Abstract
Background
Enterochromaffin (EC) cells are dispersed throughout the gastrointestinal (GI) mucosa and are the main source of 5‐hydroxytryptamine (5‐HT) in the gut. 5‐HT has been implicated in the pathophysiology of several GI disorders, but the mechanisms regulating 5‐HT production in the gut are unknown.
Aim
To investigate the role of CD4+ T cells in the production of 5‐HT using a model of enteric parasitic infection.
Methods and results
Severe combined immunodeficient (SCID) mice and their wild‐type controls were infected with the nematode Trichuris muris and killed on various days after infection to study colonic EC cells and 5‐HT production. The number of EC cells and the amount of 5‐HT produced were significantly higher in infected wild‐type mice than in non‐infected mice. The number of EC cells and the amount of 5‐HT after infection were significantly lower in SCID mice after infection than in wild‐type mice. The number of EC cells and the amount of 5‐HT was significantly increased after reconstitution of SCID mice with CD4+ T cells from infected mice and this was accompanied by an upregulation of colonic CD3 T cells and T helper 2 (Th2) cytokines. Laser capture microdissection‐based molecular and immunofluorescence techniques revealed the presence of interleukin 13 receptor α1‐chain on EC cells.
Conclusion
These results show an important immunoendocrine axis in the gut, where secretory products from CD4+ T cells interact with EC cells to enhance the production of 5‐HT in the gut via Th2‐based mechanisms. These results show new insights into the mechanisms of gut function, which may ultimately lead to improved therapeutic strategies in functional and inflammatory disorders of the GI tract.
The gastrointestinal (GI) mucosa contains an extensive system of endocrine cells that represent the largest contingent of hormone‐producing cells in the body, in terms of both number of endocrine cells and number of hormones.1,2 Interactions among the immune, endocrine and enteric nervous systems are considered to comprise an integrated network of host defence in the GI tract.3 Enteroendocrine cells are specialised cells of the GI epithelium, which release various biologically active compounds such as cholecystokinin, chromogranin A (CGA) and serotonin (5‐hydroxytryptamine, 5‐HT).4,5
The best characterised subset of enteroendocrine cells are enterochromaffin (EC) cells, which synthesise and release 5‐HT.6,7 The GI tract contains about 95% of the body's 5‐HT, and EC cells are its main source.8,9 EC cells have specialised microvilli that project into the lumen and contain enzymes and transporters that are present in the apical parts of the enterocytes.10 EC cells function as sensors for the contents of the gut lumen and respond to luminal stimuli directly through these transporters and/or indirectly through mediators from the surrounding cells. Granules containing 5‐HT are concentrated around the basolateral pole of the EC cells, and 5‐HT produced from these cells is implicated in the neuroimmunoendocrine networks of both humans and rodents.11 5‐HT is also found in enteric neurones, but the amount present seems to be small in comparison to that present in EC cells.9 5‐HT is released from EC cells into the blood, into the surrounding tissue and into the gut lumen and plays an important part in gut physiology (motor and secretory).12 5‐HT has been implicated in several GI diseases, including inflammatory bowel disease and functional disorders such as irritable bowel syndrome (IBS).13,14,15,16 In addition, alteration in EC cells is also reported in several bacterial, viral and parasitic infections of the GI tract.17,18,19,20 The association between alteration in 5‐HT and various GI disorders emphasises the significance of 5‐HT in intestinal homeostasis. Nevertheless, the precise mechanisms regulating the changes in EC cells and 5‐HT content in the gut during infection and inflammation are still not clear.
Owing to the strategic location of EC cells in the GI mucosa, the infection‐induced changes in EC cells are likely to be modulated by the immune system as a component of host defence. There is emerging evidence of immune activation (an increase in CD3 T cells) in patients with post‐infectious IBS (PI IBS) in whom increased numbers of EC cells have been reported.21,22 This is also indicated in an animal model of PI IBS.23,24 Therefore, immune activation may increase the number of EC cells in the clinically relevant context of functional bowel disease such as IBS. The reduced number of EC cells in mice with targeted disruption of interleukin (IL)225 and T cell receptor α,26 and the presence of EC cells in contact with, or in close proximity to, lymphocytes,27 further suggests the existence of immunological control on EC cells, although the precise regulatory mechanisms remain to be determined.
In this study, we investigated the role of CD4+ T cells in EC cell biology during enteric infection, by using the well‐defined model of large intestinal nematode infection, T muris in mouse. After infection with T muris, mouse strains (C57BL/6, BALB/c) resistant to the infection generate a T helper 2 (Th2) immune response and expel the parasites.28,29 Our study clearly shows that the infection with T muris notably increased the number of colonic EC cells and the production of 5‐HT in the immunocompetent resistant mice but not in the severe combined immunodeficient (SCID) mice. However, adoptive transfer of purified CD4+ T cells from T muris‐infected mice into SCID mice significantly upregulated numbers of EC cells and the amount of 5‐HT in SCID mice. In addition, the present study also showed the presence of the IL13 receptor on EC cells. These observations provide evidence for immunological control of EC cell biology and show a new mechanism by which the immune system regulates changes in gut physiology via 5‐HT from EC cells.
Materials and methods
Animals
C57BL/6 and SCID (C57BL/6 background) mice (Jackson Laboratories, Bar Harbor, Maine, USA) were kept in sterilised, filter‐topped cages under specific pathogen‐free conditions and fed autoclaved food; only male mice aged 8–10 weeks were used. The protocols used were in direct accordance with guidelines drafted by the McMaster University Animal Care Committee and the Canadian Council on the Use of Laboratory Animals.
Parasitological technique
The techniques used for the maintenance and infection of T muris have been described previously.30 Mice were infected with approximately 300 eggs, and worm burdens were assessed as described previously.31
CD4+ T cell reconstitution
Splenocytes from non‐infected and T muris‐infected (day 14 pi) euthymic C57BL/6 mice were collected in Hank's balanced salt solution containing 10% fetal bovine serum and 1% antibiotic/antimycotic agent. CD4+ T cells were isolated from the mixed splenocytes by negative selection using an EasySep mouse CD4+ T cell enrichment cocktail with magnetic nanoparticles (Stem Cell Technologies, Vancouver, BC, Canada). Purified CD4+ T cells were resuspended in phophate‐buffered saline (PBS), and each mouse received 2.5×106 cells intraperitoneally. Cell purity was 90%as determined by flow cytometry, using anti‐mouse CD4 (LT34) monoclonal antibody (BD Pharmingen, San Diego, California, USA).
Determination of CD4+ T cell reconstitution by fluorescence activated cell sorter analysis
To confirm reconstitution of SCID mice with CD4+ T lymphocytes, isolated splenocytes were incubated with phycoerythrin‐conjugated antibody to CD4 and analysed by flow cytometry (FACScan Flow Cytometer, Becton Dickinson, California, USA).
Immunohistochemistry/immunofluorescence
Immunohistochemical studies on 5‐HT‐expressing EC cells and CD3 cells were performed on formalin‐fixed, paraffin‐wax‐embedded samples. Sections were deparaffinised in CitriSolv (Fisher Scientific, Ontario, Canada), and rehydrated through a graded series of ethanol and PBS. Endogenous peroxide was blocked by incubation in peroxidase‐blocking reagent (DakoCytomation, Ontario, Canada) for 15 min. After washing, sections were subjected to antigen retrieval in citrate buffer after heating in a microwave or were predigested with proteinase K solution (DakoCytomation) for 15 min. After blocking of non‐specific binding with 1% bovine serum albumin in PBS, sections were incubated with 5‐HT rabbit antibody (Immunostar; 1:5000, 1 h at room temperature (RT)) or with polyclonal rabbit anti‐CD3 (DakoCytomation, 1:500, 1 h at RT). After washing, sections were incubated with Envision (horse radish peroxidase‐coupled anti‐rabbit secondary reagent; DakoCytomation) for 30 min. Sections were developed with 3,3′‐diaminobenzidine and counterstained with Meyer's haematoxylin. The numbers of CD3 and 5‐HT‐expressing cells were expressed per 10 glands. The numbers of CD3 and 5‐HT‐expressing cells were counted at original magnification ×400 and numbers were expressed per 10 glands.
For detecting IL13 receptor α1‐chain (IL‐13Rα1) on EC cells, double staining for IL13Rα1 and 5‐HT/CGA was carried out by a immunofluorescence technique using mouse anti‐IL13Rα1 (Gene Tex, San Antonio, Texas, USA) and rabbit anti‐5‐HT (Immunostar, Hudson, Wisconsin, USA; 1:5000 dilution) or rabbit anti‐CGA antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA; 1:400 dilution) as primary antibodies, respectively. Alexa Fluor 633‐goat anti‐mouse IgG and Alexa Fluor 488‐goat anti‐rabbit IgG were used as secondary antibodies. Propidium iodine was diluted in secondary antibody solution to a final concentration of 0.5 µg/ml for nuclear staining. The sections were studied with a Zeiss fluorescence microscope.
Determination of colonic 5‐HT content
Segments of colon were homogenised in 0.5 ml of 0.2 M perchloric acid, and centrifuged at 10 000 g for 5 min. The supernatants were neutralised with 0.5 ml of 1.0 M borate buffer (pH 9.25), and centrifuged at 10 000 g for 1 min. The 5‐HT content in the supernatant was analysed by enzyme immunoassay using commercially available kit (Beckman Coulter, Fullerton, California, USA). The 5‐HT content of the tissue was expressed as a function of wet weight (in mg).
Laser capture microdissection
Laser capture microdissection (LCM) was used to isolate 5‐HT‐expressing EC cells from colonic tissues. Cryosectioned tissues were fixed in cold acetone, dried and incubated with 5‐HT rabbit antibody (Immunostar; 1:20, 3 min at RT). After washing, sections were incubated with Envision for 3 min. After washing again, the sections were developed with 3,3′‐diaminobenzidine, rinsed with RNase‐free water and then dehydrated. LCM was performed on a PixCell II (Arcturus Engineering, Mountain View, California, USA) within a maximum of 2 h and captured cells were transferred to Cap‐Sure LCM Caps. Approximately 500–1000 EC cells were captured onto each cap.
RNA extraction, DNase treatment and RT‐PCR
Pico Pure RNA Isolation Kit (Arcturus Engineering) was used for RNA extraction and DNase treatment. RNA was finally dissolved in 11 μl of RNase‐free water. Reverse transcription (RT)‐PCR was performed by using the SuperScript one‐step RT‐PCR System (Invitrogen). Both complementary DNA synthesis and PCR were performed in a single tube using gene‐specific primers. Primer sets used were as follows: (1) for IL4 receptor α (IL4Rα), (sense) 5′‐GAG TGA GTG GAG TCC TAG CAT C‐3′; (antisense) 5′‐GCT GAA GTA ACA GAA CAG GC‐3′ and (2) for IL13Rα1, (sense) 5′‐GAA TTT GAG CGT CTC TGT CGA A‐3′; (antisense) 5′‐GGT TAT GCC AAA TGC ACT TGA G‐3′.32,33 PCR products were loaded onto a 2.5% agarose gel and then visualised under ultraviolet light after ethidium bromide staining.
Evaluation of in vitro cytokine production from splenocytes
Single‐cell suspensions of spleen were prepared in RPMI 1640 containing 10% fetal calf serum, 5 mM L‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 25 mM Hepes, 0.05 mM 2‐mercaptothanol (all Gibco‐BRL, Burlington, Ontario, Canada). Cells (107) were incubated in the presence of 5 μg/ml Concanavalin A. IL4 and IL13 levels in the supernatant were measured by enzyme immunoassay using commercially available kit (R&D Systems, Minneapolis, Minnesota, USA).
Statistical analysis
Data were analysed by one‐way analysis of variance (ANOVA) with Dunnett's post hoc test for comparisons with a control and by two‐way ANOVA using MINITAB Release V.14.1 Statistical Software for Windows (Minitab, State College, Pennsylvania, USA), where p<0.05 was considered significant. All results are expressed as mean (SEM).
Results
Infection with T muris upregulated the number of 5‐HT‐expressing EC cells and 5‐HT content in colon
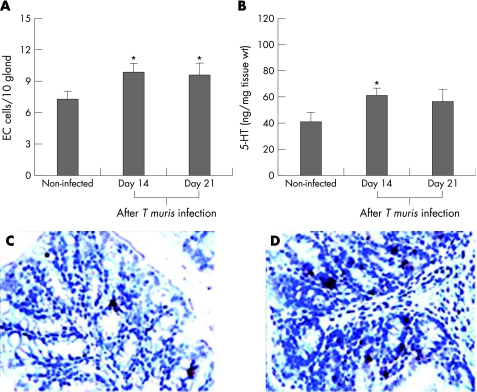
C57BL/6 mice are resistant to T muris infection, and clear the parasite by day 35 after infection.34 To investigate the number of colonic EC cells and the amount of 5‐HT in T muris infection, C57BL/6 mice were infected and killed on different days after infection. There was a significant increase in the number of 5‐HT‐expressing EC cells in the colon over the course of time as revealed by the one‐way ANOVA test, and we observed significantly higher numbers of EC cells on days 14 and 21 after infection compared with non‐infected controls (fig 1). The amount of colonic 5‐HT was significantly increased on day 14 after infection but not on day 21 after infection (fig 2) compared with non‐infected controls. We also observed a significant increase in IL4 and IL13 production from in vitro concanavalin A‐stimulated spleen cells from T muris‐infected mice on day 14 after infection as compared with that in non‐infected control mice (data not shown).
Figure 1 Infection of mice with Trichuris muris upregulated the numbers of enterochromaffin (EC) cells and the amount of 5‐hydroxytryptamine (5‐HT) in the colon. C57BL/6 mice were infected orally with 300 eggs of T muris and were killed on different days after infection to study 5‐HT‐expressing EC cells and the amount of 5‐HT in the colon. (A) Number of EC cells in the colon of non‐infected and infected mice. (B) Amount of 5‐HT in the colonic tissue of non‐infected and infected mice. (C) Representative micrograph showing 5‐HT‐expressing EC cells in the colon of non‐infected mice. (D) Representative micrograph showing 5‐HT‐expressing EC cells in the colon of T muris‐infected mice on day 14 after infection. Each bar represents mean (SEM) from six mice. *Significantly (p<0.05) higher than that in non‐infected mice as analysed by Dunnett's post hoc test.
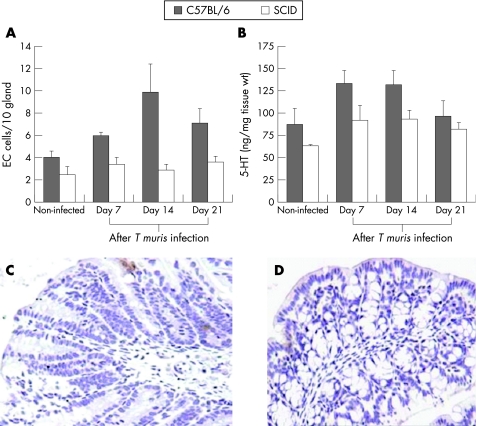
Figure 2 Severe combined immunodeficient (SCID) mice exhibited markedly lower numbers of enterochromaffin (EC) cells and lower amounts of 5‐hydroxytryptamine (5‐HT) after Trichuris muris infection than wild‐type controls. SCID mice (on C57BL/6 background) and control C57BL/6 mice were infected orally with 300 eggs of T muris and were killed on different days after infection to study 5‐HT‐expressing EC cells and the amount of 5‐HT in the colon. (A) Number of EC cells in the colon of non‐infected and infected SCID and C57BL/6 mice. (B) Amount of 5‐HT in the colonic tissue of non‐infected and infected SCID and C57BL/6 mice. (C) Representative micrograph showing 5‐HT‐expressing EC cells in the colon of non‐infected SCID mice. (D) Representative micrograph showing 5‐HT‐expressing EC cells in the colon of T muris‐infected SCID mice on day 14 after infection. Each bar represents mean (SEM) from five mice. Two‐way analysis of variance revealed significant difference in numbers of EC cells and the amount of 5‐HT between the SCID and C57BL/6 mice after T muris infection.
SCID mice exhibited markedly lower numbers of EC cells and lower amount of 5‐HT after T muris infection than wild‐type control mice
SCID mice are susceptible to T muris infection and exhibit impaired ability to generate the characteristic Th2 predominant immune response and clear the infection.35,36 Therefore, we next investigated whether the immunodeficiency in SCID mice affected the generation of infection‐induced increase in EC cell responses. We found significantly lower numbers of colonic EC cells and 5‐HT content in the SCID mice after T muris infection than in wild‐type mice (fig 2). The reduced number of EC cells and the amount of 5‐HT was associated with an inhibition in worm expulsion in SCID (data not shown). This impaired EC cell response in infected SCID mice suggests that immune cells such as lymphocytes play an important role in the development of EC cell hyperplasia in this infection.
CD4+ T cells play an important role in the development of EC cell hyperplasia and in the upregulation of 5‐HT production
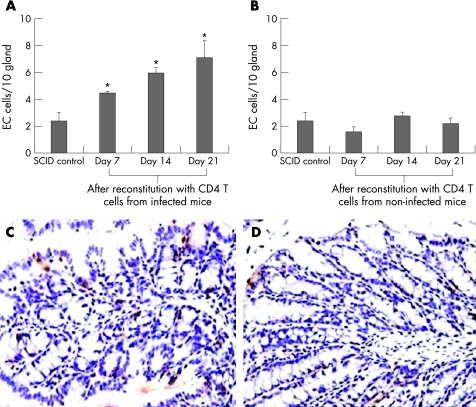
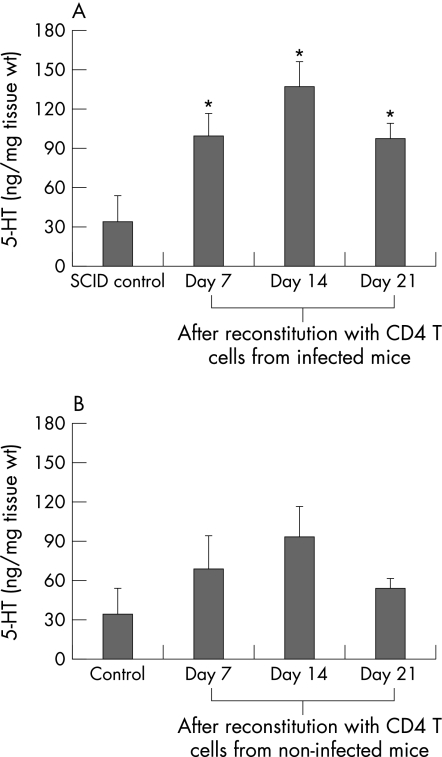
Considering the increase in EC cells and activation of CD4+ T cells in T muris infection, we next investigated the role of CD4+ T cells in the regulation of EC cell function and 5‐HT production in the gut during this infection. We transferred purified CD4+ T cells from T muris‐infected and non‐infected C57BL/6 mice into SCID mice and investigated the number of EC cells and the 5‐HT content in the colon. As fig 3 shows, the number of EC cells was significantly higher after reconstitution of SCID mice with purified CD4+ T cells from infected mice as compared with SCID controls on days 7, 14 and 21 after reconstitution. However, the number of EC cells was not significantly altered in SCID mice reconstituted with cells from non‐infected mice as compared with that in SCID controls. We also observed that the colonic 5‐HT content was markedly increased in SCID mice reconstituted with cells from infected mice on days 7, 14 and 21 after reconstitution (fig 4). However, the colonic 5‐HT content was not significantly altered in SCID mice reconstituted with cells from non‐infected mice as compared with that in SCID controls.
Figure 3 Number of enterochromaffin (EC) cells in the colon of severe combined immunodeficient (SCID) mice increased after reconstitution with CD4+ T cells from Trichuris muris‐infected mice. SCID mice (C57BL/6 background) were reconstituted with purified CD4+ T cells from non‐infected and T muris‐infected euthymic C57BL/6 mice and were killed on different days after reconstitution to study 5‐hydroxytryptamine (5‐HT)‐expressing EC cells. (A) Number of EC cells in the colon of SCID mice reconstituted with CD4 cells from infected euthymic mice. (B) Number of EC cells in the colon of SCID mice reconstituted with CD4 cells from non‐infected euthymic mice. (C) Representative micrograph showing EC cells in the colon of SCID mice reconstituted with CD4+ T cells from infected C57BL/6 mice on day 14 after reconstitution. (D) Representative micrograph showing EC cells in the colon of SCID mice reconstituted with CD4+ T cells from non‐infected C57BL/6 mice on day 14 after reconstitution. Each bar represents mean (SEM) from five mice. *Significantly (p<0.05) higher than that in SCID control mice by Dunnett's post hoc test.
Figure 4 Amount of 5‐hydroxytryptamine (5‐HT) in the colon of severe combined immunodeficient (SCID) mice increased after reconstitution with CD4+ T cells from Trichuris muris‐infected mice. SCID mice (C57BL/6 background) were reconstituted with purified CD4+ T cells from non‐infected and T muris‐infected euthymic C57BL/6 mice and were killed on different days after reconstitution to study 5‐HT in SCID mice reconstituted with CD4+ T cells from infected (A) and non‐infected (B) C57BL/6 mice by enzyme immunoassay. Each bar represents mean (SEM) from five mice. *Significantly (p<0.05) higher than that in SCID control mice by Dunnett's post hoc test.
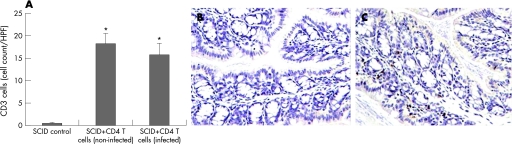
To confirm the effectiveness of the reconstitution, fluorescence‐activated cell sorter analysis of isolated spleen cells was performed. A markedly higher percentage of CD4+ T cells (0.81% (0.1%) vs 7.43% (1.4%), unreconstituted vs reconstituted) was observed in the SCID mice reconstituted with CD4 cells from euthymic control mice. In addition, immunohistochemical studies clearly revealed an upregulation of colonic CD3 T cells in the reconstituted SCID mice (fig 5).
Figure 5 CD3 cells increased in the colon of severe combined immunodeficient (SCID) mice after reconstitution with CD4+ T cells. SCID mice (on C57BL/6 background) were reconstituted with purified CD4+ T cells from non‐infected and Trichuris muris‐infected euthymic C57BL/6 mice and were killed on day 14 after reconstitution to study CD3 cells. (A) Number of CD3 cells in the colon of SCID mice reconstituted with CD4 cells from non‐infected and infected euthymic mice. (B) Representative micrograph showing CD3 immunostaining of colon tissue of control SCID mice. (C) Representative micrograph of CD3 immunostaining of colon tissue of SCID mice after reconstitution with CD4 cells from infected euthymic mice. Data were expressed in cell counts per high‐power field (HPF). *Significantly (p<0.05) higher than that in SCID control mice by Dunnett's post hoc test.
Reconstitution of SCID mice with CD4+ T cells increased Th2 cytokine production
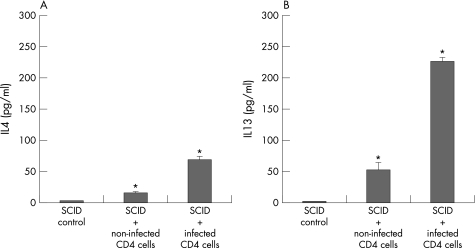
The increase in EC cells and 5‐HT in reconstituted SCID mice was associated with an increase in Th2 cytokine production. Measurement of in vitro cytokine production from spleen cells after concanavalin A stimulation revealed an upregulation of both IL4 and IL13 production in reconstituted SCID mice (fig 6). The increase in IL4 and IL13 production was significantly higher in the SCID mice reconstituted with CD4+ T cells isolated from infected mice than in the SCID mice reconstituted with cells from non‐infected mice.
Figure 6 Cytokines produced by in vitro concanavalin A stimulated spleen cells. Severe combined immunodeficient (SCID) mice (on C57BL/6 background) were reconstituted with purified CD4+ T cells from non‐infected and Trichuris muris‐infected euthymic C57BL/6 mice and were killed on day 21 after reconstitution to investigate the cytokine response in spleen cells. Spleen cells were stimulated with concanavalin A for 24 h and the levels of interleukin (IL)4 (A) and IL13 (B) present in the supernatant was investigated by ELISA. Each value (pg/ml) represents mean (SEM) from four mice. *Significantly (p<0.05) higher than that in SCID control mice by Dunnett's post hoc test.
EC cells in the colon express IL‐13 Rα1
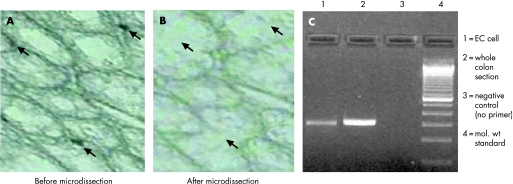
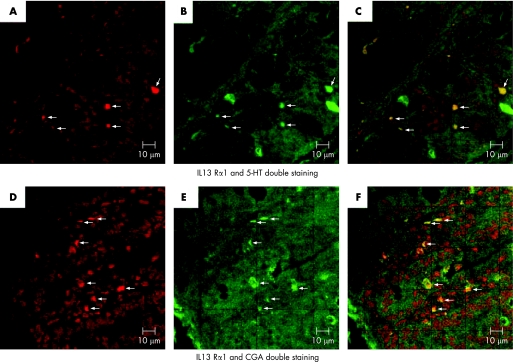
As increase in Th2 cytokines is associated with T muris infection, and as we observed an upregulation of EC and 5‐HT content during the infection it seems likely that Th2 cytokines play an important part in EC cell biology. Therefore, we studied the expression of receptors for IL4 and IL13 on EC cells using LCM and RT‐PCR. EC cells were microdissected by LCM from colonic tissue sections of non‐infected mice, immunostained with anti‐5‐HT antibody and were assessed for the expression of receptors for IL4 and IL13Rα1 by RT‐PCR. Expression of IL13Rα1 was observed in the isolated EC cells (fig 7). However, we could not detect expression of the IL4 receptor in the isolated cells. These data were confirmed by double staining for IL13Rα1 and 5‐HT/CGA. Figure 8 shows the presence of IL13Rα1 on both 5‐HT and CGA‐expressing cells in the colon of infected mice. We also observed expression of IL13Rα1 on 5‐HT and CGA‐expressing cells in the colon of non‐infected mice (data not shown).
Figure 7 Isolation of 5‐hydroxytryptamine (5‐HT)‐expressing enterochromaffin (EC) cells and expression of interleukin (IL)13 and IL4 receptors in isolated EC cells. EC cells were microdissected by laser capture microdissection from colonic tissue sections of non‐infected C57BL/6 mice after immunostaining with 5‐HT antibody. Isolated EC cells were investigated for expression of IL13 receptor α1 (IL13Rα1) and IL4 receptor α (IL4Rα) by reverse transcription (RT)‐PCR. (A) 5‐HT immunostained colonic tissue section before microdissection. (B) Colonic section after microdissection. (C) Expression of IL13Rα1 by RT‐PCR. (D) IL4Rα mRNA expression by RT‐PCR. Mol wt, molecular weight. Representative data from two experiments.
Figure 8 Presence of interleukin 13 receptor α1‐chain (IL‐13Rα1) on enterochromaffin (EC) cells in the colon as detected by immunofluorescence technique. Colonic sections from infected C57BL/6 mice (day 14 after infection) were double immunostained for IL‐13Rα1 and 5‐hydroxytryptamine (5‐HT)/chromogranin A (CGA).(A) Representative micrograph showing expression of IL13Rα1 in colonic section. (B) Representative micrograph showing 5‐HT‐expressing cells in colonic section. (C) Representative micrograph showing 5‐HT and IL13Rα1‐expressing cells in colonic section. (D) Representative micrograph showing IL‐13 Rα1‐expressing cells in colonic section. (E) Representative micrograph showing CGA‐expressing cells in colonic section. (F) Representative micrograph showing CGA and IL13Rα1‐expressing cells in colonic section.
Discussion
The present study provides evidence of an important role of CD4+ T cells in the development of enteric infection‐induced EC cell hyperplasia and in the upregulation of 5‐HT production. Infection with T muris generated EC cell hyperplasia and an increase in 5‐HT content in the colon. This infection‐induced change in EC cells and in 5‐HT was not evident in immunodeficient SCID mice but was restored after reconstitution with purified CD4+ T cells from infected immunocompetent mice. In addition, our results show for the first time, the presence of cytokine receptors on colonic EC cells. Taken together, it is surmised that cytokines from activated CD4+ T cells in enteric infection lead to changes in EC cells acting directly through receptors expressed on EC cells.
Enteric infection‐induced mucosal inflammation induces various changes in GI physiology, which include increased propulsive activity and mucus secretion. These changes are considered to be under direct immunological control rather than a non‐specific consequence of the inflammatory reaction to the infective agent.37 It is likely that immune‐mediated changes in EC cell function play an important role in gut physiological changes in enteric infections and inflammatory conditions. Alteration in EC cells is reported in many GI disorders, including infections by a variety of infectious agents. 5‐HT has been shown to be important in cholera toxin‐induced fluid and mucin secretion.17,38 Recently, it has been demonstrated that 5‐HT3 antagonist attenuates rotavirus‐induced diarrhoea, indicating a critical role for 5‐HT in the pathogenesis of this viral diarrhoea.19 The number of EC cells and the amount of 5‐HT are also increased in the small intestine of Nippostrongylus brasiliensis‐infected rats20 and Trichinella spiralis‐infected mice39,40, respectively. Although these studies in nematode infections suggest an alteration in 5‐HT response in the small intestine after infection, no information is available on 5‐HT response in the large intestine. The present study clearly showed an increase in the number of EC cells and 5‐HT content in the colon of mice after infection with T muris.
Considering the location of EC cells in the GI mucosa, infection‐induced changes in EC cells and 5‐HT are likely to be modulated by immune cells such as lymphocytes. Recently, it has been shown that T spiralis infection‐induced upregulation of EC cells is attenuated in T cell receptor (β×δ) KO mice.39 It has also been demonstrated that after T spiralis infection, CD4+ T cells regulate cholecystokinin‐expressing endocrine cells in the small intestine of mice.40 The role of host's immune response underlying changes in EC cells and 5‐HT has been also demonstrated in mice infected with bacterial pathogen, Citrobacter rodentium.41 The number of EC cells and the amount of 5‐HT in the colon was significantly reduced in C rodentium‐infected immunocompetent mice. However, this infection‐induced alteration in EC cells and 5‐HT was not evident in SCID mice. Moreover, in vitro studies using BON tumour cells (model of human EC cells) have shown that interferon γ exerts antiproliferative effects on these cells and inhibits 5‐HT production from these cells.42 Our present results provide clear evidence of CD4+ T cell‐mediated immunological control of EC cell hyperplasia and 5‐HT production in intestinal infection and inflammation. The transfer of purified CD4+ T cells from infected mice into naive SCID mice significantly increased the number of EC cells and the amount of 5‐HT. The increase in EC cells and 5‐HT in reconstituted SCID mice was associated with an upregulation of colonic CD3 T cells and Th2 cytokine production. However, EC cells or colonic 5‐HT was not significantly altered in SCID mice reconstituted with cells from non‐infected mice. These observations suggest that activated CD4+ T cells play a key role in the alteration of EC cell function after infection.
A noticable observation of this study is the presence of cytokine receptors on EC cells. By combining both microdissection‐based molecular and immunohistochemical techniques we have shown the presence of IL13Rα1 on EC cells. IL13 is a major cytokine in Th2‐type immune responses in helminth infection and plays a critical role in host defence in T muris infection.43 In addition, IL13 exhibits a broad range of other biological activities including regulation of airway hyper‐responsiveness, tissue eosinophilia, mastocytosis, goblet cell hyperplasia and fibrosis.44 IL13 exerts its activity on target cells via the dimeric IL13 receptor, which comprises the IL13Rα1 as a specific component.45,46 In this study, we observed expression of IL13Rα1 mRNA in the microdissected EC cells and could also colocalise the expression of IL13Rα1 and 5‐HT or CGA in the colonic tissue. CGA is a member of the granin family, and studies on both humans and mice have identified CGA as a reliable marker for enteric endocrine cells.47 5‐HT can also be produced by mast cells in rodents, which are also increased in nematode infection.48 Therefore, by double immunostaining for IL13Rα1 and CGA, we have precisely detected IL13Rα1 on EC cells. Upregulation of EC cells and Th2 cytokine responses in the SCID mice after reconstitution with CD4+ T cells from infected mice and presence of IL13Rα1 on EC cells as depicted in this study, strongly suggest that cytokines such as IL13 from CD4+ T cells play an important role in EC cell biology by acting directly on specific cytokine receptors on EC cells.
Alteration in EC cells and 5‐HT production occurs in a variety of clinical settings including enteritis, IBS, inflammatory bowel disease and colon carcinoma.13,14,15,16,19,39,49 5‐HT is an important enteric mucosal signalling molecule and is considered critical for maintaining intestinal homeostasis. This is supported by reports of serious complications such as ischaemic colitis after treatment with a 5‐HT3 receptor antagonist, alosetron in some patients with IBS who were not ostensibly at ischaemic risk.50,51 The case of alosetron suggests that 5‐HT is critical for intestinal homeostasis, and interference in its activities may result in deleterious consequences, and also prompts a rethinking of our approaches to the modulation of serotoninergic pathways. Our present data provide valuable information in understanding immunological control of EC cell function and 5‐HT production in the gut. In addition to enhancing our understanding of EC cell biology in enteric infection and inflammatory conditions, this study provides new information on this cell in association with intestinal homeostasis. Considering the importance of 5‐HT in gut physiology, these results provide new insights into the mechanisms of gut immunoendocrine interaction, which may ultimately lead to improved therapeutic strategies in various GI disorders, such as PI‐IBS, where hyperplasia of EC cells is seen in association with immune activation in the gut.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Canadian Association of Gastroenterology (CAG)/Crohn's and Colitis Foundation of Canada (CCFC) to Dr WIKhan.
We thank Dr Stephen Collins (McMaster University) for his valuable discussion, Dr Jack Gauldie (McMaster University) for support in laser capture microdissection, and Daniel Coyen‐Lyons (McMaster University) and Shazia Kazmi (University of Manchester) for technical support.
Abbreviations
ANOVA - analysis of variance
CGA - chromogranin A
EC - enterochromaffin
GI - gastrointestinal
5‐HT - 5‐hydroxytryptamine
IBS - irritable bowel syndrome
IL - interleukin
IL4R - interleukin 4 receptor
IL‐13Rα1 - interleukin 13 receptor α1‐chain
LCM - laser capture microdissection
PBS - phophate‐buffered saline
PI - post‐infectious
RT - room temperature
RT‐PCR - reverse transcription PCR
SCID - severe combined immunodeficient
Th2 - T helper 2
Footnotes
Competing interests: None.
References
- 1.Walsh J H, Dockray G J.Gut peptides. New York: Raven, 19941–884.
- 2.Rehfield J F. The new biology of gastrointestinal hormones. Physiol Rev 1998781087–1108. [DOI] [PubMed] [Google Scholar]
- 3.Sharkey K A, Mawe G M. Neuroimmune and epithelial interactions in intestinal inflammation. Curr Opin Pharmacol 20022669–677. [DOI] [PubMed] [Google Scholar]
- 4.Gershon M D. Review article: roles played by 5‐hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 199913(Suppl 2)15–30. [PubMed] [Google Scholar]
- 5.Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol 200290109–120. [DOI] [PubMed] [Google Scholar]
- 6.Erspamer V. Occurrence of indole alkylamines in nature. In: Eicher O, Farah A, eds. Handbuch der Experimentellen Pharmakologie. Berlin, Germany: Springer Verleg, 1996; 132–81,
- 7.Cetin Y, Kuhn M, Kulaksiz H.et al Enterochromaffin cells of the digestive system: cellular source of guanylin, a guanylate cyclase‐activating peptide. Proc Natl Acad Sci USA 1994912935–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon M D. Serotonin and its implication for the management of irritable bowel syndrome. Rev Gastroenterol Disord 20033(Suppl 2)S25–S34. [PubMed] [Google Scholar]
- 9.Kim D Y, Camilleri M. Serotonin: a mediator of the brain‐gut connection. Am J Gastroenterol 2000952698–2709. [DOI] [PubMed] [Google Scholar]
- 10.Buchan A M. Nutrient tasting and signaling mechanisms in the gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol 1999277G1103–G1107. [DOI] [PubMed] [Google Scholar]
- 11.Mossner R, Lesch K P. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun 199812249–271. [DOI] [PubMed] [Google Scholar]
- 12.Hansen M B. Neurohumoral control of gastrointestinal motility. Physiol Res 2003521–30. [PubMed] [Google Scholar]
- 13.Camilleri M, Northcutt A R, Kong S.et al Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo‐controlled trial. Lancet 20003551035–1040. [DOI] [PubMed] [Google Scholar]
- 14.Ahonen A, Kyosola K, Penttila O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res 197681–7. [PubMed] [Google Scholar]
- 15.Coates M D, Mahoney C R, Linden D R.et al Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 20041261657–1664. [DOI] [PubMed] [Google Scholar]
- 16.Belai A, Boulos P B, Robson T.et al Neurochemical coding in the small intestine of patients with Crohn's disease. Gut 199740767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore B A, Sharkey K A, Mantle M. Role of 5‐HT in cholera toxin‐induced mucin secretion in the rat small intestine. Am J Physiol 1996270(Pt 1)G1001–G1009. [DOI] [PubMed] [Google Scholar]
- 18.Grondahl M L, Jensen G M, Nielsen C G.et al Secretory pathways in Salmonella typhimurium‐induced fluid accumulation in the porcine small intestine. J Med Microbiol 199847151–157. [DOI] [PubMed] [Google Scholar]
- 19.Kordasti S, Sjovall H, Lundgren O.et al Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut 200453952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer S G, Laniyonu A A. Effects of p‐chlorophenylalanine on the sensitivity of rat intestine to agonists and on intestinal 5‐hydroxytryptamine levels during Nippostrongylus brasiliensis infection. Br J Pharmacol 198482883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadwick V S, Chen W, Shu D.et al Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 20021221778–1783. [DOI] [PubMed] [Google Scholar]
- 22.Collins S M, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut 200149743–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbara G, De Giorgio R, Deng Y.et al Role of immunologic factors and cyclooxygenase 2 in persistent postinfective enteric muscle dysfunction in mice. Gastroenterology 20011201729–1736. [DOI] [PubMed] [Google Scholar]
- 24.Barbara G, Vallance B A, Collins S M. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology 19971131224–1232. [DOI] [PubMed] [Google Scholar]
- 25.Qian B F, El‐Salhy M, Melgar S.et al Neuroendocrine changes in colon of mice with a disrupted IL‐2 gene. Clin Exp Immunol 2000120424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin D C, Zhang H, Qian P.et al Altered enteroendocrine cell expression in T cell receptor alpha chain knock‐out mice. Microsc Res Tech 200051112–120. [DOI] [PubMed] [Google Scholar]
- 27.Yang G B, Lackner A A. Proximity between 5‐HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5‐HT in mucosal immunity. J Neuroimmunol 200414646–49. [DOI] [PubMed] [Google Scholar]
- 28.Else K J, Grencis R K. Helper T‐cell subsets in mouse trichuriasis. Parasitol Today 19917313–316. [DOI] [PubMed] [Google Scholar]
- 29.Grencis R K. Th2‐mediated host protective immunity to intestinal nematode infections. Philos Trans R Soc Lond B Biol Sci 19973521377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakelin D. Acquired immunity to Trichuris muris in the alnino laboratory mouse. Parasitology 196757515–517. [DOI] [PubMed] [Google Scholar]
- 31.Else K J, Wakelin D, Wassom D L.et al The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology 1990101(Pt 1)61–67. [DOI] [PubMed] [Google Scholar]
- 32.Chilton P M, Fernandez‐Botran R. Regulation of the expression of the soluble and membrane forms of the murine IL‐4 receptor. Cell Immunol 199715104–115. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita M, Yamamoto T, Nishioka K. Upregulation of interleukin‐13 and its receptor in a murine model of bleomycin‐induced scleroderma. Int Arch Allergy Immunol 2004135348–356. [DOI] [PubMed] [Google Scholar]
- 34.Deschoolmeester M L, Else K J. Cytokine and chemokine responses underlying acute and chronic Trichuris muris infection. Int Rev Immunol 200221439–467. [DOI] [PubMed] [Google Scholar]
- 35.Else K J, Grencis R K. Antibody‐independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infect Immun 1996642950–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan W I, Richard M, Akiho H.et al Modulation of intestinal muscle contraction by interleukin‐9 (IL‐9) or IL‐9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun 2003712430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins S M. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 19961111683–1699. [DOI] [PubMed] [Google Scholar]
- 38.Beubler E, Horina G. 5‐HT2 and 5‐HT3 receptor subtypes mediate cholera toxin‐induced intestinal fluid secretion in the rat. Gastroenterology 19909983–89. [DOI] [PubMed] [Google Scholar]
- 39.Wheatcroft J, Wakelin D, Smith A.et al Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil 200517863–870. [DOI] [PubMed] [Google Scholar]
- 40.McDermott J R, Leslie F C, D'Amato M.et al Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut 200655492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Hara J R, Skinn A C, MacNaughton W K.et al Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol 20068646–660. [DOI] [PubMed] [Google Scholar]
- 42.Hopfner M, Sutter A P, Huether A.et al A novel approach in the treatment of neuroendocrine gastrointestinal tumors: additive antiproliferative effects of interferon‐gamma and meta‐iodobenzylguanidine. BMC Cancer. 2004;21;423. [DOI] [PMC free article] [PubMed]
- 43.Bancroft A J, McKenzie A N, Grencis R K. A critical role for IL‐13 in resistance to intestinal nematode infection. J Immunol 19981603453–3461. [PubMed] [Google Scholar]
- 44.Wynn T A. IL‐13 effector functions. Annu Rev Immunol 200321425–456. [DOI] [PubMed] [Google Scholar]
- 45.Aman M J, Tayebi N, Obii N I.et al cDNA cloning and characterization of the human interleukin‐13 receptor alpha chain. J Biol Chem 199627129265–29270. [DOI] [PubMed] [Google Scholar]
- 46.Myrtek D, Knoll M, Mattiesen T.et al Expression of interleukin‐13 receptor alpha 1‐subunit on peripheral blood eosinophils is regulated by cytokines. Immunology 2004112597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Facer P, Bishop A E, Lloyd R V.et al Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology 1985891366–1373. [DOI] [PubMed] [Google Scholar]
- 48.Nagata K, Fujimiya M, Sugiura H.et al Intracellular localization of serotonin in mast cells of the colon in normal and colitis rats. Histochem J 200133559–568. [DOI] [PubMed] [Google Scholar]
- 49.Ulich T R, Cheng L, Glover H.et al A colonic adenocarcinoma with argentaffin cells. An immunoperoxidase study demonstrating the presence of numerous neuroendocrine products. Cancer 1983511483–1489. [DOI] [PubMed] [Google Scholar]
- 50.Ladabaum U. Safety, efficacy and costs of pharmacotherapy for functional gastrointestinal disorders: the case of alosetron and its implications. Aliment Pharmacol Ther 2003171021–1030. [DOI] [PubMed] [Google Scholar]
- 51.Cole J A, Cook S F, Sands B E.et al Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol 200499486–491. [DOI] [PubMed] [Google Scholar]