Abstract
Background
Drug‐eluting stents (DES) have been introduced successfully in clinical practice to prevent post‐angioplasty restenosis. Nevertheless, concerns about the safety of DES still exist.
Objective
To investigate the vascular pathology and transcriptional responses to sirolimus and paclitaxel in a murine model for restenosis on underlying diseased atherosclerotic arteries.
Methods
Atherosclerotic lesions were induced by placement of a perivascular cuff around the femoral artery of hypercholesterolaemic ApoE*3‐Leiden transgenic mice. Two weeks later these cuffs were replaced either by sirolimus‐ or paclitaxel‐eluting cuffs. The vascular pathological effects were evaluated after two additional weeks.
Results
Both anti‐restenotic compounds significantly inhibited restenotic lesion progression on the atherosclerotic plaques. Vascular histopathological analyses showed that local delivery of sirolimus has no significant adverse effects on vascular disease. Conversely, high dosages of paclitaxel significantly increased apoptosis, internal elastic lamina disruption, and decreased medial and intimal smooth muscle cells and collagen content. Moreover, transcriptional analysis by real‐time RT‐PCR showed an increased level of pro‐apoptotic mRNA transcripts (FAS, BAX, caspase 3) in paclitaxel‐treated arteries.
Conclusions
Sirolimus and paclitaxel are effective in preventing restenosis. Sirolimus has no significant effect on arterial disease. In contrast, paclitaxel at high concentration demonstrated adverse vascular pathology and transcriptional responses, suggesting a narrower therapeutic range of this potent drug. Since the use of overlapping stents is becoming more common in DES technology, this factor is important, given that higher dosages of paclitaxel may lead to increased apoptosis in the vessel wall and, consequently, to a more unstable phenotype of the pre‐existing atherosclerotic lesion.
Keywords: atherosclerosis, animal model, pathology, restenosis, stents
Restenosis remains the major drawback of percutaneous coronary interventions.1 Recently, drug‐eluting stents (DES) were introduced in interventional cardiology, leading to a drop in the (in‐stent) restenosis.2,3 Since approval of DES, restenosis rate have decreased from 29.3% with bare‐metal stents to 8.9%.4 For DES, sirolimus and paclitaxel are the most effective anti‐restenotic drugs.5 Nonetheless, problems of safety related to the global use of DES still exist.6,7,8,9,10,11,12,13 The strong hydrophobicity of such compounds allows them to partition highly into arterial tissue, resulting in drug concentrations that exceed the applied bulk concentration.14 This highly concentrated local delivery of potent drugs may lead to increased vascular toxicity.15,16 Only limited pathological data for the use of DES on human coronary arteries exist because little histology of the stented region is available. Therefore, preclinical studies are warranted to elucidate better the DES‐related vascular pathological effects and assess potential side effects on critical mechanisms of vascular healing and stability of the underlying atherosclerotic lesion.17,18,19
One well‐defined mouse model of restenosis consists of placement of a non‐constrictive perivascular cuff around the mouse femoral artery.20,21 Previously, we showed that hypercholesterolaemic ApoE*3‐Leiden mice in combination with cuff placement results in atherosclerotic‐like lesions.22 Furthermore, we demonstrated that the non‐constrictive cuff could be constructed from a polymeric formulation suitable for controlled drug delivery. This “drug‐eluting cuff” (DEC) simultaneously induces reproducible neointimal lesions and allows local delivery of compounds to the cuffed vessel segment.23,24,25 Although these DECs are placed perivascularly instead of intraluminarly it is believed that this situation is certainly comparable to the human situation where the DES struts are pressed deeply into the vessel wall. The mouse femoral artery is only a few cell layers thick, and therefore penetration of the active compounds will be similar.
Here, we evaluated the vascular histopathological responses of murine diseased atherosclerotic arteries to sirolimus and paclitaxel. Moreover, to identify factors potentially involved in the arterial responses to sirolimus and paclitaxel, vascular transcriptional analyses were performed in inflammation‐, remodelling‐ and apoptosis‐related genes. We found that both anti‐restenotic drugs effectively inhibit neointimal lesions. Furthermore, sirolimus has no significant effects on arterial disease. In contrast, paclitaxel demonstrated at higher dosages adverse vascular pathology and transcriptional responses affecting both vessel wall and pre‐existing atherosclerotic lesions.
Methods
Drug‐eluting cuffs
Sirolimus was purchased from LC Laboratories (Woburn, USA). Paclitaxel was provided by Bristol‐Myers Squibb Company (New Jersey, USA). Poly(ε‐caprolactone)‐based DECs were manufactured as previously described.23 Sirolimus‐ (SECs) and paclitaxel‐eluting cuffs (PECs) were made from blended molten drug–polymer mixtures and designed to fit around the femoral artery of mice. Cuffs are shaped as longitudinally cut cylinders with internal diameter 0.5 mm, external diameter 1.0 mm, length 2.0 mm and weight about 5 mg. DECs were loaded with 1% and 2.5% (wt/wt) sirolimus or paclitaxel and the in vitro release profiles were determined for a 2‐week period as described previously (n = 5/group).23 Both sirolimus and paclitaxel show a sustained dose‐dependent release from our delivery system, which is representative of the concentrations of those use in DES.23,26,27 Total sirolimus release was 22.4 (0.4) µg and 40.0 (1.9) µg for the 1% and 2.5% SEC, respectively. Paclitaxel release was 32.7 (4.8) µg for the 1% PEC and 53.5 (2.2) µg for the 2.5% PEC. It should be noted that no significant differences in the in vitro release profiles between both drugs were seen (p>0.05).
Femoral artery cuff mouse model
Male ApoE*3‐Leiden mice, aged 10–12 weeks, were fed a Western‐type diet (1% cholesterol and 0.05% cholate; AB Diets, Woerden, The Netherlands) 3 weeks before and continuing after surgery. Plasma cholesterol levels were measured enzymatically (Roche, Basel, Switzerland). Experimental animals were allocated to the different groups based on cholesterol levels and body weight. After 3 weeks on the diet, animals were anaesthetised with an intraperitoneal injection of 5 mg/kg midazolam (Roche), 0.5 mg/kg medetomidine (Orion, Helsinki, Finland) and 0.05 mg/kg Fentanyl (Janssen, Geel, Belgium). Mice underwent polyethylene cuff placement (Portex, Kent, UK) to induce atherosclerotic‐like lesion formation on both femoral arteries.22 Fourteen days after surgery, the primary cuffs were removed and replaced either with a control DEC, a 1% and 2.5% SEC or PEC for two additional weeks. The cuffs on each site were from the same experimental group. The selected sirolimus and paclitaxel dosages were based on previous work from our laboratory.23 Eight animals were killed at the time of cuff replacement (t = 14 days); for the second branch of the experiment, eight mice per group were used (t = 28 days). All animal work was approved by the TNO institutional regulatory authority and carried out in compliance with guidelines issued by the Dutch government.
Quantification and histological assessment of lesions in cuffed femoral arteries
After death, femoral arteries were harvested, fixed in formaldehyde and embedded in paraffin. Histological assessment and quantification of neointimal lesions was performed as previously described.21,22,23,24 In brief, the thorax was opened and a mild pressure‐perfusion (100 mm Hg) with 4% formaldehyde in 0.9% NaCl (vol/vol) for 5 minutes was performed by cardiac puncture. After perfusion, the femoral artery was harvested, fixed overnight in 4% formaldehyde, dehydrated and paraffin embedded. Serial cross‐sections (200 μm; 5 μm thick) were used throughout the entire length of the cuffed femoral artery for histological analysis. All samples were routinely stained with haematoxylin–phloxine–saffron. Weigert's elastin stain was used to visualise elastic laminae. Ten equally spaced cross‐sections were used in all mice to quantify intimal lesions. Using image analysis software (Leica Qwin, Wetzlar, Germany), total cross‐sectional medial area was measured between the external and internal elastic lamina (IEL); total sectional intimal area was measured between the endothelial cell monolayer and the IEL. IEL disruption was assessed by evaluating the number of broken IELs throughout the entire length of the cuffed vessel segments. Apoptotic cells were detected by TUNEL assay (Roche).24 TUNEL‐labelled nuclei were expressed as a percentage of the total number of nuclei. Smooth muscle cells (SMCs) were visualised with α‐SM actin staining (1:800; Roche). Collagen content was determined using Sirius red stain. Mac3 monoclonal antibody (1:300; BD Biosciences, San Jose, USA) was used to detect tissue macrophages. The percentage of SMCs and collagen content was determined by morphometry (Leica Qwin) as the α‐SM actin‐ or Sirius red‐positive area. The number of medial Mac3‐stained cells per microscopic field was scored in a single‐blinded fashion (magnification ×100). Scoring was as follows: 1, 0–10 cells/field; 2, >10–20 cells/field; 3, >20 cells/field.
Real‐time reverse transcriptase PCR mRNA analysis in cuffed femoral arteries
Mice underwent (double) cuff placement as described above. Animals received either a control DEC, a 2.5% SEC, or a 2.5% PEC (n = 4/group) and were killed 5 days after DEC placement. Arteries were harvested and snap frozen. Total RNA was isolated using RNAeasy Fibrous Tissue Mini‐Kit (Qiagen, Venlo, The Netherlands). From all the RNA samples, cDNA was made using Ready‐To‐Go RT‐PCR beads (Amersham Biosciences, Uppsala, Sweden). Intron‐spanning primers and TaqMan probe were purchased from TaqMan Gene Expression Assays (Applied Biosystems, Foster City, USA). Hypoxanthine phosphoribosyltransferase (HPRT) was assayed to correct for cDNA input. At each time point RT‐PCR was performed in duplicate. For analysis, the average cycle threshold was subtracted from the average cycle threshold of the housekeeping gene HPRT (ΔCt). ΔΔCt was determined as the difference between ΔCt values of the SEC‐ or PEC‐treated arteries and the control DEC group. Data are presented as fold induction (normalised to the control DEC group), which was calculated as 2−ΔΔCt.25
Statistical analysis
Results were expressed as mean (SEM). Significance was determined by the Mann–Whitney U test. Differences were considered significant at p<0.05.
Results
Sirolimus and paclitaxel inhibit neointimal lesion development on pre‐existing atherosclerotic lesions
To assess the effect of perivascular delivery of both sirolimus and paclitaxel on pre‐existing atherosclerotic lesions mice underwent (double) femoral artery cuff placement. In this specific setting, atherosclerotic lesions were first induced by sheeting the femoral artery for 14 days with a primary cuff and subsequent replacement of the first cuff by a second cuff: a control DEC or a DEC loaded either with 1% and 2.5% sirolimus or paclitaxel for two additional weeks. At death, plasma cholesterol levels were 16.6 (1.7) mmol/l. No significant differences in body weights or plasma cholesterol levels were found between groups (data not shown).
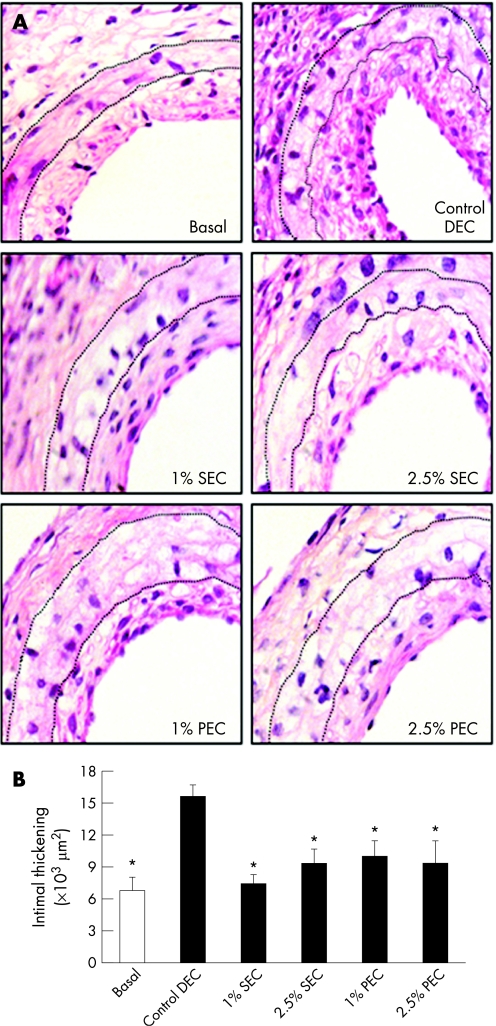
Fourteen days after primary cuff placement the intimal region thickened. Lesions were four to six cell‐layers thick consisting of both α‐SM actin‐positive cells and of foam cell‐like macrophage accumulation (fig 1A). It should be noted that this intimal thickening represents the (basal) pre‐existing atherosclerotic lesions to which, subsequently, sirolimus and paclitaxel are locally delivered.
Figure 1 (A) Representative cross‐sections of cuffed murine femoral arteries at the time of cuff replacement (basal, t = 14 days) and after an additional 14‐day control drug‐eluting cuff (DEC, t = 28 days), sirolimus‐eluting cuff (SEC, t = 28 days), and paclitaxel‐eluting cuff (PEC, t = 28 days). Haematoxylin–phloxine–saffron staining, magnification ×400 (dotted lines delineate the medial region). (B) Total intimal area of cuffed femoral artery segments at the time of cuff replacement (basal, t = 14 days; open bar) and after additional 14‐day control DEC, SEC, and PEC (filled bars, all t = 28 days). Total intimal area was quantified by image analysis using 10 sections in each cuffed artery and expressed in μm2 (mean (SEM), n = 8). *p<0.05, in comparison with control DEC.
Two weeks after control DEC placement, cuffed femoral arteries showed a profound luminal narrowing (fig 1A). Morphometric quantification disclosed a 2.3‐fold increase (p = 0.002) on lesion size as compared with the initial lesions. Local perivascular delivery of either sirolimus or paclitaxel, at both concentrations tested, resulted in a significant inhibition of the neointimal lesions as compared with control arteries (all p<0.05, fig 1B). This detained lesion formation is comparable with the initial lesion size (all p>0.09). No significant differences in medial thickness were seen between the groups (table 1). Likewise, no differences in inner and outer elastic lamina circumference were seen between the groups (all p>0.05, data not shown).
Table 1 Comparison of morphometric measurements of cuffed artery segments at cuff replacement (basal) and from 14‐day control DEC, SEC and PEC (14 days normal cuff plus 14 days DEC).
| Intimal thickening (×103 μm2) | Intima/media ratio | Lumen stenosis (%) | Medial area (×103 μm2) | |
|---|---|---|---|---|
| Basal | 6.8 (1.2)* | 0.49 (0.10)* | 24.0 (5.0)* | 15.9 (1.3) |
| Control DEC | 15.5 (1.1) | 1.43 (0.15) | 65.3 (4.4) | 12.1 (1.1) |
| 1% SEC | 7.4 (0.9)* | 0.54 (0.07)* | 34.7 (3.3)* | 13.1 (1.3) |
| 2.5% SEC | 9.3 (1.4)* | 0.75 (0.11)* | 38.9 (3.9)* | 12.4 (0.7) |
| 1% PEC | 9.9 (1.5)* | 0.68 (0.07)* | 36.9 (7.8)* | 15.2 (0.6) |
| 2.5% PEC | 9.3 (2.1)* | 0.65 (0.14)* | 33.6 (8.9)* | 12.3 (1.0) |
Values are mean (SEM); n = 8/group.
DEC, drug‐eluting cuff; SEC, sirolimus‐eluting cuff; PEC, paclitaxel‐eluting cuff.
*p<0.05 vs control DEC.
Effect of sirolimus and paclitaxel on apoptosis and vascular integrity
To evaluate the effects of local delivery of increasing sirolimus and paclitaxel concentrations on vascular disease, apoptosis and vascular integrity were assessed. A TUNEL assay was performed to assess apoptotic cells in the cuffed artery segments 14 days after DEC placement in control cuffed segments. Low levels of TUNEL‐labelled nuclei were observed in the medial or intimal region. Similarly, local delivery of sirolimus to pre‐existing atherosclerotic lesions had no significant effect on the apoptosis rate and neither did the lowest paclitaxel concentration (all p>0.08). Conversely, cuffed vessels treated with the 2.5% PEC showed a striking 71‐fold increase (p = 0.011) in TUNEL‐positive medial nuclei compared with control arteries. Neointimal TUNEL‐labelled nuclei of the 2.5% paclitaxel‐treated arteries also registered a modest increase but did not reach significance (p = 0.737, table 2) (supplementary figure 1A, available online at http://heart.bmj.com/supplemental).
Table 2 Comparison of histological findings of cuffed femoral artery segments from 14‐day control DEC, SEC and PEC (14 days normal cuff plus 14 days DEC).
| TUNEL+ cells (%) | SMC content (%) | Collagen content (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Media | Intima | Media | Intima | Media | Intima | IEL disruption¶ | Medial macrophage¶ | ||||
| Control DEC | 0.27 (0.24) | 0.39 (0.24) | 25.2 (2.3) | 30.8 (2.7) | 28.3 (4.9) | 40.7 (2.4) | 2.2 (0.6) | 1.08 (0.08) | |||
| 1% SEC | 1.99 (0.68) | 0.58 (0.24) | 27.5 (2.7) | 28.0 (1.1) | 24.0 (3.4) | 28.0 (5.8) | 2.0 (0.5) | 1.39 (0.15) | |||
| 2.5% SEC | 1.78 (0.77) | 0.42 (0.21) | 16.9 (2.9)*† | 21.8 (2.8) | 24.0 (3.1) | 32.9 (4.9) | 2.2 (0.5) | 1.49 (0.16)* | |||
| 1% PEC | 0.84 (0.79) | 0.95 (0.56) | 2.6 (0.9)*†‡ | 10.3 (1.7)*†‡ | 18.6 (1.4) | 19.0 (2.1)*‡ | 3.4 (0.4) | 2.20 (0.39)* | |||
| 2.5% PEC | 19.2 (5.7)*†‡§ | 3.1 (3.08) | 3.8 (0.9)*†‡ | 9.4 (3.1)*†‡ | 12.7 (2.0)*†‡ | 12.4 (6.4)*‡ | 5.2 (0.6)*†‡§ | 2.26 (0.33)* | |||
Values are mean (SEM); n = 8/group.
DEC, drug‐eluting cuff; IEL, internal elastic lamina; PEC, paclitaxel‐eluting cuff; SEC; sirolimus‐eluting cuff; SMC, smooth muscle cell.
*p<0.05 vs control DEC; †p<0.05 vs 1% SEC; ‡p<0.05 vs 2.5% SEC; §p<0.05 vs 1% PEC.
¶IEL disruption was quantified as the number of broken IEL for each cuffed artery segment; medial macrophage content was assessed with a 1–3 score.
Vascular integrity was assessed by evaluating the disruption of the IEL in the cuffed vessel segments. IEL disruption in the control cuffed vessels was similar to that found in both the sirolimus‐ and the 1% paclitaxel‐treated groups (all p>0.1). In contrast, the 2.5% paclitaxel‐treated arteries showed a significant 2.4‐fold (p = 0.010) increase in IEL disruption as compared with control cuffed arteries (table 2) (supplementary figure 1B, available online at http://heart.bmj.com/supplemental).
Effect of sirolimus and paclitaxel on vascular composition
Morphometric evaluation of vascular composition was analysed by quantification of the SMC and collagen content of both medial and intimal regions. Arteries treated with the lowest sirolimus concentration showed no change on medial or intimal SMC‐positive content (both p>0.7). In contrast, the 2.5% sirolimus‐treated vessels showed a modest, but significant, 33.1% (p = 0.014) decrease in medial SMC‐positive area, but no significant effect on neointimal SMC content was found (p = 0.070). In addition, paclitaxel‐treated arteries showed a profound and significant decrease of 89.7% (p = 0.004) and 84.9% (p = 0.009) in medial and of 66.6% (p = 0.006) and 69.4% (p = 0.003) in intimal SMC content for both 1% and 2.5% paclitaxel concentrations, respectively (table 2) (supplementary figure 2A, available online at http://heart.bmj.com/supplemental).
Vascular collagen content was unchanged in the sirolimus‐treated vessels both in the medial and the intimal region (p>0.2). Medial collagen‐positive area in the 1% paclitaxel‐treated vessels was unchanged (p = 0.063), while a 51.5% decrease in the 2.5% paclitaxel concentration (p = 0.020) was observed. Conversely, the intima of paclitaxel‐treated arteries showed a significant 53.3% (p = 0.003) and 69.5% (p = 0.006) decrease in collagen for both the 1% and 2.5% paclitaxel concentrations, respectively (table 2) (supplementary figure 2B, available online at http://heart.bmj.com/supplemental).
Although a significant decrease in medial SMC and collagen content occurred, mainly in the paclitaxel‐treated arteries, no decrease in total medial area was seen. This is probably owing to an increase in medial macrophage content. Medial macrophage score showed a significant 2.1‐ (p = 0.005) and 2.0‐fold (p = 0.006) increase for the 1% and 2.5% paclitaxel‐eluting cuffs, respectively. Furthermore, the 2.5% sirolimus‐treated vessels also showed a modest, but significant, 1.4‐fold increase (p = 0.026) in medial macrophage content (table 2) (supplementary figure 3, available online at http://heart.bmj.com/supplemental). This suggests an increased influx of monocytes into the paclitaxel‐treated vessel wall that subsequently differentiate into foam cell‐like macrophages.
Vascular mRNA responses to sirolimus and paclitaxel
To identify further the potential underlying factors involved in the apparently divergent effect between both anti‐restenotic agents, mRNA analysis was performed in arteries possessing pre‐existing atherosclerotic lesions treated for 5 days either with a control cuff, a 2.5% SEC or a 2.5% PEC.
For analysis, genes were clustered in three gene‐related groups: (a) inflammation‐, (b) remodelling‐, and (c) apoptosis‐related genes (table 3). First, both sirolimus and paclitaxel significantly increased mRNA levels of the proinflammatory cytokine interleukin 6 (IL‐6) as compared with control DEC (both p<0.05). In contrast, both treatments led to a striking suppression of the anti‐inflammatory cytokine interleukin 10 (IL‐10). Also interferon gamma (IFNγ) mRNA levels were significantly decreased. Conversely, the proinflammatory chemokine (C–C motif) ligand 3 (CCL3), the granulocyte macrophage colony‐stimulating factor (GM‐CSF), and the monocyte chemotactic protein (MCP‐1) mRNA levels were solely upregulated in arteries treated with paclitaxel, but not in sirolimus‐treated arteries (all p<0.03).
Table 3 Comparison of mRNA fold induction of 5‐day cuffed femoral artery segments from 2.5% SEC or 2.5% PEC (normalised to control DEC (14 days normal cuff plus 14 days DEC).
| Gene | Fold induction | ||
|---|---|---|---|
| 2.5% SEC | 2.5% PEC | ||
| Inflammation | IL‐6 | 69.5 (26.3)* | 27.9 (3.27)* |
| IL‐10 | 0.23 (0.02)* | 0.47 (0.02)*† | |
| INFγ | 0.03 (0.01)* | 0.03 (0.01)* | |
| CCL3 | 3.18 (1.38) | 19.8 (9.02)*† | |
| GM‐CSF | 2.09 (0.33) | 2.57 (0.64)* | |
| MCP‐1 | 1.73 (0.36) | 3.19 (0.70)*† | |
| TLR‐4 | 0.99 (0.09) | 1.20 (0.21) | |
| Remodelling | α ‐SM actin | 0.94 (0.08) | 0.47 (0.06)*† |
| MMP‐9 | 1.40 (0.33) | 4.13 (0.67)*† | |
| TIMP‐1 | 7.83 (1.97)* | 36.9 (8.8)*† | |
| MMP‐2 | 1.05 (0.41) | 0.78 (0.24) | |
| MMP‐8 | 1.10 (0.17) | 1.01 (0.15) | |
| Apoptosis | BCL‐2 | 0.52 (0.06) | 0.21 (0.09)*† |
| Caspase‐3 | 0.46 (0.03)* | 2.64 (0.03)*† | |
| BAX | 0.78 (0.05) | 6.36 (0.06)*† | |
| FAS | 0.80 (0.11) | 4.08 (0.04)*† | |
DEC, drug‐eluting cuff; PEC, paclitaxel‐eluting cuff; SEC; sirolimus‐eluting cuff;
Values are mean (SEM); n = 4/group.
*p<0.05 vs control DEC; †p<0.05 vs 2.5% SEC.
Second, in the vascular composition and remodelling‐related cluster, mRNA levels of α‐SM actin, a vascular specific actin isoform expressed by SMCs, were significantly downregulated in paclitaxel‐treated arteries as compared with control‐treated and sirolimus‐treated vessel segments (p<0.03). Interestingly, matrix metalloproteinase (MMP) 9 and tissue inhibitor of metalloproteinase 1 (TIMP‐1) mRNA levels were both upregulated in the paclitaxel group as compared with the control and sirolimus group (both p<0.03). Sirolimus‐treated arteries also showed a significant increase in TIMP‐1 mRNA expression levels (p = 0.03), but showed no significant differences in MMP‐9 expression levels as compared with control (p = 0.3). MMP‐2 and MMP‐8 mRNA expression remained stable.
Lastly, apoptosis‐related mRNA transcripts were also differentially expressed in the paclitaxel versus control and sirolimus groups. The mRNA levels of the pro‐survival B cell lymphoma (BCL) 2 were strongly suppressed as compared with both control‐ and sirolimus‐treated vessels (both p<0.05). In contrast, mRNA levels of the pro‐apoptotic factor caspase‐3 were significantly upregulated in the paclitaxel‐treated vessels but, interestingly, downregulated in the sirolimus‐treated group. Likewise, the BCL‐2‐associated X protein (BAX), and the tumour necrosis factor receptor superfamily member 6 (FAS) were significantly upregulated in the paclitaxel‐treated vessels, but not in the control or sirolimus‐treated arteries (both p<0.05).
Discussion
In this study we demonstrate that local delivery of sirolimus and paclitaxel equally inhibits progression of cuff‐induced neointima formation on pre‐existing atherosclerotic lesions after secondary (drug‐eluting) cuff placement (fig 1). The newly formed neointimal lesions consist of both SMC and foam cell‐like macrophages. Here we assessed the hypothesis that the use of potent anti‐restenotic drugs, like sirolimus and paclitaxel, may affect arterial wall biology. We found deleterious effects on vascular pathology for higher concentrations of paclitaxel, but not for sirolimus (tables 1 and 2). Furthermore, here we show that the expression of diverse inflammation‐, vessel wall composition and remodelling‐, and especially apoptosis‐related genes are differently expressed in response to the two drugs (table 3). Altogether, this indicates that although the anti‐restenotic efficacy of both compounds is indisputable, the local vascular (adverse) effects of sirolimus and paclitaxel are divergent.
Since approval of DES, the restenosis rate has decreased by a striking 70% as compared with bare‐metal stents.4 Nevertheless, concerns about the safety of DES still exist. Virmani and colleagues have recently (re)started the controversy about sirolimus‐related side effects, such as stent thrombosis and delayed healing.10,11 Likewise, paclitaxel‐eluting stents have been shown to cause incomplete healing in animal studies as observed by extensive intimal fibrin deposition, fewer SMCs and collagen content, and persistent inflammation.9,12 Our findings of increased vascular expression of genes potentially involved in adverse side effects, such as an upregulation of the pro‐apoptotic factors BAX, FAS, and caspase‐3 and a decrease of α‐SM actin mRNA levels is in line with the reported effects and may further explain some of the latter experimental and clinical observations.
Additionally, a further remarkable observation of our study is that despite a significant decrease in medial SMC and collagen content, particularly in the paclitaxel‐treated arteries, no reduction in medial size was observed. A possible explanation for this fact might be the influx of monocytes/macrophages (table 2). This histological observation supports the transcriptional analysis, where we observed a significant upregulation in vascular GM‐CSF and MCP‐1 mRNA levels, genes related to increased monocyte/macrophage influx (table 3). The cause of this late medial macrophage persistence and its potential (adverse) consequences is not fully understood.
Hydrophobic drugs like sirolimus and paclitaxel partition highly into arterial tissue, resulting in some cases in arterial drug concentration more than an order of magnitude above the applied levels.14 Levin and colleagues have shown that sirolimus shows a uniform transmural arterial distribution, in stark contrast to the non‐uniform distribution of paclitaxel.15 Particularly, sirolimus is distributed uniformly throughout the media and adventitia, whereas paclitaxel is distributed heterogeneously throughout arterial tissue, implying an interplay between paclitaxel and naturally hydrophobic arterial tissue layers (such as elastic laminae and cellular membranes).16 Furthermore, the occurrence of lipid‐laden atherosclerotic plaques might be expected to favour a more heterogeneous arterial distribution of paclitaxel. Taking into account the differential characteristics of the arterial tissue distribution of sirolimus and paclitaxel, it might be conjectured that the potential vascular harmful effects observed in the present study might be attributed to a heterogeneous distribution of paclitaxel within the arterial and neointimal tissue. Reported results showing slight differences in clinical outcome between sirolimus‐ and paclitaxel‐eluting stents have been reported, but these studies focus on clinical measures and do not include any pathological analysis of the vessel wall.28,29,30
In a recent report, human vascular disease in samples of coronary arteries treated with bare‐metal stents or DES, mainly focusing on late‐stent thrombosis, was analysed. No analysis of vascular composition was performed.31 The changes seen in the medial region in our study suggest that a more thorough analysis of the vascular composition of patient samples might be needed to evaluate possible differences between DES. Furthermore, our data are in concordance with a recent report from Finn and coworkers, which showed in a rabbit model of angioplasty and stenting using overlapped stent segments that TAXUS stents induced greater fibrin deposition and medial cell loss than Cypher stents.13
Compelling experimental and clinical evidence shows that sirolimus and paclitaxel differ in important features of vascular biology. Hence, the therapeutic dose range of such drugs is of pivotal importance in the delicate balance between neointima formation inhibition and unwanted vascular pathological side effects. The importance of our findings of a narrower therapeutic range for paclitaxel in diseased atherosclerotic arteries is especially noteworthy at the point of DES overlap. Further preclinical studies, in conjunction with results of clinical evaluations, are warranted to overcome the remaining questions about current use of DES.
The assessment of DES anti‐restenotic treatments in an animal model using a perivascular delivery platform must be acknowledged. In the present experimental setting we assessed the vascular response of perivascular delivery of sirolimus and paclitaxel in a well‐established murine model for restenosis on underlying diseased atherosclerotic arteries in order to evaluate more closely the possible interaction of the anti‐restenotic drugs and the lipid‐laden atherosclerotic plaques. Although DES are endovascular delivery systems themselves, the mouse femoral artery is only a few cell layers thick and it has been shown that both sirolimus and paclitaxel can partition through arterial tissue by endovascular or perivascular delivery.15,16 Nevertheless, it should be realised that perivascular delivery may lead to a different drug arterial distribution than endovascular delivery. Although there might be a difference between sirolimus and paclitaxel arterial distribution, we believe this is not a concern in the mouse model used in the present study. The drug concentrations used were selected based on previous studies from our laboratory as the minimal effective concentration needed to inhibit neointima formation in combination with the cuff‐induced mouse model and are representative of the concentrations used in DES. Current animal models used in the assessment of DES are undoubtedly limited by their ability to replicate human conditions. Nevertheless, we believe that our studies bring new and timely insights into the fundamental mode of action of DES for vascular pathology and, more importantly, the putative adverse effects on the underlying pre‐existing atherosclerotic lesion.
Supplementary Material
Acknowledgements
We thank Jasper J Deuring for excellent technical support.
Abbreviations
BCL - B cell lymphoma
DEC - drug‐eluting cuff
DES - drug‐eluting stent(s)
IEL - internal elastic lamina
MMP - matrix metalloproteinase
PEC - paclitaxel‐eluting cuff
SEC - sirolimus‐eluting cuff
SM - smooth muscle
SMC - smooth muscle cell
TIMP - tissue inhibitor of metalloproteinase
Footnotes
Funding: Supported by a Netherlands Heart Foundation grant to N M M Pires (2001‐T‐32). D Eefting and P H A Quax (established investigator) are supported by the Molecular Cardiology Programme of the Netherlands Heart Foundation (M93.001). Professor J W Jukema is a clinical established investigator of the Netherlands Heart Foundation (2001‐D0‐32).
Conflict of interest: None.
References
- 1.Mehilli J, Kastrati A, Bollwein H.et al Gender and restenosis after coronary artery stenting. Eur Heart J 2003241523–1530. [DOI] [PubMed] [Google Scholar]
- 2.Moses J W, Leon M B, Popma J J.et al Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 20033491315–1323. [DOI] [PubMed] [Google Scholar]
- 3.Stone G W, Ellis S G, Cox D A.et al A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med 2004350221–231. [DOI] [PubMed] [Google Scholar]
- 4.Babapulle M N, Joseph L, Belisle P.et al A hierarchical bayesian meta‐analysis of randomised clinical trials of drug‐eluting stents. Lancet 2004364583–591. [DOI] [PubMed] [Google Scholar]
- 5.van der Hoeven B L, Pires N M, Warda H M.et al Drug‐eluting stents: results, promises and problems. Int J Cardiol 2005999–17. [DOI] [PubMed] [Google Scholar]
- 6.Carter A J, Aggarwal M, Kopia G A.et al Long‐term effects of polymer‐based, slow‐release, sirolimus‐eluting stents in a porcine coronary model. Cardiovasc Res 200463617–624. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Kopia G, Hayashi S.et al Stent‐based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 20011041188–1193. [DOI] [PubMed] [Google Scholar]
- 8.Heldman A W, Cheng L, Jenkins G M.et al Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 20011032289–2295. [DOI] [PubMed] [Google Scholar]
- 9.Drachman D E, Edelman E R, Seifert P.et al Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol 2000362325–2332. [DOI] [PubMed] [Google Scholar]
- 10.Virmani R, Guagliumi G, Farb A.et al Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus‐eluting stent: should we be cautious? Circulation 2004109701–705. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Farb A, Guagliumi G.et al Drug‐eluting stents: caution and concerns for long‐term outcome. Coron Artery Dis 200415313–318. [DOI] [PubMed] [Google Scholar]
- 12.Farb A, Heller P F, Shroff S.et al Pathological analysis of local delivery of paclitaxel via a polymer‐coated stent. Circulation 2001104473–479. [DOI] [PubMed] [Google Scholar]
- 13.Finn A V, Kolodgie F D, Harnek J.et al Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus‐ or paclitaxel‐eluting stents. Circulation 2005112270–278. [DOI] [PubMed] [Google Scholar]
- 14.Creel C J, Lovich M A, Edelman E R. Arterial paclitaxel distribution and deposition. Circ Res 200086879–884. [DOI] [PubMed] [Google Scholar]
- 15.Levin A D, Vukmirovic N, Hwang C W.et al Specific binding to intracellular proteins determines arterial transport properties for sirolimus and paclitaxel. Proc Natl Acad Sci USA 20041019463–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang C W, Edelman E R. Arterial ultrastructure influences transport of locally delivered drugs. Circ Res 200290826–832. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz R S, Chronos N A, Virmani R. Preclinical restenosis models and drug‐eluting stents: still important, still much to learn. J Am Coll Cardiol 2004441373–1385. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz R S, Edelman E R, Carter A.et al Preclinical evaluation of drug‐eluting stents for peripheral applications: recommendations from an expert consensus group. Circulation 20041102498–2505. [DOI] [PubMed] [Google Scholar]
- 19.Virmani R, Kolodgie F D, Farb A.et al Drug eluting stents: are human and animal studies comparable? Heart 200389133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moroi M, Zhang L, Yasuda T.et al Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest 19981011225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quax P H, Lamfers M L, Lardenoye J H.et al Adenoviral expression of a urokinase receptor‐targeted protease inhibitor inhibits neointima formation in murine and human blood vessels. Circulation 2001103562–569. [DOI] [PubMed] [Google Scholar]
- 22.Lardenoye J H, Delsing D J, de Vries M R.et al Accelerated atherosclerosis by placement of a perivascular cuff and a cholesterol‐rich diet in ApoE*3Leiden transgenic mice. Circ Res 200087248–253. [DOI] [PubMed] [Google Scholar]
- 23.Pires N M, van der Hoeven B L, de Vries M R.et al Local perivascular delivery of anti‐restenotic agents from a drug‐eluting poly(epsilon‐caprolactone) stent cuff. Biomaterials 2005265386–5394. [DOI] [PubMed] [Google Scholar]
- 24.Pires N M, Schepers A, van der Hoeven B L.et al Histopathologic alterations following local delivery of dexamethasone to inhibit restenosis in murine arteries. Cardiovasc Res 200568415–424. [DOI] [PubMed] [Google Scholar]
- 25.Monraats P S, Pires N M, Schepers A.et al Tumor necrosis factor‐α plays an important role in restenosis development. FASEB J . 2005;191998–2004. [DOI] [PubMed]
- 26.TAXUS™ Express2™ Paclitaxel‐eluting coronary stent system. Direction for use. http://www.bostonscientific.com/templatedata/imports/collateral/Coronary/dfu_taxexp_01_us.pdf (accessed 26 April 2007)
- 27.Cordis CYPHER® Sirolimus‐eluting coronary stent. Instruction for use. http://www.cordislabeling.com/pdf/5462872_8.pdf (accessed 26 April 2007)
- 28.Saia F, Piovaccari G, Manari A.et al Clinical outcomes for sirolimus‐eluting stents and polymer‐coated paclitaxel‐eluting stents in daily practice: results from a large multicenter registry. J Am Coll Cardiol 2006481312–1318. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y H, Park S W, Lee S W.et al Sirolimus‐eluting stent versus paclitaxel‐eluting stent for patients with long coronary artery disease. Circulation 20061142148–2153. [DOI] [PubMed] [Google Scholar]
- 30.Tanimoto S, Daemen J, Tsuchida K.et al Two‐year clinical outcome after coronary stenting of small vessels using 2. 25‐mm sirolimus‐ and paclitaxel‐eluting stents: Insight into the RESEARCH and T‐SEARCH registries, Catheter Cardiovasc Interv 20066994–103. [DOI] [PubMed] [Google Scholar]
- 31.Joner M, Finn A V, Farb A.et al Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 200648193–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.