be associated with improvement in endothelial function, little is currently known about the clinical and prognostic effects of therapeutic improvement in endothelial function. Cholesterol lowering with statins and therapy with ACE inhibitors are also associated with a reduction of myocardial ischemia in patients with coronary artery disease. Moreover, the improvement in coronary endothelial function by L-arginine supplementation has been associated with a reduction in anginal symptoms. Taken together, these findings point out the clinical significance of therapeutic improvement in coronary endothelial function.
Prognostic value of endothelial dysfunction
To date, 14 published studies have examined the value of endothelial dysfunction in prognosis. Five of these assessed coronary endothelial dysfunction and the remainder evaluated brachial endothelial function. Three of these studies were retrospective analyses and the rest were prospective studies (Table 2). We retrospectively evaluated the long-term outcome of patients (n = 157) with normal or mildly diseased coronary arteries who underwent coronary vascular reactivity evaluation. Patients were divided into three groups according to their response to intracoronary acetylcholine: a normal endothelial function group, a mild endothelial dysfunction group, and a moderate-to-severe endothelial dysfunction group. We found there were no significant differences between the various groups in regard to traditional cardiovascular risk factors. At a mean follow-up of 28 months, six patients in the moderate-to-severe endothelial dysfunction group developed cardiac events, suggesting that endothelial dysfunction is an independent predictor for the development of cardiac events (Al Suwaidi et al 2000).
Table 2.
Studies on the prognostic effect of coronary and peripheral endothelial dysfunction
| Reference | Study type (time of follow-up) | Patient population | Vascular bed | Marker of endothelial function | End points examined | Finding |
|---|---|---|---|---|---|---|
| Yataco et al 1999 | Prospective | 50 with PVD, 50 matched controls | Coronary | FMD | Higher incidence of CAD | |
| Al Suwaidi et al 2000 | Retrospective (28 mo) | 157 with mild CAD | Coronary | Acetylcholine response | Cardiac death, MI, CHF, CABG, or PCI | 3.8% CV event rate; acetylcholine response independent predictor of events |
| Schächinger et al 2000 | Retrospective (7.7 y) | 147 with CAD | Coronary | Acetylcholine, cold pressor test, FMD, NTG | MI, UA, ischemic stroke, CABG, PTCA, peripheral bypass | 19% event rate; vasomotor function independent predictor of events |
| Neunteufl et al 2000 | Retrospective (5 y) | 73 with CAD | Brachial | FMD | Death, MI, PTCA, or CABG | 36% of patients with event; FMD < 10% predictive of events; effect lost when controlling for extent of CAD |
| Teragawa et al 2001 | Prospective | 30 with vasospastic angina, 30 controls | Brachial | FMD | Impaired FMD | |
| Perticone et al 2001 | Prospective (32 mo) | 225 with hypertension | Brachial | Forearm blood flow response to acetylcholine | CV death, MI, stroke, TIA, UA, CABG, PTCA, PVD | 12.8% CV event rate; acetylcholine response predictor of events |
| Heitzer et al 2001 | Prospective (4.5 y) | 281 with CAD | Brachial | Forearm blood flow response to acetylcholine | CV death, MI, CABG, PTCA, peripheral bypass | 32% CV event rate; acetylcholine response independent predictor of events |
| Gokce et al 2002 | Prospective (30 d) | 187 undergoing vascular, surgery | Brachial | FMD | CV death, MI, stroke, UA | 24% CV event rate; FMD independent predictor of events |
| Halcox et al 2002 | Prospective (46 mo) | 308 referred for cardiac catheterization | Coronary | Acetylcholine response | CV death, MI, ischemic stroke, UA | 11% CV event rate; acetylcholine response independent predictor of events |
| Modena et al 2002 | Prospective (67 mo) | 400 hypertensive postmenopausal women | Brachial | FMD | Hospitalization for CV event (not otherwise specified) | 11.7% CV event rate; failure to improve FMD with 6 months of antihypertensive therapy independent predictor of events |
| Gokce et al 2003 | Prospective (1.2 y) | 199 undergoing vascular surgery | Brachial | FMD | CV death, MI, UA, stroke | 17.5% CV event rate; FMD independent predictor of long-term events |
| Schindler et al 2003 | Prospective (45 mo) | 130 with normal coronary angiograms | Coronary | Cold presser test | CV death, UA, MI, PTCA, CABG, stroke, peripheral bypass | 20% CV event rate; cold pressor test response independent predictor of events |
| Brevetti, Silvestro, Schiano, et al 2003 | Prospective | 88 with PVD, 30 controls | Brachial | FMD in relation to ankle-brachial index | Significant correlation | |
| Brevetti, Silvestro, Di Giacomo, et al 2003 | Prospective | Brachial | Noninvasive FMD | Significant correlation with CV event |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CV, cardiovascular; FMD, flow-mediated dilation; MI, myocardial infarction; NTG, nitroglycerin; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; TIA, transient ischemic attack; UA, unstable angina.
Since the publication of our findings, several other investigators have reported similar findings in patients with obstructive coronary artery disease and peripheral vascular disease. This seems to show the importance of evaluating the function of endothelial cells as an independent predictor in all patients with or without coronary artery disease in either peripheral or coronary blood vessels.
Future perspectives
The future will witness increasing interest in finding reliable methods of testing endothelial function; several large noninvasive studies are needed to determine the predictive value of brachial ultrasound testing as a potential predictor of cardiovascular disease. As the measures of endothelial dysfunction become clinically applicable, this may translate into improved methods of risk assessment that help in predicting, preventing, and treating cardiovascular disease. Inflammatory markers, such as C-reactive protein, will probably find their way into risk assessment; several therapeutic strategies aimed at improving endothelial function in a variety of cardiovascular disease states are under investigation. The future holds great promise.
References
- Aanderud S, Krane H, Nordoy A. Influence of glucose, insulin and sera from diabetic patients on the prostacyclin synthesis in vitro in cultured human endothelial cells. Diabetologia. 1985;28:641–4. doi: 10.1007/BF00291967. [DOI] [PubMed] [Google Scholar]
- Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- Al Suwaidi J, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Al Suwaidi J, Higano ST, Holmes DR, et al. Obesity is an independent predictor of coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34:631–8. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Elstein E, Haber H, et al. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35:60–6. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Gerhard MD, Meredith IT, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Andrews NP, Prasad A, Quyyumi AA. N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol. 2001;37:117–23. doi: 10.1016/s0735-1097(00)01093-7. [DOI] [PubMed] [Google Scholar]
- Annuk M, Lind L, Linde T, et al. Impaired endothelium-dependent vasodilation in renal failure in humans. Nephrol Dial Transplant. 2001;16:302–6. doi: 10.1093/ndt/16.2.302. [DOI] [PubMed] [Google Scholar]
- Arbogast BW, Lee GM, Raymond TL. In vitro injury of porcine aortic endothelial cells by very-low-density lipoproteins from diabetic rat serum. Diabetes. 1982;31:593–9. doi: 10.2337/diab.31.7.593. [DOI] [PubMed] [Google Scholar]
- Arcaro G, Cretti A, Balzano A, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–82. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Calo L, Piarulli F, et al. Effect of acute ketosis on the endothelial function of type 1 diabetic patients: the role of nitric oxide. Diabetes. 1999;48:391–7. doi: 10.2337/diabetes.48.2.391. [DOI] [PubMed] [Google Scholar]
- Bakker SJ, Ijzerman RG, Teerlink T, et al. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction and beta-cell failure? Atherosclerosis. 2000;148:17–21. doi: 10.1016/s0021-9150(99)00329-9. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-κ: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bellamy MF, McDowell IFW, Ramsey MW, et al. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation. 1998;98:1848–52. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- Berger R, Stanek B, Hulsmann M, et al. Effects of endothelin A receptor A blockade on endothelial function in patients with chronic heart failure. Circulation. 2001;103:981–6. doi: 10.1161/01.cir.103.7.981. [DOI] [PubMed] [Google Scholar]
- Bergholm R, Tiikkainen M, Vehkavaara S, et al. Lowering of LDL cholesterol rather than moderate weight loss improves endothelium-dependent vasodilatation in obese women with previous gestational diabetes. Diabetes Care. 2003;26:1667–72. doi: 10.2337/diacare.26.6.1667. [DOI] [PubMed] [Google Scholar]
- Bijlstra PJ, Smits P, Lutterman JA, et al. Effect of long-term angiotensin-converting enzyme inhibition on endothelial function in patients with the insulin-resistance syndrome. J Cardiovasc Pharmacol. 1995;25:658–64. doi: 10.1097/00005344-199504000-00021. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, et al. Mechanisms of aging-induced impairment of endothelium-dependent relaxations – role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–53. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT. Endothelial dysfunction, neuropathy and the diabetic foot, diabetic mastopathy, and erectile dysfunction. Diabetes Care. 1998;21:183–9. doi: 10.2337/diacare.21.1.183. [DOI] [PubMed] [Google Scholar]
- Blum A, Simsolo C. The antiphospholipid syndrome and endothelial function. Isr Med Assoc J. 2004;6:556–8. [PubMed] [Google Scholar]
- Boers GH, Smals AG, Trijbels FJ, et al. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. N Engl J Med. 1985;313:709–15. doi: 10.1056/NEJM198509193131201. [DOI] [PubMed] [Google Scholar]
- Bogaty P, Hackett D, Davies G, et al. Vasoreactivity of the culprit lesion in unstable angina. Circulation. 1994;90:5–11. doi: 10.1161/01.cir.90.1.5. [DOI] [PubMed] [Google Scholar]
- Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–7. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Bragulat E, de la Sierra A, Antonio MT, et al. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–8. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Silvestro A, Di Giacomo S, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. Vasc Surg. 2003;38:374–9. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Silvestro A, Schiano V, et al. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- Burke AP, Farb A, Malcolm GT, et al. Coronary risk factors and plaque morphology in men with coronary artery disease who died suddenly. N Engl J Med. 1997;336:1276–82. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- Bürrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb. 1991;11:1678–89. [PubMed] [Google Scholar]
- Caballero AE, Arora S, Saouaf R, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–62. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- Calles-Escandon J, Ballor D, Harvey-Berino J, et al. Amelioration of the inhibition of fibrinolysis in elderly, obese subjects by moderate energy intake restriction. Am J Clin Nutr. 1996;64:7–11. doi: 10.1093/ajcn/64.1.7. [DOI] [PubMed] [Google Scholar]
- Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Campia U, Kilcoyne CM, et al. Improved endothelium-dependent vasodilation after blockade of endothelin receptors in patients with essential hypertension. Circulation. 2002;105:452–6. doi: 10.1161/hc0402.102989. [DOI] [PubMed] [Google Scholar]
- Casino PR, Kilcoyne CM, Cannon RO, et al. Impaired endothelium-dependent vascular relaxation in patients with hypercholesterolemia extends beyond the muscarinic receptor. Am J Cardiol. 1995;75:40–4. doi: 10.1016/s0002-9149(99)80524-4. [DOI] [PubMed] [Google Scholar]
- Casino PR, Kilcoyne CM, Quyyumi AA, et al. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–7. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, et al. Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–15. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Cella G, Bellotto F, Tona F, et al. Plasma markers of endothelial dysfunction in pulmonary hypertension. Chest. 2001;120:1226–30. doi: 10.1378/chest.120.4.1226. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Ueland PM, Obeid OA, et al. Improved vascular endothelial function after oral B vitamins: an effect mediated through reduced concentrations of free plasma homocysteine. Circulation. 2000;102:2479–83. doi: 10.1161/01.cir.102.20.2479. [DOI] [PubMed] [Google Scholar]
- Cheetham C, Collis J, O'Driscoll G, et al. Losartan, an angiotensin type 1 receptor antagonist, improves endothelial function in noninsulin-dependent diabetes. J Am Coll Cardiol. 2000;36:1461–6. doi: 10.1016/s0735-1097(00)00933-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Chester M, Kaski JC. Clinical factors and angiographic features associated with premature coronary artery disease. Chest. 1995;108:364–9. doi: 10.1378/chest.108.2.364. [DOI] [PubMed] [Google Scholar]
- Chou EJ, Keevil JG, Aeschlimann S, et al. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am J Cardiol. 2001;88:553–5. doi: 10.1016/s0002-9149(01)01738-6. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ. Elevated glucose potentiates contraction of isolated rat resistance arteries and augments protein kinase C-induced intracellular calcium release. Metabolism. 1999;48:1015–22. doi: 10.1016/s0026-0495(99)90199-3. [DOI] [PubMed] [Google Scholar]
- Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–55. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- Clarke SC, Schofield PM, Grace AA, et al. Tamoxifen effects on endothelial function and cardiovascular risk factors in men with advanced atherosclerosis. Circulation. 2001;103:1497–502. doi: 10.1161/01.cir.103.11.1497. [DOI] [PubMed] [Google Scholar]
- Clarkson P, Celermajer DS, Donald AE, et al. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol. 1996;28:573–9. doi: 10.1016/0735-1097(96)82380-1. [DOI] [PubMed] [Google Scholar]
- Cockcroft JR, Chowienczyk PJ, Benjamin N, et al. Preserved endothelium-dependent vasodilatation in patients with essential hypertension. N Engl J Med. 1994;330:1081–3. doi: 10.1056/NEJM199404143301502. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Luscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S54–61. [PubMed] [Google Scholar]
- Creager MA, Cooke JP, Mendelsohn MP, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–34. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager MA, Gallagher SI, Girerd XJ, et al. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–53. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M, Lumaitre RN, Kuller LH, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly. The cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:493–8. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Yusuf S, Bourassa MG, et al. Effects of ramipril on coronary events in high-risk persons: results of the Heart Outcomes Prevention Evaluation Study. Circulation. 2001;104:522–6. doi: 10.1161/hc3001.093502. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Woolf N, Rowles PM, et al. Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Br Heart J. 1988;60:459–64. doi: 10.1136/hrt.60.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- De Catarina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–8. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mattia G, Bravi MC, Laurenti O, et al. Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia. 1998;41:1392–6. doi: 10.1007/s001250051082. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dimitrow PP. Coronary endothelial dysfunction in patients with hypertrophic cardiomyopathy. Chest. 2002;121:1374. doi: 10.1378/chest.121.4.1374. [DOI] [PubMed] [Google Scholar]
- Dosquet C, Wautier MP, Guillausseau PJ, et al. Monokines and endothelial cell proliferation in patients with diabetes mellitus. Diabetes Metab. 1994;20:37–42. [PubMed] [Google Scholar]
- Duffy SJ, Keaney JF, Jr, Holbrook M, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–6. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Tardif JC, Cernacek P, et al. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (Reduction of Cholesterol in Ischemia and Function of the Endothelium) trial. Circulation. 1999;99:3227–33. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- Elhadd TA, Khan F, Kirk G, et al. Influence of puberty on endothelial dysfunction and oxidative stress in young patients with type 1 diabetes. Diabetes Care. 1998;21:1990–6. doi: 10.2337/diacare.21.11.1990. [DOI] [PubMed] [Google Scholar]
- ENCORE I Study. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin on Recovery of Coronary Endothelial Function) Circulation. 2003;107:422–8. doi: 10.1161/01.cir.0000046488.52939.bf. [DOI] [PubMed] [Google Scholar]
- Evans M, Anderson RA, Graham J, et al. Ciprofibrate therapy improves endothelial function and reduces postprandial lipemia and oxidative stress in type 2 diabetes mellitus. Circulation. 2000;101:1773–9. doi: 10.1161/01.cir.101.15.1773. [DOI] [PubMed] [Google Scholar]
- Farouque HM, Meredith IT. The assessment of endothelial function in humans. Coron Artery Dis. 2001;12:445–54. doi: 10.1097/00019501-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Fasching P, Veitl M, Rohac M, et al. Elevated concentrations of circulating adhesion molecules and their association with microvascular complications in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4313–17. doi: 10.1210/jcem.81.12.8954033. [DOI] [PubMed] [Google Scholar]
- Ferri C, Bellini C, Desideri G, et al. Circulating endothelin-1 levels in obese patients with the metabolic syndrome. Exp Clin Endocrinol Diabetes. 1997;105(Suppl 2):38–40. doi: 10.1055/s-0029-1211794. [DOI] [PubMed] [Google Scholar]
- Ferri C, Desideri G, Valenti M, et al. Early up regulation of endothelial adhesion molecules in obese hypertensive men. Hypertension. 1999;34:568–73. doi: 10.1161/01.hyp.34.4.568. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S, Rosenberger G, Walter DH, et al. Elevated C-reactive protein level and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–6. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- Fuentes F, Lopez-Miranda J, Sanchez E, et al. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med. 2001;134:1115–19. doi: 10.7326/0003-4819-134-12-200106190-00011. [DOI] [PubMed] [Google Scholar]
- Gazis A, White DJ, Page SR, et al. Effect of oral vitamin E (α-tocopherol) supplementation on vascular endothelial function in type 2 diabetes mellitus. Diabet Med. 1999;16:304–11. doi: 10.1046/j.1464-5491.1999.00049.x. [DOI] [PubMed] [Google Scholar]
- Gilligan DM, Guetta V, Panza JA, et al. Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation. 1994;90:35–41. doi: 10.1161/01.cir.90.1.35. [DOI] [PubMed] [Google Scholar]
- Gilligan DM, Quyyumi AA, Cannon RO., III Effects of physiologic levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–51. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- Gokce N, Vits JA. Thrombosis and hemorrhage. In: Loscalzo J, Schafer AI, editors. Clinical manifestation of endothelial dysfunction. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 685–706. [Google Scholar]
- Goodfellow J, Bellamy MF, Ramsey MW, et al. Dietary supplementation with marine omega-3 fatty acids improves systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000;35:265–70. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Sinkey C, Barenz T, et al. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–21. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- Hamasaki S, Al Suwaidi J, Higano ST, et al. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hyperatrophy. J Am Coll Cardiol. 2000;35:1654–60. doi: 10.1016/s0735-1097(00)00594-5. [DOI] [PubMed] [Google Scholar]
- Harker LA, Harlan JM, Ross R. Effect of sulfinpyrazone on homocysteine-induced endothelial injury and arteriosclerosis in baboons. Circ Res. 1983;53:731–9. doi: 10.1161/01.res.53.6.731. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Kasai K, Nakamura T, et al. Effect of glucose and insulin on immunoreactive endothelin-1 release from cultured porcine aortic endothelial cells. Metabolism. 1991;40:165–9. doi: 10.1016/0026-0495(91)90168-v. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–19. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig B, Landmesser U, Kohler C, et al. Comparative effect of ACE inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001;103:799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- Hsueh WA, Anderson PW. Hypertension, the endothelial cell, and the vascular complications of diabetes mellitus [clinical conference] Hypertension. 1992;20:253–63. doi: 10.1161/01.hyp.20.2.253. [DOI] [PubMed] [Google Scholar]
- Hung J, Lam JYT, Lacoste L, et al. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–6. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- Husain S, Andrews NP, Mulcahy D, et al. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97:716–20. doi: 10.1161/01.cir.97.8.716. [DOI] [PubMed] [Google Scholar]
- Huszka M, Kaplar M, Rejto L, et al. The association of reduced endothelium derived relaxing factor-NO production with endothelial damage and increased in vivo platelet activation in patients with diabetes mellitus. Thromb Res. 1997;86:173–80. doi: 10.1016/s0049-3848(97)00060-1. [DOI] [PubMed] [Google Scholar]
- Huvers FC, De Leeuw PW, Houben AJ, et al. Endothelium-dependent vasodilatation, plasma markers of endothelial function, and adrenergic vasoconstrictor responses in type 1 diabetes under near-normoglycemic conditions. Diabetes. 1999;48:1300–7. doi: 10.2337/diabetes.48.6.1300. [DOI] [PubMed] [Google Scholar]
- Janka HU. Platelet and endothelial function tests during metformin treatment in diabetes mellitus. Horm Metab Res Suppl. 1985;15:120–2. [PubMed] [Google Scholar]
- Johnstone MT, Creager SJ, Scales KM, et al. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–16. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, et al. Coronary heart disease in smokers. Vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–8. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80:11-I–16-I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- Koh MS, Majewski BB, Rhodes EL. Diabetic serum stimulates the proliferation of endothelial cells in culture. Diabetes Res. 1985;2:287–9. [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer AM, Campbell B, Shin Y-K, et al. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–84. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- Lentz SR, Sobey CG, Piegors DJ, et al. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98:24–9. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman A, Burnett JC., Jr Intact and altered endothelium in regulation of vasomotion. Circulation. 1992;86(Suppl III):III-12–III-19. [PubMed] [Google Scholar]
- Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lissin LW, Cooke JP. Phytoestrogens and cardiovascular health. J Am Coll Cardiol. 2000;35:1403–10. doi: 10.1016/s0735-1097(00)00590-8. [DOI] [PubMed] [Google Scholar]
- Lorenzi M, Montisano DF, Toledo S, et al. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986;77:322–5. doi: 10.1172/JCI112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–41. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- Makimattila S, Virkamaki A, Groop PH, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–82. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- Malamitsi-Puchner A, Sarandakou A, Tziotis J, et al. Serum levels of basic fibroblast growth factor and vascular endothelial growth factor in children and adolescents with type 1 diabetes mellitus. Pediatr Res. 1998;44:873–5. doi: 10.1203/00006450-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Mancini GBJ, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing Endothelial Dysfunction) Study. Circulation. 1996;94:258–65. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Hirooka Y, Hironaga K, et al. Effect of pravastatin on endothelial function in patients with coronary artery disease (cholesterol-independent effect of pravastatin) Am J Cardiol. 2001;88:1291–4. doi: 10.1016/s0002-9149(01)02090-2. [DOI] [PubMed] [Google Scholar]
- Mather KJ, Verma S, Anderson T. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;38:2131–2. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- McFarlane R, McCredie RJ, Bonney MA, et al. Angiotensin converting enzyme inhibition and arterial endothelial function in adults with type 1 diabetes mellitus. Diabet Med. 1999;16:62–6. doi: 10.1046/j.1464-5491.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, et al. The role of vascular cell senescence in atherosclerosis: antisenescence as a novel therapeutic strategy for vascular aging. Curr Vasc Pharmacol. 2004;2:141–8. doi: 10.2174/1570161043476393. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Matsuka H, Itabe H, et al. Hemodialysis impairs endothelial function via oxidative stress. Effects of vitamin E-coated dialyzer. Circulation. 2000;101:1002–6. doi: 10.1161/01.cir.101.9.1002. [DOI] [PubMed] [Google Scholar]
- Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–10. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Mullen MJ, Clarkson R, Donald AE, et al. Effect of enalapril on endothelial function in young insulin-dependent diabetic patients: a randomized, double blind study. J Am Coll Cardiol. 1998;31:1330–5. doi: 10.1016/s0735-1097(98)00099-0. [DOI] [PubMed] [Google Scholar]
- Multiple Risk Factor Intervention Trial Group. Multiple Risk Factor Intervention Trial: risk factor changes and mortality results. JAMA. 1982;248:1465–77. [PubMed] [Google Scholar]
- Myrup B, Mathiesen ER, Ronn B, et al. Endothelial function and serum lipids in the course of developing microalbuminuria in insulin-dependent diabetes mellitus. Diabetes Res. 1994;26:33–9. [PubMed] [Google Scholar]
- Napoli C, de Nigris F, Palinski W. Multiple roles of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–82. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- Neri S, Bruno CM, Leotta C, et al. Early endothelial alterations in non-insulin-dependent diabetes mellitus. Int J Clin Lab Res. 1998;28:100–3. doi: 10.1007/s005990050027. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–10. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Njolstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction: a 12-year follow-up of the Finnmark study. Circulation. 1996;93:450–6. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- Nolan D, Watts GF, Herrmann SE, et al. Endothelial function in HIV-infected patients receiving protease inhibitor therapy: does immune competence affect cardiovascular risk? QJM. 2003;96:825–32. doi: 10.1093/qjmed/hcg145. [DOI] [PubMed] [Google Scholar]
- Nordt TK, Klassen KJ, Schneider DJ, et al. Augmentation of synthesis of plasminogen activator inhibitor type-1 in arterial endothelial cells by glucose and its implications for local fibrinolysis. Arterioscler Thromb. 1993;13:1822–8. doi: 10.1161/01.atv.13.12.1822. [DOI] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Maiorana A, et al. Improvement in endothelial function by angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1999;33:1506–11. doi: 10.1016/s0735-1097(99)00065-0. [DOI] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Rankin J, et al. Improvement in endothelial function by angiotensin converting enzyme inhibition in insulin-dependent diabetes mellitus. J Clin Invest. 1997;100:678–84. doi: 10.1172/JCI119580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–31. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- Panza JA, Casino PR, Kilcoyne CM, et al. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–74. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- Panza JA, Casino PR, Kilcoyne CM, et al. Impaired endothelium-dependent vasodilation in patients with essential hypertension: evidence that the abnormality is not at the muscarinic receptor level. J Am Coll Cardiol. 1994;23:1610–16. doi: 10.1016/0735-1097(94)90664-5. [DOI] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Callahan TS, et al. Effect of antihypertensive treatment on endothelium-dependent vascular relaxation in patients with essential hypertension. J Am Coll Cardiol. 1993;21:1145–51. doi: 10.1016/0735-1097(93)90238-v. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia. 1996;39:357–63. doi: 10.1007/BF00418354. [DOI] [PubMed] [Google Scholar]
- Peng HB, Libby P, Liao JK. Induction and stabilization of IκBα by nitric oxide mediates inhibition of NF-κB. J Biol Chem. 1995;270:14214–19. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Pistrosch F, Passauer J, Fischer S, et al. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–90. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- Prasad A, Tupas-Habib T, Schenke WH, et al. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–54. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- Prasad A, Zhu J, Halcox JP, et al. Predisposition to atherosclerosis by infections. Role of endothelial dysfunction. Circulation. 2002;106:184–90. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- Quere I, Hillaire-Buys D, Brunschwig J, et al. Effects of homocysteine on acetylcholine- and adenosine-induced vasodilation of pancreatic vascular bed in rats. Br J Pharmacol. 1997;122:351–7. doi: 10.1038/sj.bjp.0701358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyyumi AA, Dakak N, Diodati JG, et al. Effect of L-arginine on human coronary endothelium-dependent and physiologic vasodilation. J Am Coll Cardiol. 1997;30:1220–7. doi: 10.1016/s0735-1097(97)00279-9. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen N, Ihlemann T, Krarup E, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611–18. doi: 10.2337/diabetes.50.11.2611. [DOI] [PubMed] [Google Scholar]
- Rector TS, Bank AJ, Mullen KA, et al. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–41. doi: 10.1161/01.cir.93.12.2135. [DOI] [PubMed] [Google Scholar]
- Ruschitzka F, Shaw S, Gygi D, et al. Endothelial dysfunction in acute renal failure. J Am Soc Nephrol. 1999;10:953–62. doi: 10.1681/ASN.V105953. [DOI] [PubMed] [Google Scholar]
- Ryan M, McInerney D, Owens D, et al. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM. 2000;93:85–91. doi: 10.1093/qjmed/93.2.85. [DOI] [PubMed] [Google Scholar]
- Salvolini E, Rabini RA, Martarelli D, et al. A study on human umbilical cord endothelial cells: functional modifications induced by plasma from insulin-dependent diabetes mellitus patients. Metabolism. 1999;48:554–7. doi: 10.1016/s0026-0495(99)90049-5. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Higashi Y, Nakagawa K, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15:302–9. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Schindler TH, Horing B, Buser PT, et al. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 2003;23:495–501. doi: 10.1161/01.ATV.0000057571.03012.F4. [DOI] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Bostom AG, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–91. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Gaziano JM, Buring JE, et al. Coffee and tea intake and the risk of myocardial infarction. Am J Epidemiol. 1999;149:162–7. doi: 10.1093/oxfordjournals.aje.a009782. [DOI] [PubMed] [Google Scholar]
- Skrha J, Vackova I, Kvasnicka J, et al. Plasma-free N-terminal fibronectin 30-kDa domain as a marker of endothelial dysfunction in type 1 diabetes mellitus. Eur J Clin Invest. 1990;20:171–6. doi: 10.1111/j.1365-2362.1990.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Skyrme-Jones RA, O'Brien RC, Berry KL, et al. Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomized, placebo-controlled study. J Am Coll Cardiol. 2000;36:94–102. doi: 10.1016/s0735-1097(00)00720-8. [DOI] [PubMed] [Google Scholar]
- Skyrme-Jones RA, O'Brien RC, Luo M, et al. Endothelial vasodilator function is related to low-density lipoprotein particle size and lowdensity lipoprotein vitamin E content in type 1 diabetes. J Am Coll Cardiol. 2000;35:292–9. doi: 10.1016/s0735-1097(99)00547-1. [DOI] [PubMed] [Google Scholar]
- Sorensen KE, Celermajer DS, Georgakopoulos D, et al. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein (a) level. J Clin Invest. 1994;93:50–5. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–81. [PubMed] [Google Scholar]
- Stein JH, Carlsson CM, Papcke-Benson K, et al. The effects of lipid-lowering and antioxidant vitamin therapies on flow-mediated vasodilation of the brachial artery in older adults with hypercholesterolemia. J Am Coll Cardiol. 2001;38:1806–13. doi: 10.1016/s0735-1097(01)01650-3. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, et al. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–9. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlinger MC, Tsao PS, Her JH, et al. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569–75. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- Tack CJ, Ong MK, Lutterman JA, et al. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia. 1998;41:569–76. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- Taddei S, Mattei P, Virdis A, et al. Effect of potassium on vasodilation to acetylcholine in essential hypertension. Hypertension. 1994;23:485–90. doi: 10.1161/01.hyp.23.4.485. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, et al. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension. 1993;21(6 Pt 2):929–33. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–7. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tao J, Jin YF, Yang Z, et al. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 2004;17:654–9. doi: 10.1016/j.amjhyper.2004.03.678. [DOI] [PubMed] [Google Scholar]
- Teragawa H, Kato M, Kurokawa JK, et al. Endothelial dysfunction is an independent factor responsible for vasospastic angina. Clin Sci (Lond) 2001;101:707–13. [PubMed] [Google Scholar]
- Thogersen AM, Jansson J, Boman K, et al. High plasminogen activator inhibitor and tissue plasminogen activator level in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–7. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- Thompson LP, Pinkas G, Weiner CP. Chronic 17 beta-estradiol replacement increases nitric oxide-mediated vasodilation of guinea pig coronary microcirculation. Circulation. 2000;102:445–51. doi: 10.1161/01.cir.102.4.445. [DOI] [PubMed] [Google Scholar]
- Thorne S, Mullen MJ, Clarkson P, et al. Early endothelial dysfunction in adults at risk from atherosclerosis: different responses to L-arginine. J Am Coll Cardiol. 1998;32:110–15. doi: 10.1016/s0735-1097(98)00211-3. [DOI] [PubMed] [Google Scholar]
- Ting HH, Timimi FK, Haley EA, et al. Vitamin C improves endothelium dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–22. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- Vehkavaara S, Yki-Jarvinen H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2001;24:325–30. doi: 10.1161/01.ATV.0000113817.48983.c5. [DOI] [PubMed] [Google Scholar]
- Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation. 1996;94:727–33. doi: 10.1161/01.cir.94.4.727. [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Wever RMF, Kastelein JJP, et al. Effects of oral folic acid supplementation on endothelial function in familial hypercholesterolemia: a randomized placebo-controlled trial. Circulation. 1999;100:335–8. doi: 10.1161/01.cir.100.4.335. [DOI] [PubMed] [Google Scholar]
- Vita JA, Yeung AC, Winniford M, et al. Effect of cholesterol-lowering therapy on coronary endothelial vasomotor function in patients with coronary artery disease. Circulation. 2000;102:846–51. doi: 10.1161/01.cir.102.8.846. [DOI] [PubMed] [Google Scholar]
- Wakatsuki A, Okatani Y, Ikenoue N, et al. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–8. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- Walker HA, Dean TS, Sanders TA, et al. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation. 2001;103:258–62. doi: 10.1161/01.cir.103.2.258. [DOI] [PubMed] [Google Scholar]
- Wang BY, Ho HK, Lin PS, et al. Regression of atherosclerosis: role of nitric oxide and apoptosis. Circulation. 1999;99:1236–41. doi: 10.1161/01.cir.99.9.1236. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Stamenkovic D, et al. Raloxifene improves endothelial dysfunction in hypertension by reduced oxidative stress and enhanced nitric oxide production. Circulation. 2002;105:2083–91. doi: 10.1161/01.cir.0000014618.91633.67. [DOI] [PubMed] [Google Scholar]
- Watts GF, Playford DA. Dyslipoproteinaemia and hyperoxidative stress in the pathogenesis of endothelial dysfunction in non-insulin dependent diabetes mellitus: a hypothesis. Atherosclerosis. 1998;141:17–30. doi: 10.1016/s0021-9150(98)00170-1. [DOI] [PubMed] [Google Scholar]
- Webb CM, Ghatei MA, McNeill JG, et al. 17 beta-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation. 2000;102:1617–22. doi: 10.1161/01.cir.102.14.1617. [DOI] [PubMed] [Google Scholar]
- Webb CM, McNeill JG, Hayward CS, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–6. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- Woo KS, Chook P, Lolin YI, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96:2542–4. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- Xiang GD, He YS, Dai XJ, et al. Angiotensin-converting enzyme gene and the brachial artery endothelial dysfunction in type 2 diabetes without angiopathy. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:355–9. [PubMed] [Google Scholar]
- Yaqoob M, Patrick AW, McClelland P, et al. Relationship between markers of endothelial dysfunction, oxidant injury and tubular damage in patients with insulin-dependent diabetes mellitus. Clin Sci (Colch) 1993;85:557–62. doi: 10.1042/cs0850557. [DOI] [PubMed] [Google Scholar]
- Yataco AR, Corretti MC, Gardner AW, et al. Endothelial reactivity and cardiac risk factors in older patients with peripheral arterial disease. Am J Cardiol. 1999;83:754–8. doi: 10.1016/s0002-9149(98)00984-9. [DOI] [PubMed] [Google Scholar]
- Yavuz D, Deyneli O, Akpinar I, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic premenopausal women. Eur J Endocrinol. 2003;149:187–93. doi: 10.1530/eje.0.1490187. [DOI] [PubMed] [Google Scholar]
- Yokoyama I, Momomura S-I, Ohtake T, et al. Improvement of impaired myocardial vasodilatation due to diffuse coronary atherosclerosis in hypercholesterolemics after lipid-lowering therapy. Circulation. 1999;100:117–22. doi: 10.1161/01.cir.100.2.117. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Imaizumi T, Ando S, et al. Impaired forearm vasodilatation by acetylcholine in patients with hypertension. Heart Vessels. 1991;6:218–23. doi: 10.1007/BF02125100. [DOI] [PubMed] [Google Scholar]
- Zhang F, Slungaard A, Vercellotti GM, et al. Superoxide-dependent cerebrovascular effects of homocysteine. Am J Physiol. 1998;274:R1704–11. doi: 10.1152/ajpregu.1998.274.6.R1704. [DOI] [PubMed] [Google Scholar]
- Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–9. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]