Abstract
Cyclodextrins (CDs) are used to deliver hydrophobic molecules in aqueous environments. Methyl-β-cyclodextrin (MβCD), a member of this family of molecules, has been proposed to be a good carrier to deliver fatty acids to cells in culture. This report focuses on studying the in vitro effects of MβCD on nerve growth factor-differentiated PC12 (NGFDPC12) cells, a tissue culture model to study neuronal survival and differentiation. The main findings are: (1) NGFDPC12 cells have normal viability when exposed to 0.12% MβCD but showed a significant loss in cell viability at higher concentrations; (2) NGFDPC12 cells exposed to 0.25% MβCD exhibit nuclear condensation, blebbing and apoptotic bodies, and whole cell lysates exhibited an increase in caspase-3-like activity and high levels of Bax and Bcl-XL protein expression compared to control. Cultures treated with 0.25% MβCD also showed cleavage of normal 21-kDa Bax protein into a 18-kDa fragment. (3) Experiments using 0.12% MβCD to deliver oleic acid did not affect cell viability, in contrast NGFDPC12 cultures in which 0.25% MβCD concentration is used exhibited similar loss of cell viability as observed with 0.25% MβCD alone. Treating these cultures with caspase-3 inhibitor z-VAD-fmk did not protect the cells from MβCD toxic effects. (4) Immortalized Schwann cells (iSC) exposed to MβCD 0.12% did not show loss of cell viability while 0.25% MβCD triggered a significant toxicity but with a different dose and time course dynamic than NGFDPC12 cells. Thus, NGDPC12 or iSC cell cultures exposed to 0.12% MβCD exhibits normal viability while higher concentrations increase in cell death and apoptosis.
Keywords: Methyl-β-cyclodextrin, toxicity, caspase, Bcl-2, Bcl-XL, and Bax
Introduction
Delivering lipophilic substances to the central nervous system (CNS) and nerve cells cultures requires the use of carrier molecules to ensure the predictable intracellular delivery to target cells. Commonly utilized delivery methods include solubilization in ethanol, dimethylsulfoxide (DMSO), liposomes and mixed micelles, and chemical compounds such as CDs (Klaassen et al., 1999; Pfitzner et al., 2000; Loftsson et al., 2005, 2006). Each of these methods has documented disadvantages, including precipitation of the substance upon dilution in aqueous medium, decreased stability, and vehicle-mediated toxicity.
CDs enhance the solubility of nonpolar substances by non-covalent incorporation of the lipophilic portion of the molecule into their hydrophobic cavity (Bender and Komiyama, 1978).CDs are cyclic oligosaccharides consisting of 6, 7, or 8 glucose units linked through α-1,4-glucosidic bonds yielding α-β-, and γ-CD, respectively. They have been used to as carrier to deliver hydrophobic substances including long- and short-chain fatty acids, hormones, and drugs (Vicanova et al., 1999; Biddy et al., 2000; Pfitzner et al., 2000; Ulloth et al., 2003; Loftsson et al., 2006; Cappello et al., 2006; Miro et al., 2004; Jug et al., 2005). Early studies reported only minimal toxicity for CDs used to deliver lipophilic substances to cells in culture (Keller and Simons, 1998; Epa et al., 2000) as well as in the intrathecal/intracerebral delivery of hydrophobic drugs (Yaksh et al., 1991; Jang et al., 1992; Yamamoto and Yaksh, 1992). For instance, the cytotoxic effects of MβCD have been assessed in cultures of human skin fibroblasts for periods of up to 144 h without reported increase in lactate dehydrogenase activity measured in the culture media (Pfitzner et al., 2000; Boulmedarat et al; 2006). However, as the interest to use these molecules intensifies, there is an increased interest in further evaluating potential negative effects of CDs on cell viability considering the role of these molecules in depleting cholesterol from the cell membrane (Schonfelder et al., 2006; Bar-On et al., 2006; Li et al., 2006).
We report that exposure of NGFDPC12 cells to 0.12% MβCD does not affect cell viability, but 0.18% or higher concentrations trigger massive loss of cell viability and apoptotic cell death. Cell viability studies using immortalized Schwann cell cultures (iSC) confirmed that this toxic effect is not unique to PC12 cells.
Materials and Methods
Cell Culture
Undifferentiated PC12 cells were cultured in DMEM containing 10% horse serum, 5% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (all from Mediatech, Herndon, VA) at 37ºC with 95% air-5% CO2. The culture medium was replaced every 2–3 days. PC12 cells were differentiated by exposure to 50 ng/ml of 2.5S (grade II) nerve growth factor (Alomone Labs, Jerusalem, Israel) for 10–14 days in DMEM supplemented with 1% FBS, penicillin/streptomycin, and L-glutamine. Cells were trypsinized every 4 to 6 days and only low-passage cells were used for subsequent experiments. Twenty-four to 36 h prior to treatment differentiated PC12 cells were replated at 12,000 cells/cm2.
Immortalized Schwann cells (iSC) were a generous gift of Dr. Laurel Bolin (Bolin et al., 1992). Cells were cultured in DMEM/F12 50/50 containing 10% horse serum, 2mM L-glutamine, 100 units/ml of penicillin and 100 ug/ml of streptomycin. Cells were incubated at 37ºC with 5% CO2. Culture media was changed every 3 day.
Cell Viability
Cell viability was determined using the trypan blue exclusion method. NGFDPC12 cells were treated with MβCD, harvested by centrifugation at 200 × g and incubated in 0.2% trypan blue (Sigma, St. Louis, MO) for 10 min at 37ºC. Cells were then washed in PBS and counted under phase-contrast microscopy. In this assay, blue cells were concluded to have lost membrane integrity and were scored as nonviable. A minimum of 1000 cells was counted per sample and the number of trypan blue-excluding cells expressed as a percentage of the total counted. All counts were performed in triplicate. The iSC cells were plated in a T25 flask at a density of 2.50 ×105 using full serum media. Cells were placed in the incubator and allowed to attach for 5 h. After the 5 h, the media was removed and cells washed with 5ml of cold DPBS and put in a new media containing 5ml of serum free DMEM/F12 50/50, 1X N-2 Supplement (Invitrogen, CA), L-glutamine, and penicillin/streptomycin. After 12hr in serum free media, cells were treated with MBCD. Determination of cell viability and non viability was done as described above.
Caspase-2, -3, and -7 (Caspase-3-like) Activity
Caspase-2,-3 and -7 enzymatic activity was assessed by cleavage of the site-selective tetrapeptide chromogenic reporter substrates with the specificity of DEVD (caspase-2, -3 and -7) (Alexis, San Diego, CA). Cell were lysed at 4ºC in 50mM Hepes-KOH, pH 7.4, 1 mM EDTA buffer containing 75mM NaCl, 1% Triton X−100, 1mM dithiothreitol (DTT), 1mM PMSF, 10 μg/ml pepstatin A and 10 μg/ml aprotinin. Cells were then spun at 15,000 x g for 20 min at 4ºC and the supernatant recovered and stored immediately at −80 % C until use. Enzymatic reactions (100 μl final volume) were performed at 37ºC with 50 μg of cell lysate and 100 μM of chromogenic reporter substrate in 50 mM Hepes-KOH, pH 7.4 buffer containing 75 mM NaCl, 2mM DTT, and 0.1% CHAPS. Caspase catalyzed release of the chromophore p-nitroanilide was monitored spectrophotometrically at 405 nm. Optical density readings were corrected for background and normalized to lysate from untreated cells. Caspase-3 inhibition experiments with z-VAD-fmk were performed as described before using 100 μM in all experiments (Ulloth et al., 2003). The pancaspase inhibitor benzyoxycarbonyl-Val-Ala-Asp (Ome)-fluoromethylketone (z-VAD-fmk) was purchased from Enzyme Systems (Livermore, CA). Stock solutions were made in dimethyl sulfoxide (DMSO) and cells were pre-treated for 4 h prior to exposure to oleic acid.
FACScan cytometer based BRDU-TUNEL assay
The TUNEL assay was used to detect early apoptotic cleavage characteristic of apoptosis. NGFDPC12 cells were cultured for 60 h in media containing 0.25% MβCD and NGF. Cells cultures treated with etoposide were used as a positive control for apoptosis. At the end of the incubation, cell cultures were washed in PBS, fixed in 1% paraformaldehyde (+4°C, 15 min), washed in PBS, and fixed (stored) in ice-cold 70% ethanol. We used an APO-BRDU kit (PharMingen, San Diego, CA), following the manufacturer instructions, to label the cell’s DNA with BRDU-dUTP. Ethanol-fixed cells were washed twice with Wash Buffer and supernatant was discharged by centrifugation. Freshly prepared DNA labeling solution (containing TdT and Br-dUTP) was added to the cell pellet and incubated for 1 h at 37°C with occasional shaking. Cells were labeled by FITC-conjugated mAbs to BrdU, washed, and resuspended in staining solution containing PI and RNAse. Cells were incubated for 30 min at room temperature and immediately analyzed using a FACScan cytometer. The percentage of green fluorescent–positive cells with DNA strand breaks was calculated using CellQuest software.
Nuclear morphology
Chromatin condensation was detected using the fluorescent Hoechst 33342 staining. Hoechst dye was added at 1ng/ml and cells incubated for 10 min at 37oC, 100% humidity in the dark and photographed using an Olympus fluorescent microscopy (excitation/emission of 365/420 nm wave length) with a digital spot camera. Apoptotic cells were identified by the presence of highly condensed or fragmented nuclei.
Western Blot Analysis
Following various periods of exposure to MβCD (0.25%), cells were pelleted at 200 x g, washed once in ice-cold PBS at pH 7.2, and supplemented with a complete protease inhibitor (1 tablet/25 mls; Roche, Indianapolis). Cells were lysed in sample buffer (200 mM Tris-HCl, 5% SDS, and 10% glycerol) containing complete protease inhibitor cocktail. Electrophoresis was performed on 50 μg of protein sample loaded in loading buffer on 12% SDS-polyacrylamide gels (Bio-Rad). Proteins were transferred to nitrocellulose membrane (Osmonics, Westerborough, MA) and blocked with 5% dry milk in TBS-T (tris buffered saline with 0.05% Tween 20) for 1 h. Blots were then probed with antibodies to Bcl-2 (100; Santa Cruz Biotechnology), Bcl-XL (H-5; Santa Cruz Biotechnology), and Bax (polyclonal; Pharmingen). All secondary antibodies were horseradish peroxidase conjugated. Antigen-antibody complexes were visualized with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). All membranes were subsequently washed and probed again with actin (C-11; Santa Cruz Biotechnology) to assure that protein loading and transfer were approximately equal.
Statistic Analysis
All values are expressed as the mean ± SEM. Cell viabilities among the different concentrations and exposure times of CD were compared with the X square test (X2) followed by paired comparisons among different concentrations and exposure times. We used one-way ANOVA followed by Newman-Kelus Multiple Comparison Test for each pair to determine the significance of Caspase-2, 3, and -7 (Caspase-3-like) activity in response of 0.25% MβCD. Each figure shows the p value used to determined significance.
Results
Cell viability Assays
CDs have been used extensively for the delivery of hydrophobic substances to cells in culture (Vicanova et al., 1999; Grimmer et al., 2000; Pfitzner et al., 2000; Schonfelder et al., 2006; Bar-On et al., 2006; Li et al., 2006). The toxic effects reported have varied depending on the cell type used and the concentration. In our interest to use MβCD to deliver saturated free fatty acids to nerve cells and NGFDPC12 cells we performed a series of experiments to evaluate MβCD potential toxicity. We first did a dose response by exposing NGFDPC12 cells to various concentrations of MβCD for 24 h. After 24 h, 0.12% MβCD was not toxic (94.2 ± 0.7%), whereas concentrations of 0.18% (p< .05) and greater induced a significant increase in cell death (Figure 1A). Exposure of NGFDPC12 cells to 0.25%, 0.32% and 0.38% MβCD revealed a significant (p< .01) loss of cell viability by 24 hours. Time course experiments using 0.25% MβCD confirmed this significant loss of cell viability as early as 24 hours with only 9.7 ± 1.8% of cells continuing to exclude trypan blue after 60 hours of exposure (Figure 1B).
Figure 1. Cell viability of nerve growth factor-differentiated PC12 (NGFDPC12) cells following exposure to different concentrations of MβCD.
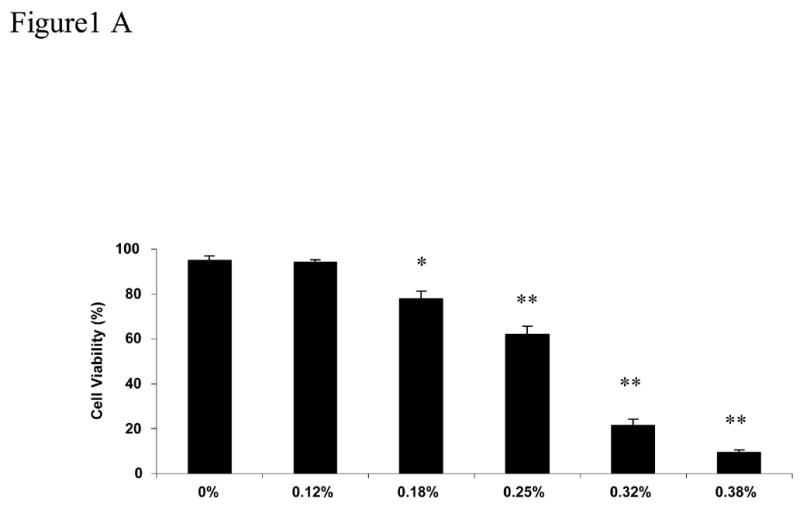
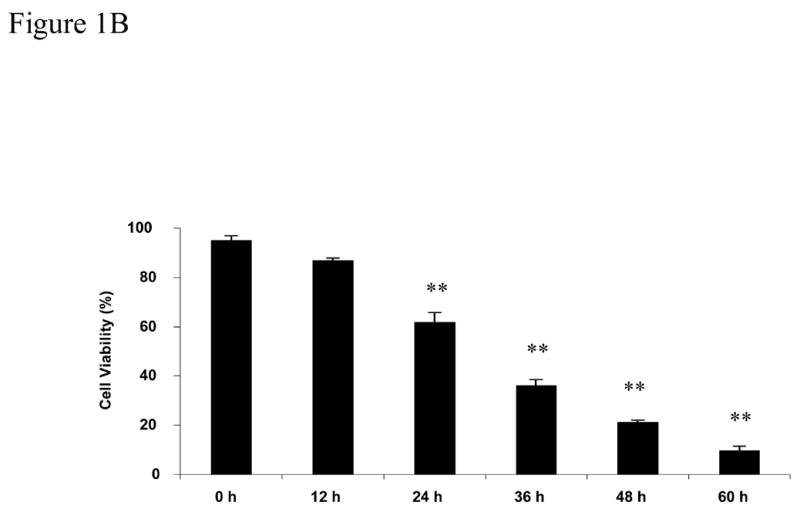
A: NGFDPC12 cells were exposed to different concentrations of MβCD (shown on the X-axis) for 24 h. Cell viability was assessed using trypan blue exclusion as described in Materials and Methods section. B: NGFDPC12 cell cultures were exposed to 0.25% MβCD for up to 60 hours (h) and cell viability assessed at each time point. Data are represented as the mean ± the standard deviation of the mean (X ± SDM) for two independent experiments performed in duplicate. Each experiment examined at least 1000 cells selected in random fields. Statistical significance was determined as described in Methods. * p<0.05, ** p<0.01.
MβCD Induces Apoptosis-like Cell death
Cellular and nuclear morphology were documented at 72 hours following exposure to 0.25% and 0.12% MβCD in undifferentiated and NGFDPC12 cells. Cultures were examining using bright field phase contrast or fluorescent microscopy for Hoechst staining. Undifferentiated PC12 cells cultures treated with 0.25% MβCD showed a significant cell loss as quantified in Figure 1 while exhibiting a cellular morphology less rounded, smaller and fragmented as compared with untreated or cultures treated with 0.12% MβCD (Figures 2A-C). NGFDPC12 cells cultures without MβCD or treated with 0.12% exhibit similar morphology, including healthy long processes forming a mature network between groups of cells. Cells in cultures treated with 0.25% MβCD had their processes shorten and fragmented, and frequent absence of neurites. Figure 2G-L shows Hoechst staining. NGFDPC12 cells without MβCD and with 0.12% CD had normal chromatin and absence of apoptotic bodies. In contrast, most of the PC12 cells exposed to 0.25% MβCD that had survived to this point (see Figure 1) exhibited strong chromatin condensation and apoptotic bodies (Figure 2 I, L). Many small (∼1/5 the size of a normal cell) bodies were also present but did not contain enough chromatin to be captured (Figure 2 I, L). These bodies likely represented apoptotic bodies that had lost their chromatin, i.e. chromatolysis (Majno and Joris, 1995). Thus, the cellular and nuclear morphology of cells dying in response to 0.25% MβCD is very similar to that described for apoptosis (Kerr, 1969; Kerr et al., 1972), suggesting that apoptosis is responsible of the loss of cell viability triggered by MβCD (see caspase-3 and TUNEL analysis below). Experiments using DAPI staining showed similar results (data not shown).
Figure 2. Cellular and nuclear morphology of naïve PC12 and NGFDPC12 cells treated with 0.12% and 0.25% MβCD.
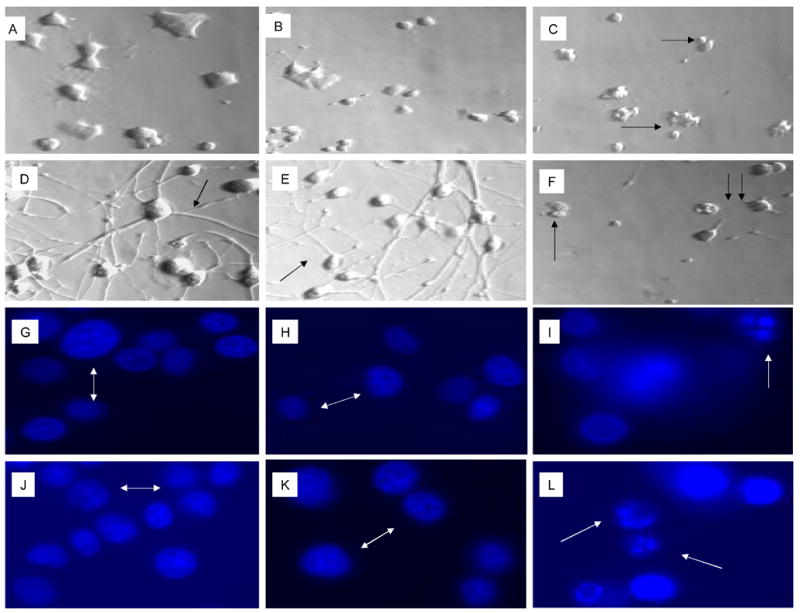
Panels A-F show representative cell morphologies with phase contrast microscopy and panels G-L show representative nuclear morphologies using Hoechst staining with fluorescent microscopy (see Materials and Methods). Naïve PC12 cells were cultured without MβCD (A,G), exposed to 0.12% (B, H) or 0.25% (C, I) MβCD for 72 hours. Similarly, NGFDPC12 cells were cultured without MβCD (D, J), exposed to 0.12% (E, K) or 0.25% (F, L) MβCD for 72 hours.) White double arrows (G, H, J, K) show normal nuclei. White arrows (I, L) indicate apoptotic and fragmented nuclei. Single black arrows (C, F) show fragmented cells. Notice the network of NGFDPC12 cells expressing neurites in D (arrow) and E (arrow) as comparison to F (twin arrows).
Figure 3 shows further DNA cleavage quantification of cultures exposed to 0.25% MβCD that exhibits a DNA damage characteristic of apoptosis (Figure 2), and a time course of caspase-3 activation. NGFDPC12 cells were exposed for 60 Hrs to 0.25% MβCD, harvested, fixed, and analyzed by flow cytometry. The BrdU-FITC Fluorescence represented in the FL-1 (X axis) is directly proportional to the amount of DNA fragmentation present (Figure 3A). Apoptotic cells have increased levels of DNA fragmentation and shifted to the right due to the increased FL-1 signal when compared to control cells (Figure 3A). Quantification of experiment in Figure 3A shows that NGFDPC12 cells exposed to MβCD for 60 hours exhibits had 4 fold increase in BrdU-FITC fluorescence when compare to control cells (Figure 3B). The same results were obtained in two independent experiments. Caspases are important mediators of apoptotic cell death (Cohn, 1997). Activation of these enzymes by treatment with MβCD would point to apoptotic cell death involvement in this process. Caspase-3-like activity in lysate of NGFDPC12 cells treated with 0.25% MβCD was found to be significantly increased after 18 hours of exposure (p <0.01, Figure 3C).
Figure 3. Tunnel assay and caspase-3 activity of NGFDPC12 cells exposed to MβCD.
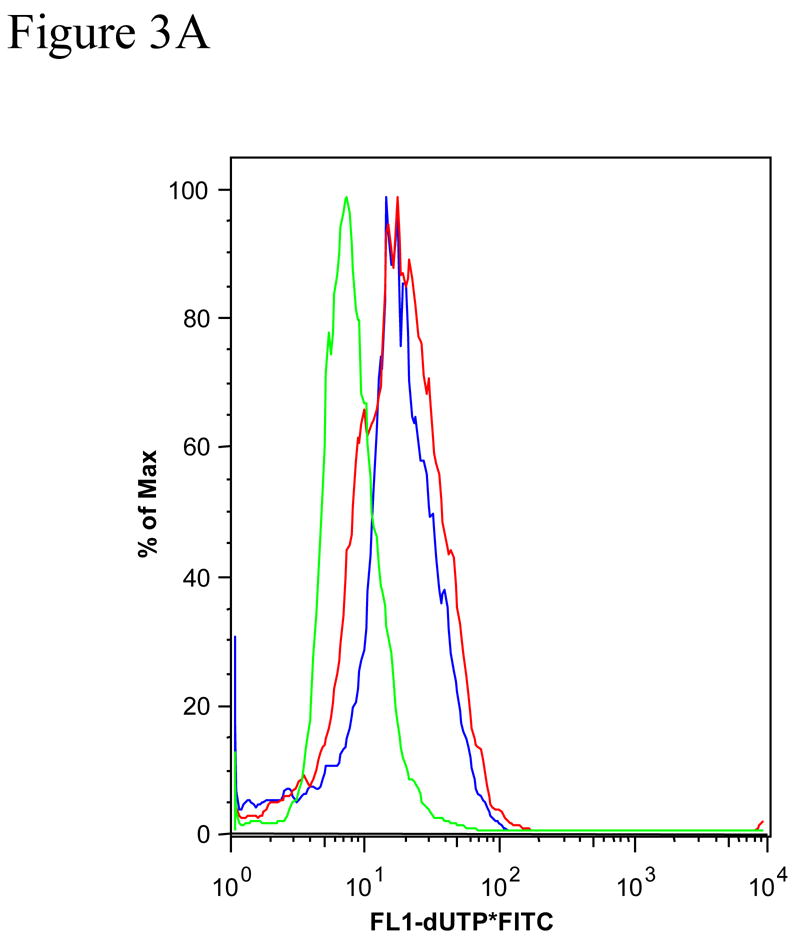
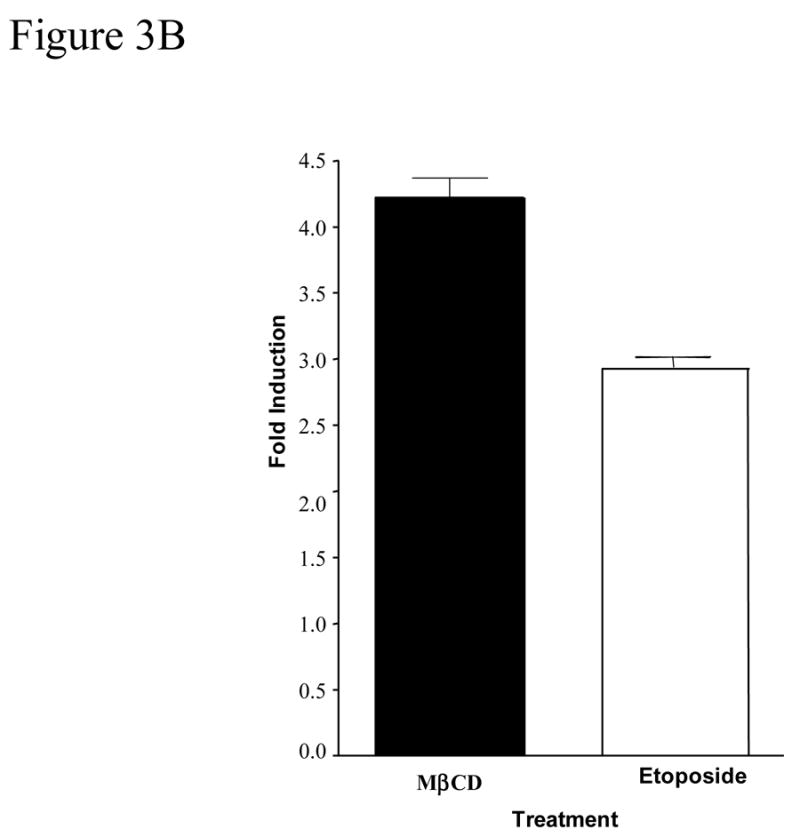
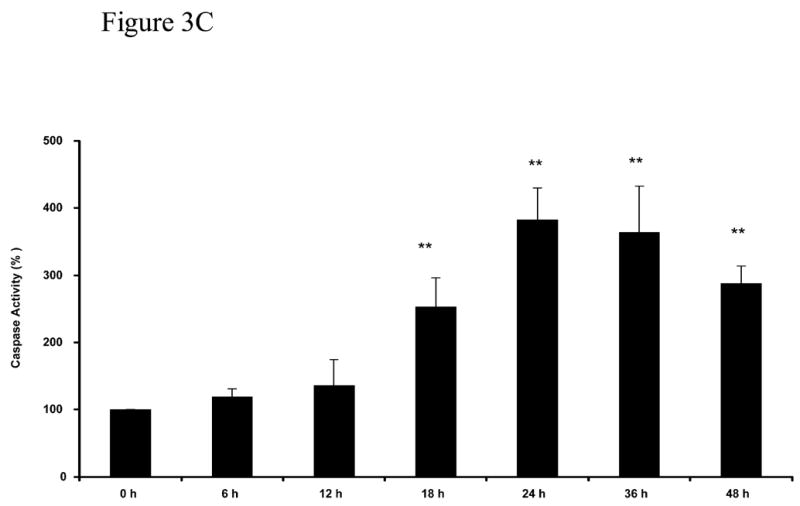
The BRDU-tunnel assay was done as described in Materials and Methods. NGFDPC12 cells were exposed for 60 h to 0.25% MβCD, or etoposide, harvested, fixed and analyzed by flow cytometry. A: Chromatogram of NGFDPC12 cells control (green) treated with MβCD (red) or with etoposide (blue). B: Quantification of MβCD and Etoposide apoptotic effect. C: Determination of caspase-3 like activity. Caspase -2, -3, and -7 (DEVDase) activities were measured in cell lysates of NGFDPC12 cells exposed to 0.25% MCD. Caspase activity is represented as the mean ± SDM of three independent experiments. Statistical significance was determined as described in Methods. * p<0.05, ** p<0.01.
An important question in evaluating the effects of MβCD on cells in culture is to examine whether toxicity is also present while delivering the specific agent to be studied. Figure 4 show cell viability under conditions that 0.25% MβCD is used to deliver oleic acid in the presence or absence of caspase-3 inhibitor z-VAD-fmk. Oleic acid, at concentration that is not toxic to NGFDPC12 cells (Ulloth et al., 2003) was delivered either with 0.12% MβCD or 0.25% MβCD. Figure 4A shows that cell viability was not affected in cultures treated with 0.12% MβCD and oleic acid. In contrast, cultures treated with 0.25% MβCD and oleic acid exhibited a significant loss of cell viability by 24 hours (p <0.01). MβCD toxic effect was not inhibited by z-VAD-fmk, a wide spread caspase-3/7 inhibitor (p <0.01) suggesting this process may be caspase-3 independent (Figure 4B).
Figure 4. Cell viability of NGFDPC12 cells with nontoxic levels of oleic acid using MβCD as carrier with or without caspase-3 inhibitor ZVAD-fnk.
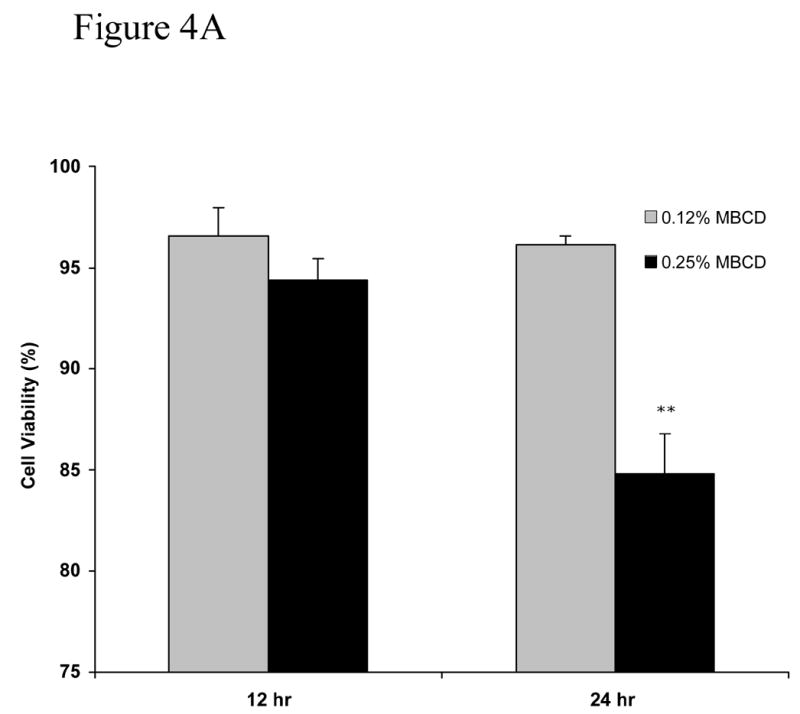
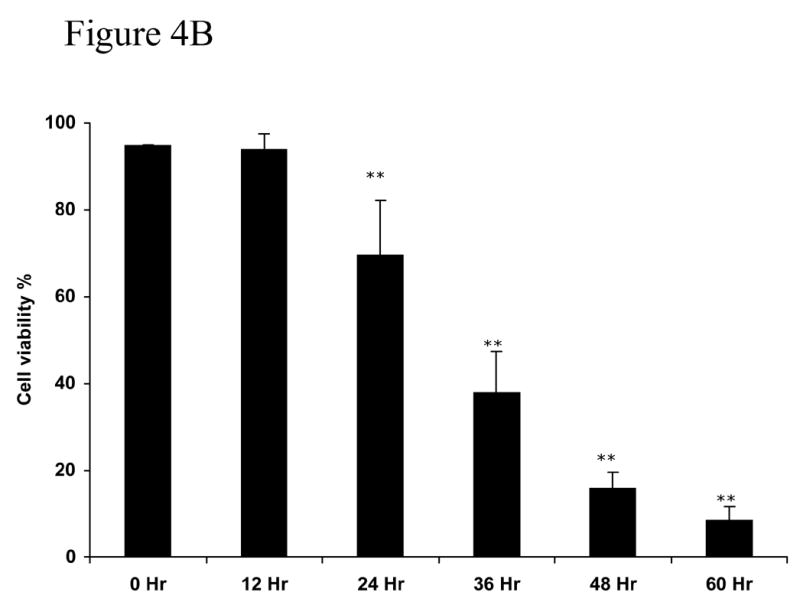
A: Viability in cell cultures treated with oleic acid and containing MβCD (0.12% or 0.25%) as carrier in the absence of zVAD-fmk. B: NGFDPC12 cells were treated with oleic acid with 0.25% MβCD in the presence of zVAD-fmk as described in Methods. Cell viability and statistical analysis were done as described in Method section. Each time point represents the mean of three independent experiments (X±SDM). ** p<0.01.
MβCD and Bcl-2 Family Members, Expression of Bcl-XL, Bax, and Bcl-2
The Bcl-2 family of proteins is a crucial regulator of cell survival and apoptosis (Adams and Cory, 1998). Members of this family of proteins (i.e., Bax, Bcl-XL, and Bcl-2) have been involved in caspase independent and caspase dependent cell death and exhibit particular expression and cleavage during apoptosis.
Figure 5A shows that the expression of Bax protein, a proapoptotic member of the Bcl-2 family, was dramatically upregulated in lysates of NGFDPC12 cultures following exposure to 0.25% MβCD. Significantly, an 18 kDa bax-immunoreactive band became apparent after 36 h of treatment with MβCD. This fragment likely represents proteolysis of the full-length 21 kDa Bax, generating a fragment of 18 kDa. Furthermore, Bcl-XL, an antiapoptosis homologue of Bcl-2, was also found to be upregulated in response to MβCD exposure (Figure 5B). Although Bcl-2 was shown to be expressed throughout MβCD treatment, the protein level was not changed (Figure 5C).
Figure 5. Western blot analysis of Bax, Bcl-XL and Bcl-2 expression in response to exposure to 0.25% MβCD.
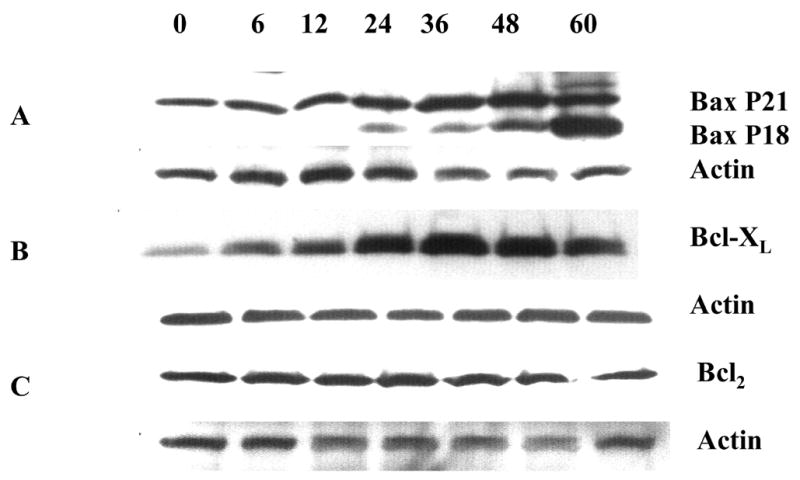
NGFDPC12 cells were exposed for varying times to 0.25% MβCD, cells harvested, whole cell lysates prepared, and Western blot analysis performed as described in Materials and Methods. We used actin protein in each Western blot as a control for loading and gel transfer. Each blot is representative of at least two independent experiments. A: Bax. B: Bcl-XL. C: Bcl-2.
MβCD toxicity in immortalized Schwann cells
The last series of experiments evaluated whether MβCD toxic effect was also observed in different type of nerve cells. We used the immortalized Schwann cells line iSC that have been shown to exhibit similar characteristics as primary Schwann cells (Bolin et al., 1992). Figure 6A and 6B show that iSC Schwann cells, although more resistance to MβCD toxicity, showed a dose-dependent and time-dependent loss in cell viability. Similarly to PC12 cells, iSC cells exhibit normal survival when treated with 0.12% MβCD even after 60 hours following treatment (Figure 6C).
Figure 6. Viability of Schwann cells cultures exposed to MβCD.
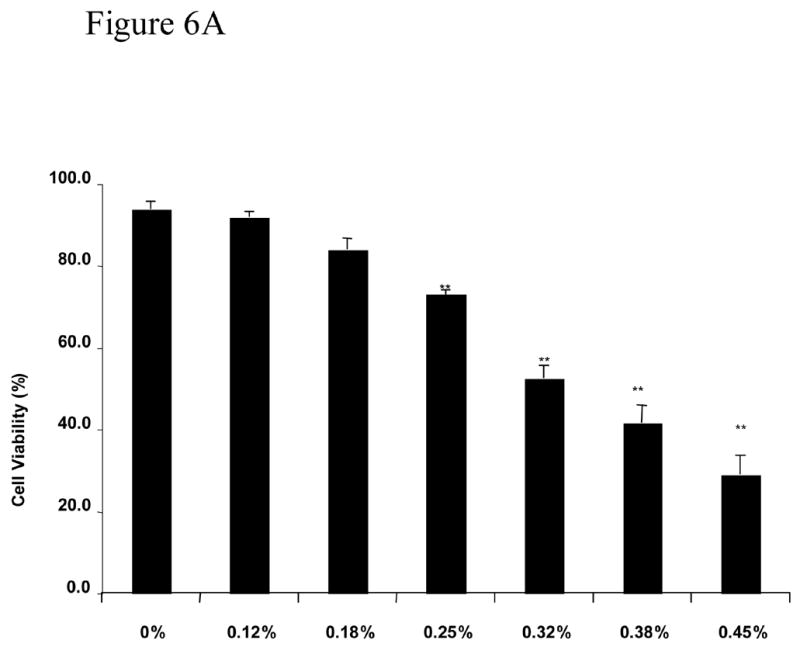
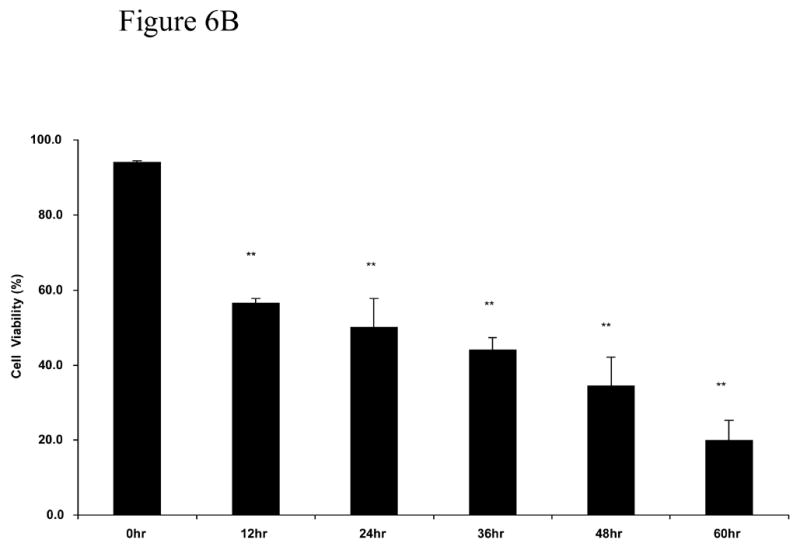
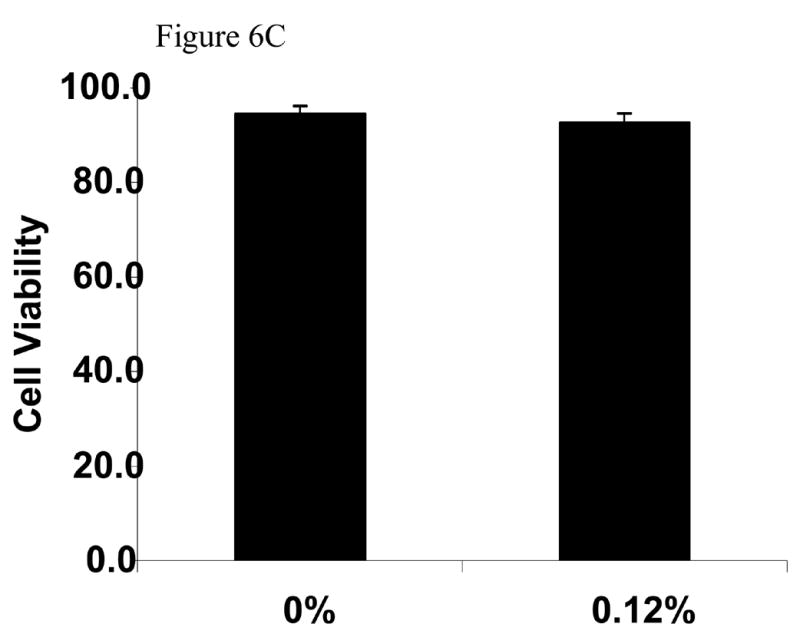
A: Concentration dependent effect of MβCD on iSC viability. Cultures were treated with different concentrations of MβCD for 48 hours and process for trypan blue assay. B: Time dependent effect of 0.45% MβCD on iSC viability. C: Determination of viability of iSC cells following exposure to continuous exposure to 0.12%MCD (the sentence before this one is deleted). Cultures were treated for 72 hours and processed for trypan blue assay. Cell viability and statistical analysis were performed as described in Methods Section. Each time point represents the mean of three independent experiments (X±SDM) with a random sampling of at least 1000 cells. Statistical analysis was performed as shown in Methods. ** p<0.01
Discussion
In summary, this study supports the use of MβCD to deliver hydrophobic molecules to tissue culture of NGFDPC12 and immortalized Schwann cells. Concentration in the range of 0.12% MβCD did not harm the cells but levels greater than or equal to 0.18% for at least 24 h resulted in a significant loss of cell viability and toxicity. Thus, MβCD can induce loss of cell viability that is dependent on the final concentration and the particular cell type used. This loss of cell viability is apoptotic, but caspase-3 independent, and correlates with elevation of Bax protein (Wood and Newcomb, 2000).
Solubilization of lipid-soluble substances in aqueous cell culture medium or body fluids is of essential importance for the effective delivery of these substances. Carriers of hydrophobic substances are essential for assuring their stability and bioavailability in the aqueous environment. CDs have been utilized for this purpose. Low concentrations (∼0.2%) of CDs have been employed to solubilize and deliver extremely hydrophobic substances to cells in culture (Janz and Shacter, 1991; Vicanova et al., 1999; Pfitzner et al., 2000). Higher concentrations (∼20%) of CD have also been used to solubilize hydrophobic drugs for delivery through intracerebral and intrathecal routes (Yaksh et al., 1991; Jang et al., 1992; Yamamoto and Yaksh, 1992). MβCD in particular, has been shown to be an effective vehicle for delivering hydrophobic substances to cells in culture (Christian et al., 1997). However, evaluation of toxic effects has been focused of attention of a number of recent studies (Schondfelder et al., 2006; Chou et al., 2003; Li et al., 2006). This study was undertaken to further evaluate MβCD and establish the levels that is nontoxic to NGFDPC12 cells.
The toxicity documented herein was found to induce loss of cell viability through apoptotic cell death. The apoptotic character of the cell death was confirmed by cell morphology, Tunnel assay, caspase-3 activation and induction of mitochondrial apoptotic related genes. These experimental findings as a whole provide a strong evidence of apoptosis as opposed to necrotic cell death Of interest are the early increases in Bcl-XL and Bax protein that occur in response to MβCD treatment. These changes preceded activation of caspase-3-like activity and may play an important role in toxicity. Bax and Bcl-XL are of particular importance in the nervous system (Motoyama et al., 1995: Deckwerth et al., 1996) and have been shown to interact in determining cell survival (Gross et al., 2000). Overexpression of Bcl-XL, furthermore, has been shown to inhibit Bax-induced apoptotic cell death (Finucane et al., 1999). Of particular significance is our observation of a high level of PC12 cell death in response to MβCD exposure—even in the presence of high levels of Bcl-XL protein. There are two possible explanations for this phenomenon. It is possible that both Bcl-XL and Bax protein elevations are not involved in the MβCD-induced death process. Another, possible explanation is that Bax is overcoming the documented protective effects of Bcl-XL. The cleavage of Bax in response to exposure to MβCD may provide an important clue for distinguishing between these two possibilities.
Bax cleavage into the 18-kDa fragment starts at 24 h following the initial incubation with MβCD and it becomes more extensive during the remainder of the incubation period. Cleavage of the full-length 21 kDa Bax into an 18 kDa (p18) fragment has been described in several systems, including staurosporine-induced apoptosis in MN9D dopaminergic cells and SH-SY5Y neuroblastoma cells (Kim et al., 1999; McGinnis et al., 1999), induced overexpression of Bax in yeast cells (Priault et al., 1999; Gross et al., 2000) and in SK-NSH neuroblastoma cells treated with ionizing radiation (McGinnis et al., 1999). Although the p18 fragment has been shown to appear in response to staurosporine-induced apoptosis in MN9D, it was not observed in staurosporine-treated NGF-differentiated PC12 cells (Kim et al., 1999). The characteristic of cell death induced by the 18 kDa fragment of Bax is different than those of the full length. Cell death induced by the p18 fragment of Bax can be partially or totally inhibited pan-caspase inhibition with z-VAD-fmk (Wood and Newcomb, 2000; Decker et al., 2004). Therefore, cleavage of Bax into the 18 kDa fragment may result in a more potent ability to induce cell death. This conclusion is further supported by the finding that 18-kDa Bax does not interact with Bcl-2, rendering Bcl-2 incapable of inhibiting Bax/p18-induced death (Gao and Dou, 2000). The possibility exists that the cleavage of Bax observed after MβCD treatment potentates the cytotoxicity of MβCD. The cleavage of Bax has been shown to occur by both caspase-dependent and -independent activation of calpain (Vanags et al,.1997; Villa et al., 1998; Wood et al., 1998; Choi et al, 2001; Oh et al., 2004; Toyota et al., 2003). Further, cadmium-induced apoptosis has been shown to be mediated by a caspase-dependent Bid cleavage and by a calpain-mediated mitochondrial Bax cleavage (Oh et al., 2004). Calpain-induced Bax-cleavage product can be a more potent inducer of apoptotic cell death than wild-type Bax (Toyota et al; 2003). Our finding that z-BAD-fmk failed to inhibit the loss of cell viability suggest that MβCD toxicity under present conditions may occur independently of caspase activation and is consistent with our observation that BAX protein elevation in cell lysates of MβCD treated cultures preceded caspase-3 activation. Future studies may clarify whether calpains or other factors are involved in this process.
MβCD toxicity is consistent with MβCD’s documented ability to deplete membrane cholesterol in neurons (Bruses et al., 2001; Simons et al., 1998), including PC12 cells (Peiro et al., 2000). MβCD depletes selectively cholesterol from the plasma membrane (Kilsdonk et al., 1995; Christian et al., 1997; Ilangumarn and Hoessli, 1998) and cholesterol-CDs complexes have been shown to increase the cellular content of cholesterol (Bernard et al., 1990; Christian et al., 1997; Racchi et al., 1997; Jennings et al., 1999). Depletion of plasma membrane cholesterol with MβCD is usually accomplished by incubations for short (∼1 h) periods with high (∼1–2%) concentrations of MβCD (Ilangumaran and Hoessli, 1998; Abrami et al., 1999). Cholesterol-depleting attribute of MβCD may be at least attenuated when it has been saturated with a hydrophobic substance. Our results showing that NGFDPC12 cells exposed to oleic acid- MβCD with 0.25% but not 0.12% MβCD exhibit similar toxicity to MβCD alone suggest that at least in this case the MβCD toxic effect is not neutralized.
Immortalized Schwann cells were more resistant to MβCD toxicity than naïve or NGFDPC12 cells. This finding suggests that the experimental cell model used may influence MβCD’s differential effect. For instance, 0.2% MβCD toxicity in fibroblasts cell lines (Pfitzner et al., 2000) exhibit lactate dehydrogenase activity in media culture comparable to control. However, 1% concentrations of MβCD triggered a significant induction of cell death and apoptosis in human keratinocytes cell lines (Schonfelder et al., 2006) and 2 mM of MβCD increases the vulnerability of hippocampal glia cells to glutamate-induced excitotoxicity (Chou et al., 2003). It has been proposed that the variability of the toxic effects of CDs observed in different cell system studied is correlated with the concentration of cholesterol in the membrane. For instance cancer cells may be more sensitive to cholesterol depleting agents because their membranes are more enriched with cholesterol (Li et al., 2006).
Acknowledgments
We acknowledge Dr. Carlos A. Casiano for advice and discussions and Dr. Sandy Hilliker for reading and helping preparing the manuscript. This study was supported in part by grants NSF IBN-9728662, NIH 5R25GM060507 and P20 MD001632-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrami L, van der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication of aerolysin. J Cell Biol. 1999;147(1):175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Cory S. The bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1325. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Bar-On P, Rockenstein E, Adame A, Ho G, Hashimoto M, Masliah E. Effect of the cholesterol-lowering compound methyl-β-cyclodextrin in models of -synucleinopathy. 2006;98:1032–1045. doi: 10.1111/j.1471-4159.2006.04017.x. [DOI] [PubMed] [Google Scholar]
- Bender ML, Komiyama M. Cyclodextrin Chemistry. Heidelberg: Springer-Verlag; 1978. pp. 10–27. [Google Scholar]
- Bernard DW, Rodriguez A, Rothblat GH, Glick JM. Influence of high density lipoprotein on esterified cholesterol stores in macrophages and hepatoma cells. Arteriosclerosis. 1990;10:135–144. doi: 10.1161/01.atv.10.1.135. [DOI] [PubMed] [Google Scholar]
- Biddy DC, Davies NM, Tucker IG. Mechanisms by which cyclodextrins modify drug release from polymeric drug delivery systems. Int J Pharm. 2000;197(1–2):1–11. doi: 10.1016/s0378-5173(00)00335-5. [DOI] [PubMed] [Google Scholar]
- Bolin LM, Iismaa TP, Shooter EM. Isolation of activated adult Schwann cells and spontaneously immortal Schwann cell clone. 1992;33(2):231–8. doi: 10.1002/jnr.490330206. [DOI] [PubMed] [Google Scholar]
- Boulmedarat L, Bochot A, Lesieur S, Fattal E. Evaluation of buccal methyl-beta-cyclodextrin toxicity on human oral epithelial cell culture model. J Pharm Sci. 2005;94(6):1300–1309. doi: 10.1002/jps.20350. [DOI] [PubMed] [Google Scholar]
- Bruses JL, Chauvet N, Rutishauser U. Membrane lipid rafts are necessary for the maintenance of the 7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J Neurosci. 2001;21(2):504–512. doi: 10.1523/JNEUROSCI.21-02-00504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello B, De Rosa G, Giannini L, La Rotonda MI, Mensitieri G, Miro A, Quaglia F, Russo R. Cyclodextrin-containing Poly (ethyleneoxide) tablets for the delivery of poorly soluble drugs: Potential as buccal delivery system. Int J Pharm. 2006;319:63–70. doi: 10.1016/j.ijpharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC, Oh YJ. Cleavage of Bax is mediated by caspase-dependent or independent calpain activation in dopaminergic neuronal cells: protective role of Bcl–2. J Neurochem. 2001;77(6):1531–1541. doi: 10.1046/j.1471-4159.2001.00368.x. [DOI] [PubMed] [Google Scholar]
- Chou Y-C, Lin S-B, Tsai LH, Tsai H-I, Lin MC. Cholesterol deficiency increases the vulnerability of hippocampal glia in primary culture to glutamate-induced excitotoxicity. J Neurochem. 2003;43:197–209. doi: 10.1016/s0197-0186(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38(11):2264–2272. [PubMed] [Google Scholar]
- Cohn GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Oelsner M, Kreitman RJ, Salvatore G, Wang QC, Pastan I, Peschel C, Licht T. Induction of caspase-dependent programmed cell death in B-cell chronic lymphocytic leukemia by anti-CD22 immunotoxins. Blood. 2004;103(7):2718–26. doi: 10.1182/blood-2003-04-1317. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Snider WD, Korsmeyer SJ. Bax is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Epa WR, Greferath U, Shafton A, Rong P, Delbridge LM, Bennie A, Barrett GL. Downregulation of the p75 neurotrophin receptor in tissue culture and in vivo, using beta-cyclodextrin-adamantane-oligonucleotide conjugates. Antisense Nucleic Acid Drug Dev. 2000;10(6):469–478. doi: 10.1089/oli.1.2000.10.469. [DOI] [PubMed] [Google Scholar]
- Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- Gao G, Dou QP. N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem. 2000;80(1):53–72. doi: 10.1002/1097-4644(20010101)80:1<53::aid-jcb60>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Grimmer S, Iversen T-G, van Deurs B, Sandvig K. Endosome to golgi transport of ricin is regulated by cholesterol. Mol Biol Cell. 2000;11:4205–4216. doi: 10.1091/mbc.11.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Pilcher K, Blachly-Dyson E, Basso E, Jockel J, Bassik MC, Korsmeyer SJ, Forte M. Biochemical and genetic analysis of the mitochondrial response of yeast to BAX and BCL-XL. Mol Cell Biol. 2000;20(9):3125–3136. doi: 10.1128/mcb.20.9.3125-3136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J, Yaksh TL, Hill HF. Use of 2-hydroxypropyl-beta-cyclodextrin as an intrathecal drug vehicle with opioids. J Pharmacol Exp Ther. 1992;261(2):592–600. [PubMed] [Google Scholar]
- Janz S, Shacter E. A new method for delivering alkanes to mammalian cells: preparation and preliminary characterization of an inclusion complex between beta-cyclodextrin and pristine (2,6,10,14-tetramethylpentadecane) Toxicology. 1991;69(3):301–315. doi: 10.1016/0300-483x(91)90189-8. [DOI] [PubMed] [Google Scholar]
- Jennings LJ, Xu Q-W, Firth TA, Nelson MT, Mawe GM. Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am J Physiol. 1999;277:G1017–G1026. doi: 10.1152/ajpgi.1999.277.5.G1017. [DOI] [PubMed] [Google Scholar]
- Jug M, Becirevic-Lacan M, Kwokal A, Cetina-Cizmek B. Influence of cyclodextrin complexation on piroxicam gel formulations. Acta Pharm. 2005;55(3):223–36. [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140(6):1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JFR. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1969;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EPC, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270(29):17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Kim J-E, Oh JH, Choi W-S, Chang II, Sohn S, Krajewski S, Reed JC, O’Malley KL, Oh YJ. Sequential cleavage of poly(ADP-ribose)polymerase and appearance of a small Bax-mmunoreactive protein are blocked by Bcl-XL and caspase inhibitors during staurosporine-induced dopaminergic neuronal apoptosis. J Neurochem. 1999;72:2456–2463. doi: 10.1046/j.1471-4159.1999.0722456.x. [DOI] [PubMed] [Google Scholar]
- Klaassen I, Brakenhoff RH, Smeet SJ, Snow GB, Braakhuis BJ. Considerations for in vitro retinoid experiments: importance of protein interaction. Biochim Biophys Acta. 1999;1427(2):265–275. doi: 10.1016/s0304-4165(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Li CY, Park JM, Ye S-K, Kim C-W, Kim Y-N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302:18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Loftsson T, Konradsdottir F, Masson M. Influence of aqueous diffusion layer on passive drug diffusion from aqueous cyclodextrin solutions through biological membranes. Pharmazie. 2006;61:83–89. [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. Am J Pathol. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Wang KK. Endogenous Bax translocation in SH–SY5Y human neuroblastoma cells and cerebellar granule neurons undergoing apoptosis. J Neurochem. 1999;72(5):1899–906. doi: 10.1046/j.1471-4159.1999.0721899.x. [DOI] [PubMed] [Google Scholar]
- Miro A, Quaglia F, Sorrentino U, La Rotonda MI, D'Emmanuele Di Villa Bianca R, Sorrentino R. Improvement of gliquidone hypoglycaemic effect in rats by cyclodextrin formulations. Eur J Pharm Sci. 2004;23:57–64. doi: 10.1016/j.ejps.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Motoyama N, Wang FP, Roth H, Sawa K, Nakayama I, Negishi S, Senju Q, Zhang S, Fujii S, Loh DY. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Oh SH, Lee BH, Lim SC. Cadmium induces apoptotic cell death in WI 38 cells via caspase-dependent Bid cleavage and calpain-mediated mitochondrial Bax cleavage by Bcl-2-independent pathway. Biochem Pharmacol. 2004;68(9):1845–55. doi: 10.1016/j.bcp.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Peiro S, Comella JX, Enrich C, Martin-Zanca D, Rocamora N. PC12 cells have caveolae that contain TrkA. J Biol Chem. 2000;275(48):37846–37852. doi: 10.1074/jbc.M000487200. [DOI] [PubMed] [Google Scholar]
- Pfitzner I, Francz PI, Biesalski HK. Carotenoid:methyl-β-cyclodextrin formulations: an improved method for supplementation of cultured cells. Biochim Biophys Acta. 2000;1474:163–168. doi: 10.1016/s0304-4165(00)00014-3. [DOI] [PubMed] [Google Scholar]
- Priault M, Camougrand N, Chaudhuri B, Schaeffer J, Manon S. Comparison of the effects of bax-expression in yeast under fermentative and respiratory conditions: investigation of the role of adenine nucleotides carrier and cytochrome c. FEBS Lett. 1999;456(2):232–8. doi: 10.1016/s0014-5793(99)00957-6. [DOI] [PubMed] [Google Scholar]
- Racchi M, Baetta R, Salvietti N, Ianna P, Franceshini G, Paoletti R, Fumagalli R, Govoni S, Trabucchi M, Soma M. Secretory processing of amyloid precursor protein is inhibited by increase in cellular cholesterol content. Biochem J. 1997;322:893–898. doi: 10.1042/bj3220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondelfer U, Radestock A, Elsner P, Hipler U-C. Cyclodextrin-induced apopotosis in human keratinocytes is caspase-8 dependent and accompanied by mitochondrial cytochrome c release. Experimental Derm. 2006;15:883–890. doi: 10.1111/j.1600-0625.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons M. Cholesterol depletion inhibits the generation of -amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett. 2003;189(2):221–30. doi: 10.1016/s0304-3835(02)00552-9. [DOI] [PubMed] [Google Scholar]
- Ulloth JE, Casiano CA, De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J Neurochem. 2003;84:655–668. doi: 10.1046/j.1471-4159.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanags DM, Orrenius S, Aguilar-Santelises M. Alterations in Bcl-2/Bax protein levels in platelets form part of an ionomycin-induced process that resembles apoptosis. Br J Haematol. 1997;99:824–831. doi: 10.1046/j.1365-2141.1997.4813284.x. [DOI] [PubMed] [Google Scholar]
- Vicanova J, Weerheim AM, Kempenaar JA, Ponec M. Incorporation of linoleic acid by cultured human keratinocytes. Arch Dermatol Res. 1999;291(7–8):405–412. doi: 10.1007/s004030050430. [DOI] [PubMed] [Google Scholar]
- Villa PG, Henzel WJ, Senenbrenner M, Henderson CE, Pettman B. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998;111:713–720. doi: 10.1242/jcs.111.6.713. [DOI] [PubMed] [Google Scholar]
- Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW. Bax cleavage is mediated by calpain during drug–induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Jang JD, Nishiuchi Y, Braun KP, Ro SG, Goodman M. The utility of 2-hydroxypropyl-beta-cyclodextrin as a vehicle for the intracellular and intrathecal administration of drugs. Life Sci. 1991;48(7):623–633. doi: 10.1016/0024-3205(91)90537-l. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Effects of intrathecal capsaicin and an NK-1 antagonist, CP, 96-345, on the thermal hyperalgesia observed following unilateral constriction of the sciatic nerve in the rat. Pain. 1992;51(3):329–334. doi: 10.1016/0304-3959(92)90218-Z. [DOI] [PubMed] [Google Scholar]


