In this second overview of the current management of infertility we discuss anovulatory infertility and polycystic ovary syndrome. This syndrome (formerly known as Stein-Leventhal syndrome) is the most common hormonal disturbance in women—around one fifth of women in the United Kingdom are affected. It is also the most common reason for women not to ovulate, and the combination of being overweight and having polycystic ovary syndrome can have a profound effect on reproductive health.
Summary points
Polycystic ovary syndrome is the most common endocrine problem affecting women and the most common cause of anovulatory infertility
Oral clomifene citrate remains the first line treatment to induce ovulation
Gonadotrophin treatment needs careful monitoring to reduce risk of multiple pregnancy
Despite early promise, the role of metformin and insulin lowering agents is unclear in the management of anovulatory polycystic ovary syndrome
Sources and selection criteria
We referred to the Cochrane database of systematic reviews, The National Institute for Health and Clinical Excellence (NICE) guidelines for the investigation and management of infertility (2004), and our knowledge of the current literature.
What is polycystic ovary syndrome?
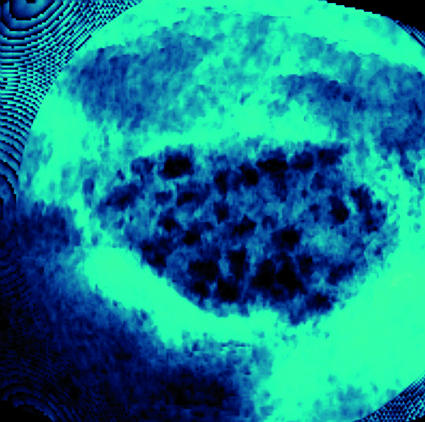
Anovulation is the cause of infertility in about a third of couples attending infertility clinics, and polycystic ovary syndrome accounts for 90% of such cases.1 Once tests have excluded other causes of androgen excess and menstrual disturbance, the syndrome can be confirmed by the presence of two of the following criteria— biochemical or clinical hyperandrogenism (hirsutism, acne, or alopecia); menstrual irregularity; and polycystic ovaries (figure).2 Symptoms, signs, and biochemical features vary greatly among affected women and may change over time in individual women.3 This review will concentrate on the management of infertility. The general practitioner should be able to start investigations and formulate a diagnosis before referral to a specialist in reproductive medicine.
Three dimensional ultrasound scan of a polycystic ovary (centre) showing multiple cysts (black) that form when follicles fail to ovulate
The endocrine abnormalities in women with polycystic ovary syndrome include raised concentrations of luteinising hormone (LH; seen in about 40% of women), testosterone, and androstenedione, in association with low or normal concentrations of follicle stimulating hormone (FSH). Androgen production from the polycystic ovary is driven predominantly by luteinising hormone in slim women and insulin in overweight women. The definition of polycystic ovary syndrome recognises obesity as an association and not a diagnostic criterion. Only 40-50% of women with the syndrome are overweight.1 3
Insulin resistance is a key pathophysiological abnormality, and women with polycystic ovary syndrome have increased risk of impaired glucose tolerance, type 2 diabetes mellitus, and the metabolic syndrome. The longer the interval between menstrual bleeds the greater the degree of insulin resistance.3 Although women with polycystic ovaries are more insulin resistant than weight matched women with normal ovaries, insulin resistance is seen in only 10-15% of slim and 20-40% of obese women with the syndrome.4
The prevalence of polycystic ovary syndrome in the general population depends on the diagnostic criteria used. In the past, using the National Institutes for Health consensus5 the definition was more exclusive and population estimates were about 7%.6 Using the more recent Rotterdam consensus2 the prevalence is estimated to be as high as 20-25% in white women in the UK,7 although symptoms are often mild. The highest reported prevalence was 52% in South Asian immigrants in the United Kingdom, of whom 49% had menstrual irregularity.8 Not all women with polycystic ovaries have the clinical and biochemical features that define the syndrome, and about 20% of women with polycystic ovaries have no symptoms. Women from South Asia living in the UK have symptoms at an earlier age than their white counterparts; they also have greater insulin resistance and more severe symptoms.9
How does obesity interact with polycystic ovary syndrome?
Obesity has a profound effect on both natural and assisted conception—it influences the chance of becoming pregnant and the likelihood of a healthy pregnancy.10 Obesity is associated with increased rates of congenital anomalies (neural tube defects and cardiac defects), miscarriage, gestational diabetes, hypertension, problems during delivery, stillbirth, and maternal mortality.11 Of the 261 deaths reported between 2000 and 2002 to the UK Confidential Enquiry into Maternal Health, 78 women (35%) were obese, compared with 23% of women in the general population, and of these more than a quarter had a body mass index (BMI) >35.11
Should obese women with polycystic ovary syndrome lose weight before treatment or should they receive treatment irrespective of the possible outcome? Several studies show that weight loss improves the endocrine profile and reproductive outcome in women with polycystic ovary syndrome.12 Even losing 5-10% of total body weight can reduce central fat by up to 30%, improve insulin sensitivity, and restore ovulation. Lifestyle modification is a key component of improving reproductive function in overweight anovulatory women with the syndrome. Treatment is harder to monitor in obese women as it is difficult to see the number of developing follicles in the ovaries; this increases the risk of multiple ovulation and multiple pregnancy. UK guidelines for the management of overweight women with polycystic ovary syndrome advise weight loss, preferably to a BMI of <30 before starting drugs for ovarian stimulation.13
How do we help women with the syndrome to ovulate?
Anovulatory infertility in polycystic ovary syndrome has traditionally been managed with clomifene citrate and then gonadotrophins or laparoscopic ovarian surgery in women who are resistant to clomifene. There has been a shift away from inducing ovulation of just one follicle to in vitro fertilisation—this is based on the false premise that in vitro fertilisation has greater cumulative rates of conception. Superovulation for in vitro fertilisation is risky in women with polycystic ovaries because of the potentially life threatening complication of ovarian hyperstimulation syndrome (although this complication can occur rarely after standard induction of ovulation). Carefully conducted induction of ovulation achieves good cumulative rates of conception, and rates of multiple pregnancy can be minimised by strict adherence to criteria that limit the number of follicles that develop.14
Does metformin have a role in fertility treatment?
Insulin sensitising agents, such as metformin, have been investigated because of the association between hyperinsulinaemia and polycystic ovary syndrome. Most studies included in the first systematic reviews had a small sample size and did not include a power calculation for the proposed effect. These reviews suggested that metformin—when used alone and compared with placebo—significantly lowered serum androgen concentrations and restored menstrual cyclicity.15 16 One of these early meta-analyses indicated that metformin can achieve ovulation either alone or when combined with clomifene.15 The meta-analysis was based on small controlled trials (not all of them double blinded), three of which came from the same centre. This illustrates the important point that meta-analysis is no substitute for adequately powered, well conducted randomised controlled trials.
Metformin seems to be less effective in significantly obese women (BMI >35). The largest appropriately powered, prospective, randomised, double blind, placebo controlled study set out to evaluate the combined effects of lifestyle modification and metformin in obese anovulatory women with polycystic ovary syndrome and a mean BMI of 38.17 All women were individually assessed by a dietitian to set a realistic goal that could be sustained, with an average reduction in energy intake of 2.09 MJ (500 kcal) a day. As a result, women in both the metformin treated and placebo groups lost weight, but the amount of weight did not differ between the two groups. Menstrual cyclicity increased in women who lost weight, but again this did not differ between the two arms of the study.17
A multicentre study comparing clomifene plus metformin with clomifene plus placebo found no significant differences in rates of ovulation or ongoing pregnancy.18 Another study randomised 676 women to three treatment arms (metformin 1000 mg twice daily plus placebo, clomifene 50 mg days three to seven of cycle plus placebo, or metformin plus clomifene).19 Live birth rates were 7.2% (5/208), 22.5% (47/209), and 26.8% (56/209), respectively—significantly lower in the group taking metformin only than in the other two groups. Miscarriage rates were higher in the metformin only group (40.0%, 22.6%, and 25.5%, respectively). Thus, metformin is significantly less effective than clomifene as first line treatment in anovulatory infertile women with polycystic ovary syndrome, and the addition of metformin to clomifene has no significant benefit. We therefore no longer recommend metformin in the routine management of anovulatory polycystic ovary syndrome.
What are the outcomes of the induction of ovulation?
The tried and tested oral agent clomifene remains the current treatment for anovulatory polycystic ovary syndrome. A recent meta-analysis confirmed that clomifene increased pregnancy rates compared with placebo as first line therapy (fixed odds ratio 5.8, 95% confidence interval 1.6 to 21.5; number needed to treat 5.9, 3.6 to 16.7).20 None the less, clomifene is associated with around an 11% risk of multiple pregnancy,21 so the ovarian response should be carefully monitored with ultrasound.22 Such monitoring is also mandatory when using gonadotrophins, which are indicated for women who have been treated with antioestrogens if they have failed to ovulate, or if their response to clomifene is likely to reduce their chance of conception (such as persistent hypersecretion of luteinising hormone). To prevent overstimulation and multiple pregnancy, standard step-up regimens have been replaced by low dose ones, and strict criteria are used before giving human chorionic gonadotrophin or luteinising hormone, which are needed for release of the eggs (no more than one or two follicles are allowed to develop).23 Expected pregnancy rates are 20% per cycle and 60-70% after six cycles.
Laparoscopic ovarian surgery (usually ovarian diathermy) is an alternative to gonadotrophins for clomifene resistant polycystic ovary syndrome, and it has replaced the more invasive and damaging ovarian wedge resection.24 Laparoscopic ovarian surgery is free of the risks of multiple pregnancy and ovarian hyperstimulation and does not need intensive ultrasound monitoring. The largest randomised controlled trial to date is a multicentre study from the Netherlands in which 168 patients resistant to clomifene were randomised to laparoscopic ovarian diathermy (n=83) or induction of ovulation with recombinant follicle stimulating hormone (n=65). The initial cumulative pregnancy rate after six months was 34% in the diathermy arm versus 67% with follicle stimulating hormone. Women who did not ovulate after diathermy were given clomifene and then follicle stimulating hormone, so the cumulative pregnancy rate was similar in each group (67%) by 12 months.25 Thus, women treated with diathermy took longer to conceive and 54% needed additional medical treatment to induce ovulation. The advantage of ovarian diathermy is a significant reduction in the rate of multiple pregnancies (0.16, 0.03 to 0.98).24 We recommend ovarian diathermy for patients who find it difficult to attend for regular ultrasound monitoring of treatment.
What are the other causes of anovulation?
At the other end of the body weight spectrum, women who are underweight—as a result of illness, anorexia nervosa, or over exercise—also become amenorrhoeic. In this situation, lack of the endocrine messenger leptin, which is secreted from fat, prevents pulses of gonadotrophin releasing hormone being released from the hypothalamus. Although ovulation can be induced by gonadotrophin injections, treatment does not benefit the mother or the baby. An undernourished mother has a high risk of miscarriage, stillbirth, and premature delivery of a growth restricted infant, with prospects both for a stormy neonatal period and significant risks to long term health. This is another example of the need to optimise health before starting fertility treatment. Psychological support is especially important in patients who are underweight as a result of anorexia nervosa.
Ongoing research questions
What are the genetics and pathophysiology of polycystic ovary syndrome?
What are the predictors of response to drugs that induce ovulation?
What is the role of insulin lowering drugs in the management of anovulatory polycystic ovary syndrome?
A detailed description of the management of all causes of anovulatory infertility is beyond the scope of this review. In essence, the underlying cause of hypothalamic anovulation should be corrected by weight gain, exercise reduction, or treatment of the underlying chronic disease. Idiopathic hypogonadotrophic hypogonadism and Kallmann's syndrome can be treated either by pulsatile gonadotrophin releasing hormone administered via a mini-infusion pump or daily injections of gonadotrophins containing both follicle stimulating hormone and luteinising hormone bioactivity. If the problem is hyperprolactinaemia then dopamine agonists are effective in most cases. Premature ovarian failure can only be treated by the use of donated oocytes in the context of an in vitro fertilisation egg donor programme.
Additional educational resources
Information resources for health professionals
National Institute for Health and Clinical Excellence. Fertility: assessment and treatment for people with fertility problems. National Collaborating Centre for Women's and Children's Health 2004. London: RCOG Press, 2004
Balen AH, Jacobs HS. Infertility in practice. 2nd ed. London: Churchill Livingstone/Harcourt Brace, 2003
Balen AH, Conway G, Homburg R, Legro R. Clinical management of polycystic ovary syndrome. London: Taylor and Francis, 2005
Cochrane Database of Systematic Reviews—Contains reviews on various aspects of infertility management (www.mrw.interscience.wiley.com/cochrane/cochrane_clsysrev_articles_fs.html)
Information resources for patients
Infertility Network UK—National UK patient support organisation for couples with infertility (www.infertilitynetworkuk.com)
Verity—Self help organisation for women whose lives are affected by polycystic ovary syndrome (www.verity-pcos.org.uk)
Conclusions
To lose weight and improve ovarian function, women with polycystic ovary syndrome should modify their lifestyle by changing their diet and exercise routine. The roles for insulin sensitising and insulin lowering drugs in the management of the syndrome and algorithms for their place in treatment are still unclear. Furthermore, no agreement exists on predictors of response or the appropriate dose of such drugs, or whether dose should be adjusted for body weight and other factors.
Contributors: AHB conceived the outline of the review and wrote the body of the text with contributions and advice from AJR. AHB is guarantor.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Balen AH, Michelmore K. What is polycystic ovary syndrome? Are national views important? Hum Reprod 2002;17:2219-27. [DOI] [PubMed] [Google Scholar]
- 2.Fauser B, Tarlatzis B, Chang J, Azziz R, Legro R, Dewailly D, et al. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41-7. [DOI] [PubMed] [Google Scholar]
- 3.Balen AH, Conway GS, Kaltsas G, Techatraisak K, Manning PJ, West C, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995;10:2107-11. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 2004;59:141-54. [DOI] [PubMed] [Google Scholar]
- 5.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome; towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, eds. Polycystic ovary syndrome Boston: Blackwell Scientific, 1992:377-84.
- 6.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745-9. [DOI] [PubMed] [Google Scholar]
- 7.Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol Oxf 1999;51:779-86. [DOI] [PubMed] [Google Scholar]
- 8.Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin Endocrinol 1998;49:91-9. [DOI] [PubMed] [Google Scholar]
- 9.Wijeyaratne CN, Balen AH, Barth J, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin Endocrinol 2002;57:343-50. [DOI] [PubMed] [Google Scholar]
- 10.Balen AH, Dresner M, Scott EM, Drife JO. Should obese women with polycystic ovary syndrome (PCOS) receive treatment for infertility? BMJ 2006;332:434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis G, ed. Why mothers die 2000-2002 London: Confidential Enquiry into Maternal and Child Health, 2004
- 12.Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update 2004;10:267-80. [DOI] [PubMed] [Google Scholar]
- 13.Balen AH. PCOS, obesity and reproductive function: RCOG Special Study Group on Obesity. 2007. London: RCOG Press (in press).
- 14.Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approaches to PCOS and other forms of anovulation. Obstet Gynecol Surv 2002;57:755-67. [DOI] [PubMed] [Google Scholar]
- 15.Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, d-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev 2003;(3):CD003053. [DOI] [PubMed]
- 16.Costello M, Eden J. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril 2003;79:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined life-style modification and metformin in obese patients with polycystic ovary syndrome (PCOS). A randomised, placebo-controlled, double-blind multi-centre study. Hum Reprod 2006;21:80-9. [DOI] [PubMed] [Google Scholar]
- 18.Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomiphene citrate plus metformin and clomiphene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ 2006;332:1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al; for the Reproductive Medicine Network. Effect of clomiphene and metformin, alone and in combination, on rate of live birth in infertile women with polycystic ovary syndrome. N Engl J Med 2007;356:551-66. [DOI] [PubMed] [Google Scholar]
- 20.Beck JI, Boothroyd C, Proctor M, Farquhar C, Hughes E. Oral anti-oestrogens and medical adjuncts for subfertility associated with anovulation. Cochrane Database Syst Rev 2005;(1):CD002249. [DOI] [PubMed]
- 21.Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update 1997;3:359-65. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Clinical Excellence. Fertility: assessment and treatment for people with fertility problems. National Collaborating Centre for Women's and Children's Health 2004. London: RCOG Press, 2004
- 23.Hamilton-Fairley D, Kiddy DS, Watson H, Sagle M, Franks S. Low-dose gonadotrophin therapy for induction of ovulation in 100 women with polycystic ovary syndrome. Hum Reprod 1991;6:1095-9. [DOI] [PubMed] [Google Scholar]
- 24.Farquhar C, Vandekerckhove P, Lilford R. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev 2005;(3):CD001122. [DOI] [PubMed]
- 25.Bayram N, van Wely M, Kaaijk EM, Bossuyt PMM, van der Veen F. Using an electrocautery strategy or recombinant FSH to induce ovulation in polycystic ovary syndrome: a randomised controlled trial. BMJ 2004;328:192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]