Abstract
Purpose: Alveolar rhabdomyosarcoma (ARMS) frequently contains the fusion transcription factor PAX3/FKHR. Therefore, clinical studies have been initiated to utilize the PAX3/FKHR translocation point area as a peptide vaccine against ARMS. Our study was directed at identifying antigenic T-lymphocyte epitopes at the PAX3/FKHR translocation point area. Experimental design: The peptide sequence surrounding the PAX3/FKHR translocation point was evaluated by MHC binding algorithms for potential T-lymphocyte antigenic epitopes (class I molecules HLA-A1, -A2 and -A3; class II molecules HLA-DR1, -DR4 and -DR7). Using in vitro techniques, dendritic cells loaded with PAX3/FKHR peptides were used to stimulate naïve T-lymphocytes. T-lymphocyte activity was then evaluated by 51Cr release and 3H-thymidine uptake assays. Results: Only one HLA-A3-restricted epitope was predicted by the algorithms. The peptide was prepared and tested for its ability to stimulate naïve cytotoxic T-lymphocytes (CTLs). Unfortunately, the peptide was unsuccessful at stimulating naïve CTL. However, induction of naïve helper T-lymphocytes (HTL) to recognize and respond to the PAX3/FKHR translocation peptide was successful. Yet, this HTL peptide activity did not translate into recognition of PAX3/FKHR-containing ARMS tumor cells. Conclusions: It appears that the fusion area of PAX3/FKHR may not be a good source of antigenic anti-tumor peptide epitopes. These results raise serious concerns about the success and applicability of future peptide-based vaccine immunotherapy directed at the PAX3/FKHR translocation point.
Keywords: Cytotoxic T-lymphocyte, Helper T-lymphocyte, Dendritic cell, Cancer vaccine, Peptide
Introduction
Rhabdomyosarcomas (RMS) are the most common types of sarcomas in childhood and are the fourth most common solid tumor during childhood and adolescence [1]. There are two main types: embryonal RMS (ERMS, 60% of all RMS cases) and alveolar RMS (ARMS, 20% of all RMS). Children with ARMS have a significantly worse prognosis than those with ERMS, with 50% of patients harboring metastatic disease at the time of presentation.
Over the past several years, some of the genetic alterations associated with the pathogenesis of ARMS have been identified. ARMS is most commonly associated with two nonrandom chromosomal translocations [2–4] The most common is the t(2;13) (q35-37;q14) translocation that occurs in approximately 75% of ARMS tumors. The other is the t(1;13) (p36;q14) translocation present in only 10% of tumors. These nonrandom translocations result in the generation of novel products that act as functional transcription factors. The t(2;13) translocation results in the expression of the hybrid transcription factor PAX3/FKHR. The 5′ PAX and the 3′ FKHR pieces are fused in-frame, resulting in a 97-kDa fusion protein that has a PAX3 DNA binding domain and a FKHR regulatory domain [5]. The FKHR regulatory domain functions at a much higher rate than the normal PAX3 regulatory domain [6, 7]. The increased activity of the PAX3/FKHR transcription factor is associated with the dysregulation of myogenic lineage, resulting in malignant transformation [8, 9].
Tumor rejection via immunotherapy is primarily mediated by T-lymphocytes recognizing unique tumor peptide antigens, resulting in tumor lysis. T-cells recognize these tumor antigens as small peptides bound to cell surface molecules encoded by the major histocompatibility complex (MHC) [10]. It would appear that both helper and cytotoxic T-lymphocyte (HTL and CTL, respectively) responses are required to insure an adequate and prolonged anti-tumor response in vivo [11–15].
The abnormal expression of the PAX3/FKHR fusion protein in ARMS tumor cells makes this molecule an attractive tumor-specific antigen (TSA) target for immunotherapy. Indeed, clinical studies have been initiated, but so far the results have been somewhat disappointing [16]. In this study the peptide sequence TIGNGLSP*QNSIRHNLSL (* = translocation junction) from the translocation point area was used to pulse monocytes and immature dendritic cells (DC) for vaccination. The vaccination was given in conjunction with IL-2. Only one of four patients had stable disease after vaccination; all the other three had progression.
The purpose of this study was to determine whether the PAX3/FKHR translocation point peptide sequence contained antigenic peptide epitopes capable of inducing T-cell responses that could be utilized as vaccines to treat ARMS. Unfortunately, our results show that peptides from the PAX3/FKHR translocation point were not able to induce CTL. However, they were able to induce HTL recognition to the peptide, but this did not translate into reactivity against PAX3/FKHR-containing tumor cells or PAX3/FKHR protein. In conclusion, the translocation point area of PAX3/FKHR may not be a good source of anti-tumor peptide epitopes for use in clinical vaccine trials.
Methods
Peptides
We used the combination of two previously described MHC binding algorithms to evaluate the PAX3/FKHR translocation area for potential antigenic epitopes [17, 18]. Peptides identified as potential antigens were then synthesized according to standard solid-phase synthesis methods using Applied Biosystems apparatus and purified by HPLC. The purity (>95%) and identity of peptides were determined by analytical HPLC and mass spectrometry analysis. Peptides were dissolved at 10 mg/ml in dimethylsulfoxide containing 0.1% TFA and were aliquoted in small volumes to be maintained frozen at −20°C until further use.
Cell lines
The T2 (ATCC CRL-1992) T-B lymphoblast hybrid cell line is class II MHC antigen negative, and HLA-A2 and -CD7 positive. Characterization of tumor cell lines and normal donors used in these experiments are described in Tables 1 and 2. Cell lines were kept in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum, L-glutamine, nonessential amino acids, sodium pyruvate, and gentamicin (complete RPMI medium). All of the culture materials were purchased from Life Technologies Inc. (Rockville, MD, USA). To increase the level of MHC class I expression, tumor cell lines (except for T2) were treated with 1,000 U/ml IFN-gamma for 48 h prior to assays.
Table 1.
Characterization of tumor cell lines used in this study (ARMS alveolar rhabdomosarcoma, ERMS embryonal rhabdomyosarcoma)
| Tumor name | Type of malignancy | PAX3/FKHR expression | HLA expression |
|---|---|---|---|
| Rh41 | ARMS | + | -A11, -A68 |
| Rh28 | ARMS | + | -A3, -A32 |
| RDA | ERMS | − | -A1 |
| Mel 624 | Melanoma | − | -A2, A3 |
Table 2.
Characterization of normal donors used in this study
| Assay | Donor | HLA expression |
|---|---|---|
| CTL | 1 | -A3, -A11 |
| 2 | -A3, -A11 | |
| 3 | -A3, -A2 | |
| HTL | 1 | -DR4, -DR7 |
| 2 | -DR7, -DR53, -DQ3, -DQ9 | |
| 3 | -DR4, -DR15, -DR51, -DR53, -DQ6, -DQ8 | |
| 4 | -DR4, -DR11, -DR51, -DR53, -DQ3 |
Generation of DC and peptide pulsing
The DC were generated using peripheral blood mononuclear cells (PBMC) from appropriate HLA type normal volunteers that had been purified using Ficoll gradient centrifugation as previously described [18]. The Institutional Review Board on Human Subjects (Mayo Foundation) approved this research, and informed consent for blood donation was obtained from all volunteers. Monocytes were isolated and incubated with ex-vivo medium containing 280 U/ml of GM-CSF and 50 ng/ml of IL-4. On days 3 and 5 additional media + IL-4 and GM-CSF were added. The DC were then ready for CTL induction on day 7. The tissue culture-generated DC (1–2×106 cells/ml) were pulsed with 10 μg/ml of peptide and 3 μg/ml β2-microglobulin at room temperature for 4 h and then irradiated (4,200 rad). The DC were seeded to a 48-well plate at 2.5×104 per well (1×105/ml) in 0.25 mls RPMI 1640 medium with 5% HS and 10 ng/ml IL-7.
T-lymphocyte induction
The methodology of T-cell induction and functional assays using in vitro methods have been well established in this laboratory [19, 20]. The first peptide-recognition functional screening assays were performed after three rounds of peptide stimulation.
Culture medium for all procedures consisted of RPMI 1640 medium supplemented with 5% human male AB serum, 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, and 50 μg/ml gentamicin.
Evaluation of T-lymphocyte activity
The cytotoxic activity of the CTL cultures was determined by 51Cr-release assay [20]. The PAX3/FKHR peptide-pulsed (5 μg/ml) T2 target cells and PAX3/FKHR containing tumor cells that express HLA-A2 were labeled with 300 μCi 51Cr-sodium chromate (Amersham Pharmacia Biotech, Piscataway, NJ, USA) for 2 h at 37°C. The target cells were then mixed with the effector cells (CTL) at ratios of 1:3 up to 1:100. After 4 h incubation at 37°C, supernatant was collected from each well and the percentage of specific lysis was determined using a gammacounter. Percent lysis is calculated as follows:
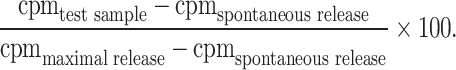 |
For the first screening assay, the labeled target cells were mixed with 10–20 times as many cold (unlabeled) K562 cells to decrease the nonspecific killing due to NK cells.
The HTL activity was determined using the 3H-thymidine proliferation assay [21]. Briefly, HTL are cocultured with PAX peptide (5 μg/ml), PAX protein (10 μg/ml), or PAX tumor lysate (1:1 cell ratio) pulsed autologous irradiated PBMC. Cell proliferation assays were incubated at 37°C for 72 h, and during the last 16 h, each well was pulsed with 0.5 μCi 3H-thymidine (Amersham Pharmacia Biotech). The radioactivity incorporated into DNA, which correlates with cell proliferation, was measured.
T-Lymphocytes that demonstrated activity against targets were selected for cloning by limiting dilution and expansion [20]. On day 21, the cultures were re-tested for activity using the 51Cr release or 3H-thymidine proliferation assays. Cells that were positive then underwent nonspecific expansion [20].
HLA restriction for T-lymphocyte activity
The HLA restriction was determined as previously described [19, 20]. To identify the MHC restriction molecules involved in antigen presentation, inhibition of the antigen-induced proliferative response was determined by the addition of anti-HLA-DR Mab L243 (IgG2a; prepared from hybridoma supernatants obtained from the American Type Culture Collection) or anti-HLA-DQ Mab SPVL3 (IgG2a; Beckman Coulter Inc., Fullerton, CA, USA). Both antibodies were used at a final concentration of 10 μg/ml throughout the 72 h assay. We also used as antigen-presenting cells (APC) several HLA-DR- or -DQ-expressing L cell lines (3×104 cells/well) and allogeneic PBMC that expressed the MHC molecule being evaluated.
Transient PAX3/FKHR transfections
293T cells were grown in six-well plates until approximately 80% confluent. Fugene6 (Roche, Nutley, NJ, USA) was used for transfection using the manufacturer’s guidelines. The media from the 293T transfected cells and controls was aspirated. Whole cell extract buffer (PBS, 1% NP-40, 0.1% SDS, and 0.5% deoxycholate) with inhibitors (aprotinin, leupeptin, pepstatin, DTT, and PMSF) was added directly to cells and lightly agitated to loosen and lyse cells. The extract was transferred to tubes and centrifuged at 20,000×g for 10 min. The intracellular protein supernates were saved. Protein concentration was determined using Bio-Rad Protein Assay reagent. SDS page was performed using premade 10% acrylamide gels with the Criterion electrophoresis system (BioRad Laboratories, Hercules, CA, USA). Proteins were transferred from the gel to PVDF membrane (Millipore, Billerica, MA, USA). Membranes were blocked overnight in Tris-buffered saline plus 0.2% Tween-20 (TBS-T) and 2% BSA plus 5% milk. The following day the membranes were washed with TBS-T and blotted with either anti-FKHR monoclonal antibody (mAb) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), horseradish peroxidase (HRP)-conjugated anti-actin mAb (Santa Cruz Biotechnology) or anti-V5-HRP mAb (Invitrogen, Carlsbad, CA) for at least 1 h. The membranes were washed again with TBS-T. The secondary antibody protA-HRP (Amersham, Buckinghamshire, England) was added for 30 min. The membranes were washed again. The membranes were then wet with ECL (Roche) for 2 min and exposed to film.
Results
Identification of potential PAX3/FKHR peptide antigens
Since T-lymphocytes are only able to recognize peptide antigens presented by specific MHC molecules, we first evaluated the PAX3/FKHR translocation point segment (ten amino acids proximal and distal to the breakpoint) for the presence of motifs that predict whether peptides bind to MHC molecules from various alleles. The MHC alleles evaluated included the common alleles HLA-A1, -A2, -A3 and -B7, as well as a large selection of less common alleles, for MHC class I and HLA-DR1, -DR4 and -DR7 for MHC class II. MHC binding algorithms were used to predict potential peptide antigens. No potential MHC class I CTL-restricted epitopes were identified that scored above the minimum values previously established (BIMAS score ≥80 and SYFPEITHI score ≥23) [18]. However, one potential CTL MHC class I epitope (PAX3/FKHR-387, peptide sequence GLSPQNSIR) was predicted for HLA-A3 by both algorithms, but the algorithm values were still below the cutoff values (Table 3). There is an approach of modifying the MHC binding anchors to optimal binding residues to improve the ability of the altered peptide to be antigenic while still maintaining recognition of the native peptide sequence in tumor cells [22, 23]. Therefore, the potential HLA-A3-restricted CTL epitope GLSPQNSIR was modified to GLSPQNSIK (PAX3/FKHR-387.9K) in an attempt to improve its potential MHC binding (Table 3). By exchanging one of the binding anchors both the BIMAS and SYFPEITHI scores for PAX3/FKHR-387.9K improved dramatically.
Table 3.
Algorithm prediction of potential antigenic epitopes at the PAX3/FKHR fusion area for MHC class I-restricted peptides
| MHC allele | Start position | Peptide sequence | SYFPEITHI score | BIMAS score |
|---|---|---|---|---|
| HLA-A1 | 387 | GLSPQNSIR | 8 | 1 |
| HLA-A*0201 | 386 | NGLSPQNSI | 14 | 0.25 |
| HLA-A3 | 387 | GLSPQNSIR | 20 | 18 |
| 387.9 V | GLSPQNSIK | 26 | 90 |
The predictive algorithm, “BIMAS”, scores potential MHC binders according to the half time disassociation of peptide/MHC complexes. The second algorithm, “SYFPEITHI”, scores the peptides according to the presence of primary and secondary MHC-binding anchor residues. The larger the score, the longer or better the peptide should bind to the MHC molecule. Table 3 lists the scores for both SYFPEITHI and BIMAS binding algorithms; however, Table 4 only presents the SYFPEITHI scores since BIMAS is not designed to evaluate for MHC class II alleles. NA indicates that the peptide did not achieve a score for that MHC allele
More promising prediction results were obtained for HTL MHC class II epitopes for which several 15 amino acid sequences were predicted to bind to HLA-DR1, HLA-DR4 and HLA-DR7 (Table 4). Using the MHC binding algorithms to predict epitopes that could be recognized by all three common HLA-DR alleles increases the likelihood that the peptide epitope will be promiscuous [19, 21, 24]. Promiscuity implies that the peptide is recognized in the context of several different MHC alleles, allowing broad applicability of these antigens in vaccines. To take advantage of the multiple potential epitopes, we synthesized the entire 21-mer peptide sequence around the PAX3/FKHR translocation point (PAX3/FKHR-381 peptide sequence NPTIGNGLSPQNSIRHNLSLH).
Table 4.
Algorithm prediction of potential antigenic epitopes at the PAX3/FKHR fusion area for MHC class II-restricted peptides
| Start position | Peptide sequence | DR1 | DR4 | DR7 |
|---|---|---|---|---|
| 381 | NPTIGNGLSPQNSIR | 14 | 20 | 16 |
| 385 | GNGLSPQNSIRHNLS | 20 | 14 | 16 |
| 382 | PTIGNGLSPQNSIRH | 23 | 12 | NA |
| 383 | TIGNGLSPQNSIRHN | 9 | 12 | 14 |
The predictive algorithm, “BIMAS”, scores potential MHC binders according to the half time disassociation of peptide/MHC complexes. The second algorithm, “SYFPEITHI”, scores the peptides according to the presence of primary and secondary MHC-binding anchor residues. The larger the score the longer or better the peptide should bind to the MHC molecule. Table 3 lists the scores for both SYFPEITHI and BIMAS binding algorithms; however, Table 4 only presents the SYFPEITHI scores since BIMAS is not designed to evaluate for MHC class II alleles. NA indicates that the peptide did not achieve a score for that MHC allele
CTL induction to the HLA-A3 peptide epitope
For CTL induction, we used naïve HLA-A3 CD8+ T-lymphocytes from three donors exposed to the modified peptide PAX3/FKHR-387.9K. Only 2% (5/288) of CTL cultures were considered positive (more than 20% specific lysis of peptide-loaded target cells), and none of these CTL cultures could be successfully maintained beyond induction to assess recognition of tumor cells. CTL induction to the native peptide sequence PAX3/FKHR-387 was completely unsuccessful (0/288 wells positive). These results are not surprising given the low algorithm scores for these peptide epitopes. In contrast, CTL induced with a positive control HLA-A2-restricted peptide QLMAFNHLV (BIMAS score 977, SYFPEITHI score 23) from elsewhere in PAX3 had 48% of CTL cultures that were positive and could be maintained beyond induction, demonstrating the effectiveness of the CTL induction methodology and that the APC and CD8+T cells from these donors were functionally viable. Thus, between the MHC binding algorithm results and the PAX3/FKHR-387 induction results, it appears that peptides from the PAX3/FKHR translocation point region may not be immunogenic for CTL induction.
HTL induction to the 21-mer translocation peptide
Helper T-lymphocyte induction to the PAX3/FKHR fusion 21-mer peptide sequence PAX3/FKHR-381 (NPTIGNGLSPQNSIRHNLSLH) was successful at generating multiple T-cell lines and clones. T-cell clones were isolated from four separate healthy naïve donors. These clones were able to recognize and proliferate in response to peptide pulsed autologous PBMC (Fig. 1). The amount of peptide required to elicit the proliferative response was approximately 0.5 ng/ml, indicating that the HTL clones were able to recognize and respond to small quantities of the peptide antigen suggesting a high affinity of the HTL to the peptide epitope. This is important since it indicates that the HTL should be able to recognize and respond to APC that express only small quantities of the peptide antigen derived from processed tumor cells.
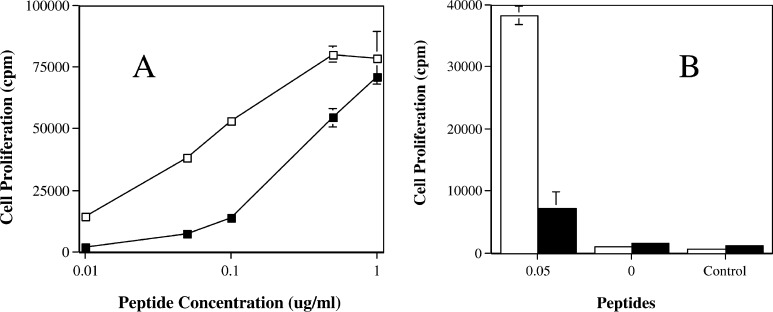
Fig. 1.
Peptide titration response of HTL clones specific for peptide PAX3/FKHR-381. HTL clones were selected from normal donors by weekly stimulation of CD4+ T cells with peptide-pulsed autologous irradiated PBMC. The HTL clones were then cocultured with peptide-pulsed autologous irradiated PBMC at 37°C for 72 h, and during the last 16 h, each well was pulsed with 0.5 μCi 3H-thymidine. HTL proliferation was measured by 3H-thymidine uptake. Two clones are shown from two different donors (open square clone 2H and filled square clone 11F). Frame a is the HTL response to various concentrations of peptide pulsed PBMC. Frame bis the response of the clones to the 0.05 and 0 μg/ml concentrations of PAX3/FKHR-381 peptide-pulsed PBMC and a negative control peptide FSSYTDSFVPPSGPS at 1 μg/ml.
MHC restriction of HTL peptide recognition
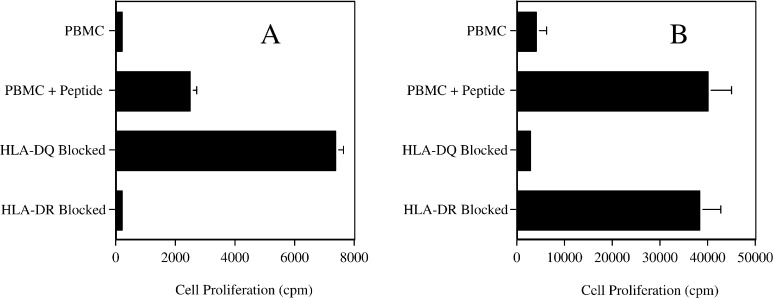
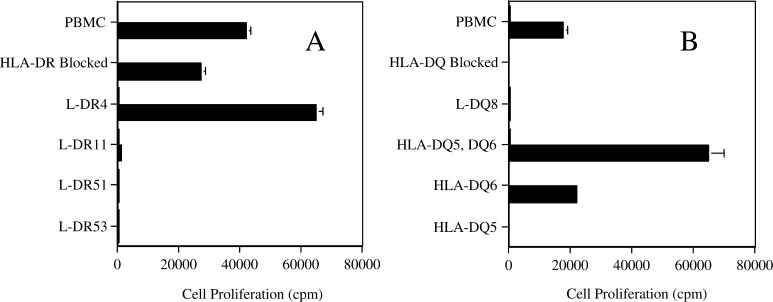
Since the PAX3/FKHR-381 peptide had the potential to be promiscuous (bound to several MHC alleles), it was important to determine which MHC alleles would present recognizable peptide to the HTL clones. Therefore, a pair of HTL clones, from different donors, was used to determine the MHC restriction for antigen recognition. For clone 5B autologous PBMC pulsed with peptide elicited a proliferative response that could be partially blocked with antibody to HLA-DR molecules (Fig. 2a). Further evaluation using fibroblasts that had been transfected with specific HLA-DR molecules and pulsed with peptide clarified that the 5B clone proliferation response was limited to HLA-DR4 (Fig. 3a). Similarly, proliferation of clone 9E to autologous PBMC pulsed with peptide was blocked with anti-HLA-DQ antibody (Fig. 2b). Further investigation with HLA transfected fibroblasts and allogeneic PBMC determined that the proliferation response was limited to HLA-DQ6 (Fig. 3b). In conclusion, it appears that HTL induction by peptide PAX3/FKHR-381 was able to generate a promiscuous response with peptide being recognized in the context of both HLA-DR4 and HLA-DQ6.
Fig. 2.
MHC restriction analysis of two peptide PAX3/FKHR-381 specific HTL lines (clone 5B in frame a and clone 9E in frame b) from different donors. Monoclonal antibodies specific for HLA-DR or HLA-DQ at 10 μg/ml were cocultured with peptide-pulsed (5 μg/ml) irradiated autologous PBMC and HTL. HTL proliferation was determined using the same culture conditions as described in Fig. 1. Proliferation was observed in response to peptide (PBMC + peptide) but not in the absence of peptide (PBMC). Values represent mean; bars SD.
Fig. 3.
MHC restriction analysis of two peptide PAX3/FKHR-381 specific HTL lines (clone 5B in frame a and clone 9E in frame b) from different donors. HTL proliferation, as measured by 3H thymidine uptake, was assessed using APC alone (open square) or loaded with peptide PAX3/FKHR-381 (filled square; 5 μg/ml) using the methods previously described. Several APC (autologous PBMC, L cells and allogeneic PBMC) were utilized based on the results from Fig. 2. Autologous PBMC in the presence of the effective HLA blocking antibody were used to confirm the results in Fig. 2. To further define the HLA molecules responsible for antigen presentation, L cells, which express individual MHC molecules, were used as APC. If L cells were not available then allogeneic PBMC expressing the appropriate HLA molecules were used as APC. Clone 5B (expressing HLA-DR4, DR11, DR51, and DR53 shown in panel a) is DR4 restricted and 9E (expressing HLA-DQ8 and DQ6 shown in panel B) is DQ6 restricted.
HTL recognition of tumor and PAX3/FKHR protein
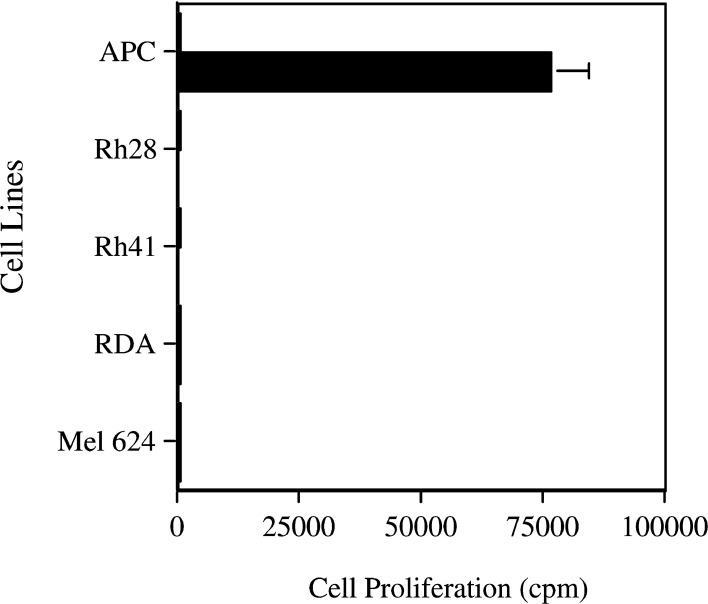
The fact that helper T-lymphocytes are able to recognize and respond to peptide-loaded target cells does not indicate that these HTL will also recognize antigen derived from tumor cells that is naturally processed by APC. Therefore, we evaluated the ability of the peptide-induced HTL to recognize APC that had been loaded with PAX3/FKHR-containing tumor cells. We evaluated Rh28 and Rh41, both of which have been previously demonstrated to contain the PAX3/FKHR translocation [3]. As negative controls we examined RDA (embryonal RMS) and Mel624 (melanoma), both of which contain PAX3 but not the translocation PAX3/FKHR. Neither apoptotic tumor cells nor tumor lysate processed by autologous PBMC were able to induce any HTL proliferation (Fig. 4). Since HTL recognition of antigen is dependent upon the quantity of MHC molecules and other co-stimulatory molecules, the quality of the APC can affect the proliferation response. DC are thought to be the best APC because they express both classes I and II MHC molecules in addition to the costimulatory molecules such as B7 and CD40 [25]. Therefore, the experiments were repeated using DC. The same lack of HTL proliferation occurred when DC, rather than PBMC, were used as the APC (data not shown).
Fig. 4.
Helper T-lymphocyte clones specific for peptide PAX3/FKHR-381 cannot recognize naturally processed PAX3/FKHR antigen present in tumor cells. HTL proliferation, as measured by 3H-thymidine uptake, to APC incubated 16 h with no peptide (open square) or peptide (filled square), demonstrated appropriate proliferative responses. However, there was no HTL proliferation to tumors (Rh28 and Rh41, positive samples containing PAX3/FKHR nor RDA and Mel 624 as negative controls) using APC incubated with either tumor lysate supernate, or apoptotic tumor cells at a one-to-one ratio (product from one tumor cell per APC) for 16 h at 37°C. This graph from clone 9E is representative of several HTL clones using either autologous PBMC or DC as APC. Values represent mean; bars SD.
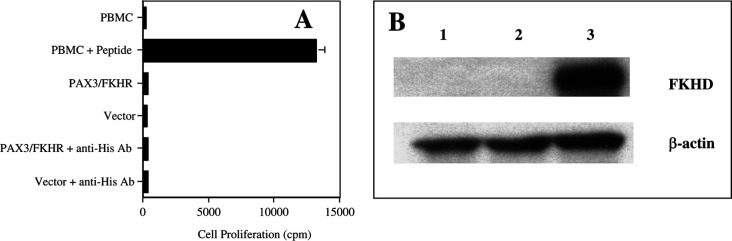
To test the possibility that the tumor cells contained insufficient PAX3/FKHR protein for HTL recognition, 293T cells were transfected with PAX3/FKHR, resulting in the production of large quantities of PAX3/FKHR protein (Fig. 5b). When lysate from these cells was loaded into autologous PBMC the HTL lines still did not proliferate (Fig. 5a). It has previously been demonstrated that incubating protein with an appropriate antibody can increase its uptake into PBMC [26, 27]. The PAX3/FKHR vector contains a HIS tag. Therefore, prior to PBMC loading, the lysate was incubated with anti-HIS antibody to increase the uptake of PAX3/FKHR protein into PBMC. Again this did not result in any HTL proliferation to protein-loaded APC (Fig. 5a). In conclusion, both of these experiments would indicate that the peptide epitope recognized by the HTL was either not processed as such or was lost/destroyed during processing of the PAX3/FKHR protein by APC.
Fig. 5.
a Helper T-lymphocyte clones specific for peptide PAX3/FKHR-381 cannot recognize naturally processed PAX3/FKHR antigen from PAX3/FKHR transfected cells. HTL proliferation, as measured by 3H-thymidine uptake, to APC incubated 16 h with no peptide or peptide (APC), demonstrated appropriate proliferative responses. However, there was no HTL proliferation to APC incubated with lysate from PAX3/FKHR transfected 293T cells at a one to one ratio (product from one transfected cell per APC) for 16 h at 37°C. In addition, lysate from 293T cells that had been transfected with vector alone was used as a negative control. Because the PAX3/FKHR vector has a His tag, a His antibody was used to increase PAX3/FKHR protein uptake into the APC. This graph from clone 9E is representative of several HTL clones using either autologous PBMC or DC as APC. Values represent mean; bars SD. b This Western blot using anti-FKHR mAb demonstrates the production of PAX3/FKHR protein (115 kDa) by 293T cells alone (lane 1), 293T cells transfected with vector alone (lane 2) and 293T cells transfected with the PAX3/FKHR gene (lane3). β-Actin (40 kDa) is used as a positive loading control.
Discussion
The purpose of this study was to evaluate the PAX3/FKHR translocation point area for possible antigenic peptide epitopes that could be used for an anticancer peptide vaccine. Peptides must satisfy several criteria to be effective as a vaccine that can induce a significant immune response resulting in antitumor activity. First, the peptide antigen must bind to the MHC and be immunogenic. Second, the peptide must bind to a common MHC molecule so that the vaccine may be broadly applicable to the general population. Third, there should be no “self-antigen” tolerance to the peptide. Fourth, the protein containing the peptide antigen must be present in tumor cells in sufficient quantities to stimulate T-lymphocytes. Fifth, the protein must be naturally processed by the tumor cell or APC to allow presentation of the appropriate peptide antigen by the MHC. And last, the peptide antigen should not be expressed in normal tissues to prevent the induction of an autoimmune response after vaccination.
There are several requirements that must be fulfilled in order for a peptide to be immunogenic and induce anti-tumor T-lymphocyte activity. First, the peptide needs to be present on the target cell surface bound to the appropriate MHC. This requires that the peptide must be present in sufficient quantities within the cell. Also, the protein must be processed appropriately so that the peptide remains intact for presentation by the MHC. Second, the peptide needs to bind to the MHC at a high enough concentration and for enough time to stimulate the immune response. And third, the T-lymphocytes must recognize the peptide as foreign. Since the PAX3/FKHR protein has a novel amino acid sequence at the translocation point, it is likely that peptide sequences from this area may be immunogenic since they would not be recognized by the immune system as a “self-antigen” and thus should not have an existing tolerance.
We postulate that the reason the PAX3/FKHR translocation point peptide sequence would not be an effective vaccine against PAX3/FKHR tumors is related to several issues. For the CTL antigens there are two main problems. First is the lack of any potential antigenic peptide epitopes associated with common MHC molecules, as predicted by the MHC binding algorithms. And second, our results show that the only peptide predicted by the MHC binding algorithms, the HLA-A3-restricted peptide epitope PAX3/FKHR-387, was not immunogenic enough to induce a significant T-cell response. Another possibility is that there were too few CTL precursors that recognized this peptide epitope to induce a detectable response.
However, the HTL peptide satisfied many of the criteria for an effective vaccine (peptide was immunogenic, peptide was expressed by common MHC molecules, and there was no T-lymphocyte tolerance). However, the peptide-reactive HTL did not respond to PAX3/FKHR-containing cells that had been naturally processed by APC. There are two potential explanations for this observation. First, the amount of protein present in tumor cells was maybe insufficient to allow HTL recognition. However, arguing against this is the lack of HTL activity towards PAX3/FKHR-transfected cells that by Western blot contained significant quantities of PAX3/FKHR protein. In addition, even when an attempt was made to increase protein uptake by the target cells, by forming immunocomplexes with specific antibody, there was no HTL proliferation to the PAX3/FKHR-loaded APC. This strategy of increasing protein uptake has proven to be effective in other studies [26, 27]. And last, given the low concentration of peptide needed to stimulate HTL proliferation the lack of PAX3/FKHR protein in tumor cells most likely was not an issue in these experiments. Second, more likely the lack of HTL activity to tumor cell-loaded APC is related to loss or destruction of the antigenic peptide epitope during antigen processing by the APC. As proteins are degraded by proteases, in preparation for binding to MHC molecules, it is possible that the predicted PAX3/FKHR-387 peptide epitope is cleaved, thus destroying the peptide antigen and subsequently preventing HTL recognition.
It has previously been suggested that the PAX3/FKHR translocation point area was an effective antigen in a mouse tumor model [28]. In this study the authors evaluated the PAX3/FKHR translocation area as a peptide vaccine. They pulsed syngeneic mouse splenocytes with the human PAX3/FKHR translocation peptide and then injected the splenocytes back into the mice. They were able to show that after 1 week, mouse splenocytes were able to lyse peptide-pulsed target cells. In addition, mouse adenocarcinomas that had been transfected with human PAX3/FKHR protein were recognized by these same mouse splenocytes. Subsequently, prophylactic vaccination with peptide-pulsed splenocytes prevented the development of PAX3/FKHR-transfected adenocarcinoma tumor growth.
These results formed the basis for a NIH-sponsored clinical trial using the translocation peptide in a vaccine trial for pediatric ARMS [16]. In this study, patients with Ewing’s sarcoma and ARMS were treated with their respective translocation peptide vaccines. All patients had disease progression, and only one patient demonstrated any immunologic response after the vaccine. The trial was subsequently closed. There were several reasons cited by the authors for these results, including large tumor burdens, decreased T-lymphocyte counts, diminished immune function after chemotherapy, and the choice of APC.
So why did the pre-clinical mouse data not accurately reflect the subsequent human clinical trial? We postulate that the human PAX3/FKHR protein in the mouse may not have been an optimal model to test the antigenicity of the peptide vaccine. Even though mouse and human PAX3 proteins have significant homology they are from different species and are therefore likely to be antigenic. This increased antigenicity could lead to improved activity as a vaccine. In addition, mouse MHC classes I and II have different binding motifs, compared to humans, and therefore may present different peptide epitopes that have been processed appropriately (to maintain the antigenic epitopes) and bind well to the MHC.
Our work suggests the lack of any clinically effective peptide antigens for CTL or HTL within the translocation point area. The methods utilized in this study have been used successfully in this lab to identify many CTL and HTL antigenic peptide epitopes from several tumor-associated antigens [18–21]. Therefore, we feel that our investigation would have revealed if any peptide antigens were present within the PAX3/FKHR translocation point area. Although negative data should always be taken with some caution, our study is important in that it suggests the lack of effective peptide antigens at the PAX3/FKHR translocation point area for both CTL and HTL. This provides a possible explanation for the lack of efficacy in the clinical trial discussed previously and highlights the problems with any future trials using the PAX3/FKHR translocation point peptide sequence.
References
- 1.Arndt CAS, Crist WM. Common musculoskeletal tumors of childhood and adolescence. NEJM. 1999;341:342–352. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro DN, Sublett JE, Li B, Downing JR, Naeve CW. Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res. 1993;53:5108–12. [PubMed] [Google Scholar]
- 3.Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;3:113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- 4.Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994;54:2869–72. [PubMed] [Google Scholar]
- 5.Galili N, David RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, III, Emmanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–5. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 6.Fredericks WJ, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr FG, Rauscher FJ., III The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcoma in a more potent transcriptional activator than PAX3. Mol Cell Biol. 1995;15:1522–35. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benicelli JL, Edwards RH, Barr FG. Mechanism for transcriptional gain of function resulting from chromosomal translocation in alveolar rhabdomyosarcoma. Proc Natl Acad Sci U S A. 1995;93:5455–5459. doi: 10.1073/pnas.93.11.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein JA, Lam P, Jepeal L, Maas RL, Shapiro DN. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem. 1995;270:11719–11722. doi: 10.1074/jbc.270.20.11719. [DOI] [PubMed] [Google Scholar]
- 9.Scheidler S, Fredericks WJ, Rauscher FJ, III, Barr FG, Vogt PK. The hybrid PAX3-FKHR fusion protein of alveolar rhabdomyosarcoma transforms fibroblasts in culture. Proc Natl Acad Sci U S A. 1996;93:9805–9. doi: 10.1073/pnas.93.18.9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardoll DM. Therapeutic vaccination for cancer. Clin Immunol. 2000;95:S44–S62. doi: 10.1006/clim.1999.4819. [DOI] [PubMed] [Google Scholar]
- 11.Bennett SRM, Carbone FR, Karamalis F, Miller JFAP, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surman D, Dudley M, Overwijk W, Restifo N. Cutting edge: CD4 + T cell control of CD8 + T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll D, Topalian S. The role of CD4 + T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/S0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 14.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL. MHC class II restricted tumor antigens and the role of CD4+ T cells in cancer immunotherapy. Curr Opin Immunol. 1994;6:741. doi: 10.1016/0952-7915(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 16.Dagher R, Long LM, Read EJ, Leitman SF, Carter CS, Tsokos M, Goletz TJ, Avila N, Berzofsky JA, Helman LJ, Mackall CL. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter-institute NIH study. Med Ped Oncol. 2002;38:158–164. doi: 10.1002/mpo.1303. [DOI] [PubMed] [Google Scholar]
- 17.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stenanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Celis E. Use of two predictive algorithms of the World Wide Web for the identification of tumor-reactive T-cell epitopes. Cancer Res. 2000;60:5223–5227. [PubMed] [Google Scholar]
- 19.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/Neu tumor antigen. Cancer Res. 2000;60:5228–5236. [PubMed] [Google Scholar]
- 20.Lu J, Wettstein PJ, Higashimoto Y, Appella E, Celis E. TAP-independent presentation of CTL epitopes by trojan antigens. J Immunol. 2001;166:7063–7071. doi: 10.4049/jimmunol.166.12.7063. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Song Y, Hoon D, Appella E, Celis E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 2001;61:4773–8. [PubMed] [Google Scholar]
- 22.Smith JW, II, Walker EB, Fox BA, Haley D, Wisner KP, Doran T, Fisher B, Justice L, Wood W, Vetto J, Maecker H, Dols A, Meijer S, Hu H-M, Romero P, Alvord WG, Urba WJ. Adjuvant immunization of HLA-A2–positive melanoma patients with a modified gp100 peptide induces peptide-specific CD8+ T-cell responses. J Clin Oncol. 2003;21:1562–1573. doi: 10.1200/JCO.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Huarte E, Sarobe P, Lu J, Casares N, Lasarte JJ, Dotor J, Ruiz M, Prieto J, Celis E, Borras-Cuesta F. Enhancing immunogenicity of a CTL epitope from carcinoembryonic antigen by selective amino acid replacements. Clin Cancer Res. 2002;8:2336–2344. [PubMed] [Google Scholar]
- 24.Kobayashi H, Omiya R, Ruiz M, Huarte E, Sarobe P, Lasarte JJ, Herraiz M, Sangro B, Prieto J, Borras-Cuesta F, Celis E. Identification of an antigenic epitope for helper T lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8:3219–3225. [PubMed] [Google Scholar]
- 25.Fernandez N, Duffour M-T, Perricaudet M, Lotze MT, Tursz T, Zitvogel L. Active specific T-cell-based immunotherapy for cancer: nucleic acids, peptides, whole native proteins, recombinant viruses, with dendritic cell adjuvants or whole tumor cell-based vaccines. principles and future prospects, cytokines. Cell Mol Ther. 1998;4:53–65. [PubMed] [Google Scholar]
- 26.Celis E. Regulation of T-cell function by antibodies: enhancement of the response of human T-cell clones to hepatitis B surface antigen by antigen-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1984;81:6846–50. doi: 10.1073/pnas.81.21.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyama K, Ebihara S, Yada A, Matsumura K, Aiba S, Nukiwa T, Takai T (2003) Targeting apoptotic tumor cells to FcyR provides efficient and versatile vaccination against tumors by dendritic cells. J Immunol 1641–1648 [DOI] [PubMed]
- 28.Mackall CL. Targeting tumor specific translocations in sarcomas in pediatric patients for immunotherapy. Clin Orthop. 2000;373:25–31. doi: 10.1097/00003086-200004000-00005. [DOI] [PubMed] [Google Scholar]