Abstract
Purpose of review
While cognitive dysfunction including memory and attentional deficits are well known in schizophrenia, recent work has also shown basic sensory processing deficits. Deficits are particularly prominent in the visual system and may be related to cognitive deficits and outcome. This article reviews studies of early-stage visual processing in schizophrenia published during the past year. These studies reflect the growing interest and importance of sensory processing deficits in schizophrenia.
Recent findings
The visual system is divided into magnocellular and parvocellular pathways which project to dorsal and ventral visual areas. Recent electrophysiological and behavioral investigations have found preferential magnocellular/dorsal stream dysfunction, with some deficits in parvocellular function as well. These early-stage deficits appear to be related to higher level cognitive, social, and community function. Structural studies of occipital cortex and particularly optic radiations provide anatomical support for early visual processing dysfunction.
Summary
These findings highlight the importance of sensory processing deficits, in addition to higher cognitive dysfunction, for understanding the pathophysiology of schizophrenia. Understanding the nature of sensory processing deficits may provide insight into mechanisms of pathology in schizophrenia, such as N-methyl-d-aspartate dysfunction or impaired signal amplification, and could lead to treatment strategies including sensory processing rehabilitation that may improve outcome.
Keywords: magnocellular, parvocellular, schizophrenia, visual evoked potentials
Introduction
Patients with schizophrenia have severe neurophysiological deficits not only at cognitive [1-4] but also at perceptual [5-8] levels of processing. Perceptual deficits have become increasingly well documented in the visual system in schizophrenia [9-17,18•,19-21] and may contribute to higher level cognitive impairments and community outcome [12,22-24]. An early report by Saccuzzo et al. [25] of visual backward masking dysfunction in schizophrenia was particularly important not only because it indicated deficits in the earliest components of visual information processing [16,26,27], but also because it suggested dysfunction of a particular visual pathway – the psychophysically defined transient visual pathway [27-30].
The classic transient/sustained psychophysical dichotomy has been supplanted over recent years by anatomically and physiologically based distinctions between magnocellular and parvocellular pathways, which roughly correspond to properties of transient and sustained pathways, respectively. The magnocellular system, in general, conducts low-resolution visual information rapidly to cortex, and is involved in attentional capture and processing of overall stimulus organization [31-33]. The parvocellular system, in contrast, conducts high-resolution visual information to cortex, and is involved in processing of fine-grained stimulus configurations and object identification [31,34]. A focus of this review is to examine the differential functional roles of magnocellular and parvocellular systems in visual pathway dysfunction, including their contribution to ‘upstream’ cognitive impairments and outcome.
Both the magnocellular and parvocellular pathways begin in the retina and project, by means of the lateral geniculate nucleus (LGN), to primary visual cortex (striate cortex, V1). These pathways have very different properties, which can be manipulated to preferentially activate the magnocellular or parvocellular components of the system. For instance, magnocellular neurons are more sensitive than parvocellular neurons to low luminance contrast [35,36]. In addition, magnocellular cells are activated vigorously by stimulus elements that are relatively large, whereas parvocellular cells are activated more strongly by stimulus elements that are relatively small [37,38]. Magnocellular cells are also relatively unresponsive to chromatic (color) contrast while parvocellular cells are not [35].
Magnocellular information is conveyed preferentially to parieto-occipital cortex and other dorsal stream visual areas which are involved in motion and space perception (the ‘where’ pathway) [31,39]. Parvocellular information is conveyed preferentially to temporo-occipital cortex and other ventral-stream areas which are involved in color and form perception (the ‘what’ pathway) [31,39]. Crossover occurs at multiple levels, including from higher-order dorsal stream to higher-order ventral stream areas [40,41]. Transmission is faster through the dorsal stream, perhaps permitting it to prime ventral stream areas. A fundamental role of the magnocellular system/dorsal stream may be to ‘spotlight’ relevant information for transmission to ventral stream areas, so that crossover inputs from the dorsal stream may modulate activity within ventral stream structures [15,32].
In accord with the original theory of ‘transient channel’ dysfunction in schizophrenia, deficits are particularly prominent in processes, such as motion detection, velocity discrimination, spatial localization, trajectory, and eye-tracking tasks that depend mainly upon magnocellular input to the dorsal visual stream, and in detection of low contrast and small stimuli [9,13,14,16,17,18•,20,42•,43,44]. However, deficits have also been observed even in processing of parvocellular-biased stimuli [20]. In addition, while some ventral stream object identification deficits have also been found, several studies provide evidence that the latter are due to aberrant crossover input from dorsal to ventral stream areas [15,19]. Advances in understanding visual processing dysfunction in schizophrenia have occurred due to use of basic neurophysiological properties of magnocellular and parvocellular neurons to selectively activate these pathways. Recent papers examining early visual processing dysfunction, with an emphasis on those related to magnocellular and parvocellular as well as dorsal and ventral stream processing, will be reviewed.
Electrophysiological studies
A primary approach to analyzing integrity of visual processing is use of visual evoked potentials (VEPs). VEPs have the advantage that they are nonbehavioral and so provide an objective measure of brain function. In particular, steady-state VEPs (ssVEPs) have been used in two recent studies [45,46]. In the ssVEP approach, stimuli are presented rapidly and may produce habituation of the higher-level cortical response and thus increase sensitivity for evaluating lower-level responses. In this approach, physical stimulus features such as luminance contrast can be manipulated to preferentially activate the magnocellular and parvocellular components of the system. ssVEP studies provide a definitive demonstration of magnocelslular dysfunction in schizophrenia.
In one recent ssVEP study, Butler et al. [45] used luminance contrast to bias processing towards the magnocellular versus parvocellular pathway. In the magnocellular-selective condition, stimuli appeared and disappeared, a manipulation that preferentially engages the magnocellular system. Conversely, in the parvocellular-selective condition, stimuli were modulated around a high 48% level of luminance contrast (‘pedestal’) that saturates the magnocellular response, and so isolates the additional parvocellular activation [9,47]. Patients generated evoked potentials that were significantly reduced in response to magnocellular but not parvocellular-biased stimuli. In terms of the pattern of responses in the magnocellular condition, controls showed a steeply rising increase in response to low luminance contrast stimuli (~1–10% contrast) which reached a saturation level once luminance contrast reached ~16–32%, similar to what is seen in single-cell recordings from magnocellular neurons in monkey LGN [35]. However, responses of patients showed a much shallower gain at low luminance contrast and a much lower plateau. Visual pathways within the brain use glutamate as their primary neurotransmitter. The decreased contrast gain and plateau in the magnocellular-biased ssVEP contrast response curve for schizophrenia patients in this study closely resembles results seen following microinfusion of an N-methyl-d-aspartate (NDMA) antagonist into cat LGN and visual cortex [48,49], consistent with glutamatergic theories of schizophrenia [50-52]. ssVEP m easures also predicted community functioning.
In a second ssVEP study, Kim et al. [46] utilized windmill–dartboard and partial-windmill stimuli to investigate responses across harmonic levels (Fig. 1). In most studies utilizing ssVEPs, the first harmonic response, extracted by Fourier transform, is used to analyze data. The first harmonic refers to responses at the stimulus input frequency. However, second harmonic responses can also be extracted. The second harmonic refers to responses at twice the stimulus input frequency. The windmill–dartboard condition used in this study produces ssVEP responses with a dominant first harmonic and an attenuated second harmonic, whereas the partial-windmill condition produces an ssVEP that contains a dominant second harmonic response. Second harmonic responses are preferentially elicited by achromatic [53] and low spatial frequency [54,55] stimuli and are thus thought to be mediated primarily by magnocellular system activity. In contrast, first harmonic responses are elicited by contrast above the magnocellular-specific range of function [56], which indicates a dominant role of the parvocellular pathway in the generation of the first harmonic [57]. Schizophrenia patients showed reduced amplitude and coherence of second harmonic responses in both conditions, but intact first harmonic responses in the windmill–dartboard condition (Fig. 2). This indicates a significant loss in the magnocellular pathway, which contributes to the generation of the second harmonic component under these conditions.
Figure 1. Partial-windmill and windmill–dartboard conditions.
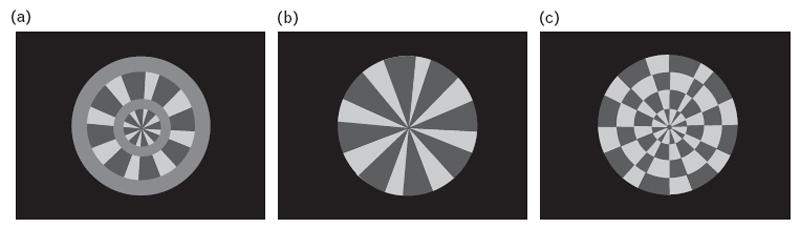
(a) The partial-windmill condition. The pattern elements in the central disk and second annulus contrast reverse to produce a partial windmill. (b, c) Windmill–dartboard condition. The windmill–dartboard stimulus has two distinct phases: windmill, shown in (b), and dartboard, shown in (c). The contrast of the first and third annuli are held constant. Contrast reversal of the pattern elements in the central disk and second annulus result in the change of appearance from a windmill to a dartboard. Reprinted with permission from Elsevier [46].
Figure 2. Partial-windmill and windmill–dartboard conditions.
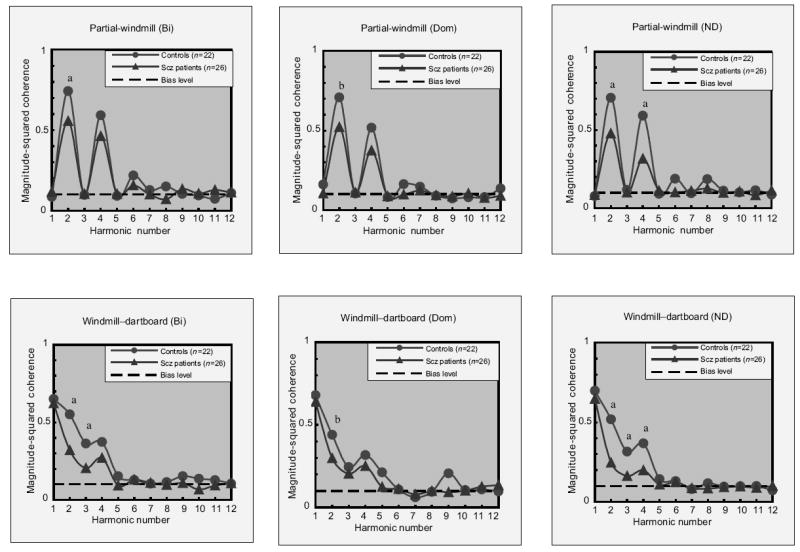
Patients with schizophrenia (Scz) showed significantly reduced second harmonic responses but intact first harmonic responses. This finding of a differential deficit may indicate a significant loss in the magnocellular pathway. Bi, binocular eye condition; DO, dominant eye condition; ND, nondominant eye condition. a, P < 0.01; b, P < 0.05. Reprinted with permission from Elsevier [46].
During the past year transient VEP studies also addressed early visual cortical responses in schizophrenia. In this approach, stimuli are presented more slowly and responses are analyzed in the time domain. Butler et al. [58] reported decreased amplitude of the P1 component, consistent with the earlier findings of Doniger et al. [15]. In addition, Haenschel et al. [59] reported decreased P1 amplitude in patients with early-onset schizophrenia. In contrast, van der Stelt et al. [60] did not find decreased P1 or N1 in schizophrenia.
Thus, ssVEP studies provide strong evidence of magnocellular dysfunction in schizophrenia, and transient VEP studies, in general, support early visual cortical dysfunction.
Behavioral studies
Visual pathway function has also been studied using behavioral methods. While studies using luminance and chromatic contrast to bias processing towards magnocellular and parvocellular pathways have generally shown preferential magnocellular dysfunction, studies utilizing stimulus size to bias processing have been more equivocal. The latter studies assess the amount of contrast (i.e. contrast threshold) needed to detect stimuli of varying sizes with large (i.e. low spatial frequency) stimuli biasing processing towards the magnocellular pathway and small (i.e. high spatial frequency) stimuli biasing processing towards the parvocellular pathway. Lower thresholds indicate better performance. One issue is that high spatial frequency stimuli can produce low contrast thresholds. Because the magnocellular system responds well to low luminance contrast (~1–10% contrast), whereas parvocellular neurons do not start responding until stimuli reach higher contrast (~10%), contrast threshold levels below 10% will be biased toward the magnocellular system regardless of spatial frequency. In addition, responses to spatial frequency are not specific to striate versus extrastriate areas [39].
Several studies in the last year have used contrast to examine magnocellular and parvocellular function. Using a backward masking paradigm, Schechter et al. [18•] used low luminance contrast to bias processing towards the magnocellular system and chromatic contrast at isoluminance to bias processing towards the parvocellular system. Backward masking dysfunction was found when low luminance but not chromatic contrast stimuli were used as masks, supporting a role of magnocellular-system dysfunction in backward masking deficits in schizophrenia. In a vernier acuity task, Keri et al. [61••] used low contrast and low spatial frequency to bias processing towards the magnocellular system and high contrast and chromatic contrast at isoluminance to bias processing towards the parvocellular system. Patients showed deficits in vernier acuity in the low spatial frequency and low contrast conditions, but not in the high contrast and isoluminant color contrast conditions, consistent with magnocellular dysfunction. In a far-out jerk paradigm, Slaghuis and Thompson [62•] examined the ability of moving objects in the periphery to decrease detection of a central object. The far-out jerk response is thought to be mediated by long-range transient or magnocellular function. Patients with predominantly negative, rather than positive, symptoms of schizophrenia showed a decreased far-out jerk response, indicating magnocellular dysfunction.
Most backward masking studies in schizophrenia have been done using masks that spatially overlap the targets [13,26,63-65]. Rassovsky et al. [66] recently examined masking in schizophrenia using masks that surround, rather than overlap, the target. This technique limits the mechanism of masking to interruption. Patients with schizophrenia showed deficits in masking by interruption, consistent with magnocellular dysfunction [16,28].
While studies within the past year using stimulus size to bias processing have consistently found contrast threshold deficits to low spatial frequency stimuli, reflecting magnocellular dysfunction [42•,45,62•,67•], results are less clear at medium to higher spatial frequencies. Slaghuis [62•,67•] reported a threshold detection deficit at medium and higher spatial frequencies of 4 and 8 c deg−1, indicative of parvocellular dysfunction. Butler et al. [45], however, reported a deficit at spatial frequencies up to 7 c deg−1 but not at higher spatial frequencies of 10 and 21 c deg−1, suggesting intact parvocellular function. Differences in absolute contrast levels at medium and high spatial frequencies may underlie variant findings.
Contrast detection has also been utilized to examine whether visual processing deficits are related to early-stage processing dysfunction. Slaghuis [67•] found that backward masking dysfunction was related to deficits in contrast threshold, which supports a role of early-stage visual processing deficits in backward masking dysfunction in schizophrenia. They commented that an earlier study by Keri et al. [68] did not find such a relationship, which may have been due to measurement of contrast thresholds in central vision and backward masking in four separate parafoveal locations in that study, whereas Slaghuis [67•] measured both in the same central retinal location.
Chen and colleagues have found motion processing deficits in schizophrenia as seen in impaired velocity discrimination [14,42•,69] and have also used contrast to examine whether this deficit is intrinsic to dorsal stream motion areas or due to impaired early-stage input [42•]. They pointed out that neurophysiological and lesion studies show that a velocity discrimination deficit may be related to early-stage motion processing (i.e. LGN, striate cortex) if it is contrast dependent and later stage processing (i.e. extra-striate) if it is contrast independent. Thus, they assessed velocity discrimination at high and low contrast, using each participant’s contrast detection threshold to equate contrast levels. Patients showed impaired velocity discrimination that did not improve with high contrast, whereas performance of controls did improve with increased contrast. The contrast independence of the deficit in schizophrenia indicates that it is mediated by later-stage extra-striate areas.
With regard to effects on higher level function, contrast threshold deficits were found to be related to impaired neuropsychological as well as community function [45], reminiscent of findings by Gold et al. [22]. Furthermore, backward masking deficits are related to impaired social perception [24].
In addition to deficits in visual pathway function in schizophrenia, there may also be abnormal lateral interactions in visual cortex in schizophrenia [70].
Medication effects
An unresolved issue is the degree to which medication may affect visual processing. Chen et al. [71•] have suggested that decreased contrast detection thresholds are related to medication such that they are found only in patients taking typical, rather than atypical, antipsychotics. In other studies, however, ssVEP and contrast detection thresholds as well as visual masking dysfunction were observed even in patients receiving atypical antipsychotics alone [45,66], suggesting that patient characteristics rather than medication type may be the primary predictor of visual dysfunction. In addition, decreased magnocellular-biased vernier acuity as well as aberrant lateral interactions were found in patients who had been off medications for several weeks [61••,70].
Patient characteristics
Several recent studies have examined early visual processing in various groups of participants. Within schizophrenia groups, Slaghuis [62•,67•] found that deficits in contrast detection, backward masking, and far-out jerk tasks are more prominent in patients with predominantly negative, rather than positive, symptoms of schizophrenia. Rund et al. [64] reported that patients with schizophrenia, but not unipolar depression, show impaired backward masking compared with controls and concluded that backward masking is a sensitive measure of the visual processing dysfunction in schizophrenia. Decreased magnocellular-mediated vernier acuity has also been found in first-degree relatives of patients with schizophrenia [61••], as has decreased magnocellular/dorsal stream function using functional magnetic resonance imaging (MRI) [72], suggesting that early visual processing deficits may be a possible endophenotype.
Anatomical studies
Inputs to primary visual cortex project from LGN to V1 via optic radiations. These white matter tracts can be examined by MRI using the technique of diffusion tensor imaging. Recently, decreased integrity of occipital white matter adjacent to the corpus callosum, in the region of the optic radiations, has been found in adults with schizophrenia [73•], in agreement with an earlier study [74]. Decreased occipital white matter integrity has also been found in adolescents with early onset schizophrenia [75•]. In addition, a significant relationship has been found between decreased magnocellular-biased ssVEP responses and decreased white matter integrity in the optic radiations, but not in higher level visual areas [45] (Fig. 3). The latter finding provides direct support for the hypothesis that magnocellular dysfunction occurs at the earliest stages of visual responsivity.
Figure 3. Fractional anisotropy (FA) image and scatter plot.
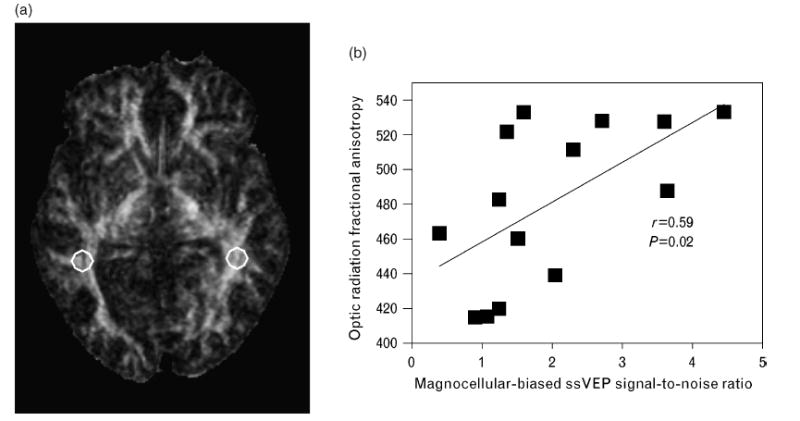
(a) FA image with circles representing regions of interest based on their placement in the optic radiations on the b = 0 image (not shown). (b) Scatter plot showing relationship between magnocellular-biased steady-state visual evoked potential (ssVEP) responses and FA of optic radiation white matter tracts for patients with schizophrenia. FA measures range from 0 to 1 with 0 representing complete isotropic diffusion (no directional selectivity of water diffusion and hence decreased white matter integrity) and 1 representing complete anisotropy. FA values have been multiplied by 1000. Reprinted with permission from Elsevier [45].
Conclusion
Results from behavioral and electrophysiological studies support early visual processing dysfunction in schizophrenia, with preferential deficits being found in the magnocellular pathway, though parvocellular deficits have been found as well. Preferential magnocellular dysfunction may provide a substrate for dorsal stream dysfunction as well as higher level cognition deficits and outcome. Structural deficits in occipital cortex, particularly in optic radiations, and their relationship to early visual processing deficits, document the importance of subcortical as well as cortical dysfunction in schizophrenia.
Acknowledgments
This review was supported in part by a Lieber Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (PDB), USPHS grants RO1 MH66374 (PDB), R37 MH49334 and K02 MH01439 (DCJ), and a Burroughs Wellcome Translational Scientist Award (DCJ).
Abbreviations
- LGN
lateral geniculate nucleus
- ssVEP
steady-state visual evoked potential
- VEP
visual evoked potential
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg TE, Gold JM. Neurocognitive functioning in patients with schizophrenia: an overview. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology, the fourth generation of progress. New York: Raven Press; 1995. pp. 1245–1257. [Google Scholar]
- 3.Weinberger DR, Gallhofer B. Cognitive function in schizophrenia. Int Clin Psychopharmacol. 1997;12(Suppl 4):S29–S36. doi: 10.1097/00004850-199709004-00006. [DOI] [PubMed] [Google Scholar]
- 4.Green MF. Schizophrenia from a neurocognitive perspective. Boston, MA: Allyn & Bacon; 1998. [Google Scholar]
- 5.Adler LE, Freedman R, Ross RG, et al. Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol Psychiatry. 1999;46:8–18. doi: 10.1016/s0006-3223(99)00085-2. [DOI] [PubMed] [Google Scholar]
- 6.Braff DL, Saccuzzo DP, Geyer MA. Information processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituation. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia. Vol. 5. New York: Elsevier; 1991. pp. 303–334. [Google Scholar]
- 7.Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophr Bull. 1999;25:763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- 8.Turetsky BI, Moberg PJ, Owzar K, et al. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003;53:403–411. doi: 10.1016/s0006-3223(02)01865-6. [DOI] [PubMed] [Google Scholar]
- 9.Butler PD, Schechter I, Zemon V, et al. Dysfunction of early stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 10.Butler PD, DeSanti LA, Maddox J, et al. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2002;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 11.Braus DF, Weber-Fahr W, Tost H, et al. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- 12.Brenner CA, Lysaker PH, Wilt MA, O’Donnell BF. Visual processing and neuropsychological function in schizophrenia and schizoaffective disorder. Psychiatry Res. 2002;111:125–136. doi: 10.1016/s0165-1781(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 13.Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Palafox GP, Nakayama K, et al. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 15.Doniger GM, Foxe JJ, Murrary MM, et al. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 16.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II: Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- 17.Li CS. Impaired detection of visual motion in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:929–934. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- 18•.Schechter I, Butler PD, Silipo G, et al. Magnocellular and parvocellular 3 contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. This paper utilizes magnocellular and parvocellular-specific stimuli to examine the hypothesis that backward masking deficits are related to magnocellular dysfunction.
- 19.Schwartz BD, Maron BA, Evans WJ, Winstead DK. High velocity transient visual processing deficits diminish ability of patients with schizophrenia to recognize objects. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:170–177. [PubMed] [Google Scholar]
- 20.Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Keri S, Antal A, Szekeres G, et al. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- 22.Gold JM, Goldberg RW, McNary SW, et al. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002;159:1395–1402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- 23.Kee KS, Kern RS, Green MF. Perception of emotion and neurocognitive functioning in schizophrenia: what’s the link? Psychiatry Res. 1998;81:57–65. doi: 10.1016/s0165-1781(98)00083-3. [DOI] [PubMed] [Google Scholar]
- 24.Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2002;59:233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- 25.Saccuzzo DP, Hirt M, Spencer TJ. Backward masking as a measure of attention in schizophrenia. J Abnorm Psychol. 1974;83:512–522. doi: 10.1037/h0037072. [DOI] [PubMed] [Google Scholar]
- 26.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I: Specifying a mechanism. Arch Gen Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 27.Schuck JR, Lee RG. Backward masking, information processing, and schizophrenia. Schizophr Bull. 1989;15:491–500. doi: 10.1093/schbul/15.3.491. [DOI] [PubMed] [Google Scholar]
- 28.Breitmeyer B, Ganz L. Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression and information processing. Psychol Rev. 1976;83:1–36. [PubMed] [Google Scholar]
- 29.Merritt RD, Balogh DW. Backward masking spatial frequency effects among hypothetically schizotypal individuals. Schizophr Bull. 1989;15:573–583. doi: 10.1093/schbul/15.4.573. [DOI] [PubMed] [Google Scholar]
- 30.Slaghuis WL, Curran CE. Spatial frequency masking in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1999;108:42–50. doi: 10.1037//0021-843x.108.1.42. [DOI] [PubMed] [Google Scholar]
- 31.Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? In: Cowan WM, Shooter EM, Stevens CF, Thompson RF, editors. Annual review of neuroscience. Palo Alto, CA: Annual Reviews; 1993. pp. 369–402. [DOI] [PubMed] [Google Scholar]
- 32.Vidyasagar TR. A neuronal model of attentional spotlight: parietal guiding the temporal. Brain Res Brain Res Rev. 1999;30:66–76. doi: 10.1016/s0165-0173(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 33.Steinman B, Steinman S, Lehmkuhle S. Transient visual attention is dominated by the magnocellular stream. Vision Res. 1997;37:17–23. doi: 10.1016/s0042-6989(96)00151-4. [DOI] [PubMed] [Google Scholar]
- 34.Norman J. Two visual systems and two theories of perception: an attempt to reconcile the constructivist and ecological approaches. Behav Brain Sci. 2002;25:73–96. doi: 10.1017/s0140525x0200002x. discussion 96–144. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan E. The receptive field structure of retinal ganglion cells in cat and monkey. In: Leventhal AG, editor. Vision and visual dysfunction. Boston, MA: CRC Press; 1991. pp. 10–40. [Google Scholar]
- 36.Tootell RB, Hamilton SL, Switkes E. Functional anatomy of macaque striate cortex. IV: Contrast and magno-parvo streams. J Neurosci. 1988;8:1594–1609. doi: 10.1523/JNEUROSCI.08-05-01594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dacey DM, Petersen MR. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proc Natl Acad Sci U S A. 1992;89:9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jindra LF, Zemon V. Contrast sensitivity testing: a more complete assessment of vision. J Cataract Refract Surg. 1989;15:141–148. doi: 10.1016/s0886-3350(89)80002-1. [DOI] [PubMed] [Google Scholar]
- 39.Tootell RBH, Hadjikhani NK, Mendola JD, et al. From retinotopy to recognition: fMRI in human visual cortex. Trends Cogn Sci. 1998;2:174–183. doi: 10.1016/s1364-6613(98)01171-1. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb Cortex. 1998;8:575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- 41.Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 42•.Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage 3 motion processing in schizophrenia. Biol Psychiatry. 2004;55:834–841. doi: 10.1016/j.biopsych.2003.12.024. Patients with schizophrenia show motion processing deficits that may either be intrinsic to dorsal stream dysfunction or due to impaired magnocellular input to the dorsal stream This paper finds evidence that motion processing dysfunction is intrinsic to the dorsal stream.
- 43.Brenner CA, Wilt MA, Lysaker PH, et al. Psychometrically matched visual-processing tasks in schizophrenia spectrum disorders. J Abnorm Psychol. 2003;112:28–37. [PubMed] [Google Scholar]
- 44.Stuve TA, Friedman L, Jesberger JA, et al. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- 45.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. doi: 10.1001/archpsyc.62.5.495. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Zemon V, Saperstein AM, et al. Dysfunction of early stage visual processing in schizophrenia: harmonic analysis. Schizophr Res. doi: 10.1016/j.schres.2004.10.011. in press. [DOI] [PubMed] [Google Scholar]
- 47.Greenstein VC, Seliger S, Zemon V, Ritch R. Visual evoked potential assessment of the effects of glaucoma on visual subsystems. Vision Res. 1998;38:1901–1911. doi: 10.1016/s0042-6989(97)00348-9. [DOI] [PubMed] [Google Scholar]
- 48.Kwon YH, Nelson SB, Toth LJ, Sur M. Effect of stimulus contrast and size on NMDA receptor activity in cat lateral geniculate nucleus. J Neurophysiol. 1992;68:182–196. doi: 10.1152/jn.1992.68.1.182. [DOI] [PubMed] [Google Scholar]
- 49.Fox K, Sato H, Daw N. The effect of varying stimulus intensity on NMDA-receptor activity in cat visual cortex. J Neurophysiol. 1990;64:1413–1428. doi: 10.1152/jn.1990.64.5.1413. [DOI] [PubMed] [Google Scholar]
- 50.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 51.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 52.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 53.McKeefry DJ, Russell MH, Murray IJ, Kulikowski JJ. Amplitude and phase variations of harmonic components in human achromatic and chromatic visual evoked potentials. Vis Neurosci. 1996;13:639–653. doi: 10.1017/s0952523800008543. [DOI] [PubMed] [Google Scholar]
- 54.Murray I, MacCana F, Kulikowski JJ. Contribution of two movement detecting mechanisms to central and peripheral vision. Vision Res. 1983;23:151–159. doi: 10.1016/0042-6989(83)90138-4. [DOI] [PubMed] [Google Scholar]
- 55.Grose-Fifer J, Zemon V, Gordon J. Temporal tuning and the development of lateral interactions in the human visual system. Invest Ophthalmol Vis Sci. 1994;35:2999–3010. [PubMed] [Google Scholar]
- 56.Zemon V, Ratliff F. Visual evoked potentials: evidence for lateral interactions. Proc Natl Acad Sci U S A. 1982;79:5723–5726. doi: 10.1073/pnas.79.18.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tootell RB, Hadjikhani NK, Vanduffel W, et al. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci U S A. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler PD, Kim D, Foxe JJ, et al. Decreased signal amplification in schizophrenia: evidence from visual studies. Society for Neuroscience Abstracts. 2004 Program No. 348.1. [Google Scholar]
- 59.Haenschel C, Waltz J, Bittner RA, et al. Intertrial synchronization deficits account for reduced P1 and high-frequency EEG activity in schizophrenia. Society for Neuroscience Abstracts. 2004 Program No. 348.3. [Google Scholar]
- 60.van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- 61••.Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with 33 schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–542. doi: 10.1037/0894-4105.18.3.537. The authors used the physiological properties of magnocellular and parvocellular pathways in this behavioral study to selectively bias processing towards these pathways This study highlights the use of luminance contrast in examining visual pathway function In addition, this study examines visual processing in first-degree relatives of patients with schizophrenia.
- 62•.Slaghuis WL, Thompson AK. The effect of peripheral visual motion on focal 3 contrast sensitivity in positive- and negative-symptom schizophrenia. Neuropsychologia. 2003;41:968–980. doi: 10.1016/s0028-3932(02)00321-4. This paper utilizes a unique approach of looking at the effects of a peripheral stimulus on perception of a central stimulus to examine magnocellular dysfunction in schizophrenia.
- 63.Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40:295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 64.Rund BR, Egeland J, Sundet K, et al. Early visual information processing in schizophrenia compared to recurrent depression. Schizophr Res. 2004;68:111–118. doi: 10.1016/S0920-9964(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 65.Saccuzzo DP, Braff DL. Information-processing abnormalities: trait- and state-dependent components. Schizophr Bull. 1986;12:447–459. doi: 10.1093/schbul/12.3.447. [DOI] [PubMed] [Google Scholar]
- 66.Rassovsky Y, Green MF, Nuechterlein KH, et al. Paracontrast and metacontrast in schizophrenia: clarifying the mechanism for visual masking deficits. Schizophr Res. 2004;71:485–492. doi: 10.1016/j.schres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 67•.Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res. 2004;156:196–211. doi: 10.1007/s00221-003-1771-3. This study addresses the issue of whether backward masking dysfunction is reliant on early-stage threshold detection problems.
- 68.Keri S, Antal A, Szekeres G, et al. Visual information processing in patients with schizophrenia: evidence for the impairment of central mechanisms. Neurosci Lett. 2000;293:69–71. doi: 10.1016/s0304-3940(00)01473-7. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y, Levy DL, Nakayama K, et al. Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Arch Gen Psychiatry. 1999;56:155–161. doi: 10.1001/archpsyc.56.2.155. [DOI] [PubMed] [Google Scholar]
- 70.Must A, Janka Z, Benedek G, Keri S. Reduced facilitation effect of collinear flankers on contrast detection reveals impaired lateral connectivity in the visual cortex of schizophrenia patients. Neurosci Lett. 2004;357:131–134. doi: 10.1016/j.neulet.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 71•.Chen Y, Levy DL, Sheremata S, et al. Effects of typical, atypical, and no 3 antipsychotic drugs on visual contrast detection in schizophrenia. Am J Psychiatry. 2003;160:1795–1801. doi: 10.1176/appi.ajp.160.10.1795. An unresolved issue in schizophrenia research is medication effects. This study looks at whether contrast detection threshold deficits are related to type of antipsychotic medication.
- 72.Bedwell JS, Miller LS, Brown JM, et al. Functional magnetic resonance imaging examination of the magnocellular visual pathway in nonpsychotic relatives of persons with schizophrenia. Schizophr Res. 2004;71:509–510. doi: 10.1016/j.schres.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 73•.Ardekani BA, Nierenberg J, Hoptman MJ, et al. MRI study of white matter 3 diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. This paper utilized a voxelwise analysis approach to examine deficits in diffusion anistropy in schizophrenia and reports decreased white matter integrity in several brain areas in patients with schizophrenia compared with controls including occipital white matter.
- 74.Agartz I, Andersson JL, Skare S. Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport. 2001;12:2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- 75•.Kumra S, Ashtari M, McMeniman M, et al. Reduced frontal white matter 3 integrity in early-onset schizophrenia: a preliminary study. Biol Psychiatry. 2004;55:1138–1145. doi: 10.1016/j.biopsych.2004.02.025. This paper uses a voxel-based analysis in patients with early-onset schizophrenia and reports decreased white matter integrity in occipital white matter.


