Abstract
Studies of immune-suppressed transplant recipients and patients with biopsy-proven skin cancer have confirmed that ultraviolet (UV) radiation-induced immune suppression is a risk factor for the development of skin cancer in humans. UV radiation suppresses the immune system in several ways. The UVB spectrum inhibits antigen presentation, induces the release of immunosuppressive cytokines, and elicits DNA damage that is a molecular trigger of UV-mediated immunosuppression. It is therefore important to elucidate the mechanisms underlying UV-induced immunosuppression as a basis for developing strategies to protect individuals from this effect and subsequent development of skin cancer. Dietary botanicals are of particular interest as they have been shown to inhibit UV-induced immune suppression and photocarcinogenesis. In this review, we summarize the most recent investigations and mechanistic studies regarding the photoprotective efficacy of selected dietary agents, including, green tea polyphenols, grape seed proanthocyanidins and silymarin. We present evidence that these chemopreventive agents prevent UVB-induced immunosuppression and photocarcinogenesis through: (i) The induction of immunoregulatory cytokine interleukin (IL)-12; (ii) IL-12-dependent DNA repair; and (iii) Stimulation of cytotoxic T cells in the tumor microenvironment. The new information regarding the mechanisms of action of these agents supports their potential use as adjuncts in the prevention of photocarcinogenesis.
Keywords: Chemoprevention, DNA repair, immunosuppression, interleukins, photocarcinogenesis, ultraviolet radiation
Introduction
Skin cancer represents a major, and growing, public health problem. It has been estimated that more than one million new cases of skin cancers are diagnosed each year in the United States alone, which is equivalent to the incidence of malignancies in all other organs combined [1]. The constant increase in life expectancy, as well as changes in environmental conditions, dietary habits and lifestyle, appears to be fueling the increase in the risk of skin cancer and, according to current projections, one in five Americans will develop at least one nonmelanoma skin cancer during their life-time. The cost of treating non-melanoma skin cancer is estimated to be in excess of US$ 650 million a year, and when melanoma is included, the estimated cost of treating skin cancer in the United States is US$ 2.9 billion annually (www.cancer.org/statistics). Thus, it is important to gain an improved understanding of the mechanisms that underlie the development of cutaneous malignancies. It is well established that the primary cause of skin cancer is the ultraviolet (UV) radiation found in sunlight. In addition to its carcinogenic potential, UV radiation also exerts immune suppressive effects. Data from studies of experimental animals and patients with biopsy-proven skin cancer suggest that there is an association between these immunosuppressive effects of UV radiation and its carcinogenic potential.
Solar UV radiation and the skin
Although many environmental and genetic factors contribute to the development of various skin diseases, the most important factor is the chronic exposure of the skin to solar UV light. For experimental purposes, the UV radiation present in sun light typically is considered in terms of the effects the short-wave (UVC; 200-290 nm), mid-wave (UVB; 290-320 nm), and long-wave (UVA; 320-400 nm) components of the radiation. UVB radiation is a mutagen, and extensive epidemiological evidence has indicated that it is UVB radiation that is responsible for the induction of melanoma and nonmelanoma skin cancers [2]. UVB radiation also has been shown to be responsible for sunburn, oxidative stress, and immune suppression. Notably, decreases in the stratospheric ozone layer are permitting more UVB radiation to reach the Earth's surface [3]. In contrast, although UVC radiation is a potent mutagen and can induce immune suppression, it is absorbed by the stratospheric ozone layer and its role as a causative agent in human disease is minimal. UVA, the major component of the UV spectrum (>90%), does cause premature aging of the skin, induce oxidative stress, and can suppress immune system although its effect are less pronounced than UVB radiation.
Skin represents the first barrier that protects the body from external environmental pollutants, including solar UV radiation and environmental chemicals. The skin is the largest organ in humans, having a surface area of approximately 1.5-2.0 m2. Morphologically, it is made up of a variety of cell types and organellar bodies, each of which has a particular function. The major cell types are organized in layers, which include the epidermis, the dermis, and the hypodermis. For the most part, it is the epidermal layer that is associated with development of skin cancer. The major cell type in the epidermis is the keratinocyte, which comprises >90% of the cells of the epidermal layer. It should be noted that in laboratory animals, such as the mouse, the epidermis is about 2-3 cell layers thick, whereas in humans, it is about 8-15 cell layers thick. Thus, human skin is more protective than mouse skin against environmental factors, including the effects of UV radiation.
UV radiation and immune suppression
Both UVA and UVB radiation suppress the immune systems of humans and mice. In addition to suppressing tumor immunity, UV exposure has been shown to suppress a wide variety of other immune reactions, including contact hypersensitivity (CHS) to chemical haptens [4], and delayed-type hypersensitivity to viral [5], bacterial [6] and fungal [7] antigens. CHS represents a special form of the delayed-type hypersensitivity response. It is induced by epicutaneous application of contact allergens, and is a prototypic T-cell-mediated immune response [8]. CHS models are used to evaluate UVB-induced suppression of immune responses. Typically, two CHS models are utilized to differentiate between local and systemic effects. In the CHS model of local immune suppression, the hapten is applied directly to the UV-irradiated skin. In the CHS model of systemic immune suppression, the UV radiation is applied to one site, and the hapten or antigen is applied to a distant non-irradiated site. Using these models, it has been clearly established that UVB radiation induces both local and systemic immune suppression, although the systemic immunosuppression clearly is mediated by mechanisms than those that lead to local immunosuppression.
It is well established that UV radiation stimulates keratinocytes to release immunosuppressive soluble mediators, including interleukin (IL)-10. These soluble mediators can then enter the circulation and can thus suppress the immune system in a systemic manner. Moreover, it has been shown that intravenous injection of supernatants obtained from UV-exposed keratinocytes into naïve mice renders the recipients unresponsive to hapten sensitization [9]. Thus, UV-induced immunosuppressive mediators derived from keratinocytes may enter the circulation and inhibit immune reactions at skin sites that are not exposed directly to UV radiation.
UV radiation-induced immune suppression is a risk factor for skin cancer
UV radiation has been shown to have multiple effects on the immune system [10,11]; however, if prevention of immune suppression is to be considered an effective strategy, it is necessary to determine whether the altered immune responses contribute to the pathogenesis of UV-induced skin cancer. There now are several lines of clinical and experimental evidence that indicate that immune factors contribute to the pathogenesis of UV-induced skin cancer in mice and, most likely, in humans [10,12]. The rate of skin cancer is exceptionally high in chronically immunosuppressed patients living in regions of intense sun exposure [13]. This observation is consistent with the hypothesis that immune surveillance plays an important role in the prevention of the generation and maintenance of neoplastic cells [14]. The incidence of skin cancers, especially squamous cell carcinomas, is also elevated among organ transplant recipients [15-18]. Studies of 2,561 kidney and heart transplant recipients indicated a 66-fold higher risk of squamous cell carcinoma than the risk in the general population [19,20]. A comprehensive study of 5,356 transplant recipients in Sweden showed that they have a 100-fold higher relative risk of developing nonmelanoma skin cancer, almost exclusively in sun-exposed areas of the skin [21]. This increased frequency of squamous cell carcinomas is presumably attributable to long-term immunosuppressive therapy [22]. Although the absolute risk of squamous-cell carcinoma after renal transplantation is highest in sunny climates, the risk of these tumors is also greatly elevated in less sunny geographic areas as suggested by the Swedish study [21] and a study of more than 700 renal-transplant recipients in the Netherlands which indicated that the overall incidence of squamous-cell carcinoma was 250 times greater than that in the general Dutch population [23]. In immunosuppressed patients, cutaneous cancers are common in areas of the skin that are exposed to the sun; moreover, they are more aggressive than in patients who are not immunosuppressed, are sometimes fatal, and often require multiple surgical procedures. The reasons for the dramatic greater incidence of these tumors among immunosuppressed patients is not understood completely, although it is often attributed to a reduction in cancer surveillance owing to pan-immunosuppression resulting from drug therapy and exposure to UVB radiation.
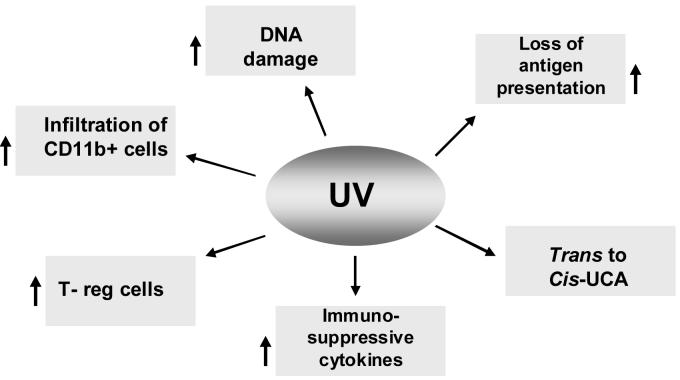
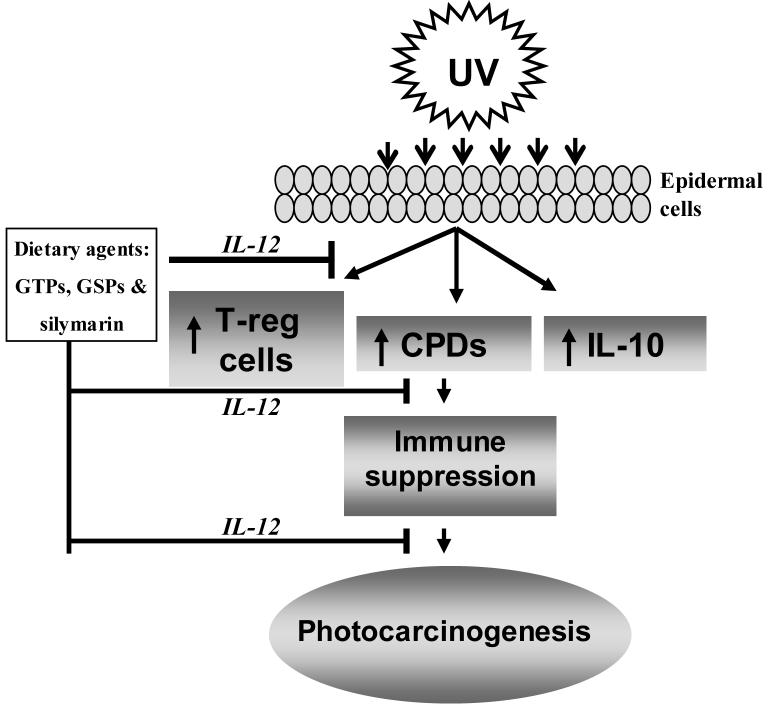
It has been suggested that the induction of DNA photoproducts [24] and reactive oxygen intermediates [25] by UVB radiation compromises cutaneous immunity. It also has been suggested that the release of cytokines following UVB radiation plays a significant role in UVB-induced immunosuppression and, thus, may be an important factor in the growth and development of immunogenic UV-induced skin tumors [10-12]. Of potential significance is the fact that a number of tumors, including some melanomas and non-melanoma skin cancer, appear to produce IL-10 [11, 26,27]. The immunosuppressive effects of IL-10 may be one of the mechanisms by which these tumors escape immunologic control [27]. The focus of this current mini-review is to highlight the effect of UV radiation on various targets that contribute to the suppression of the immune system (Fig. 1), and to summarize the chemopreventive potential and mechanisms of action of some dietary botanicals that protect the skin from UV-induced immune suppression and afford protection from UV-induced carcinogenesis (Fig. 2).
Figure 1.
Molecular and cellular targets in the skin that are affected by UV radiation resulting in induction of immune suppression. Upward arrows (↑) indicate the enhancement or stimulation.
Figure 2.
Dietary agents (GTPs, GSPs and silymarin) have the ability to block the adverse biological effects of UV radiation through the induction of the immunoregulatory cytokine, IL-12, thus preventing UV-induced immune suppression. Inhibition of UV-induced immune suppression contributes to the prevention of photocarcinogenesis. Upward arrows (↑) indicate the enhancement or stimulation.
UV radiation alters the function of Langerhans cells thereby affecting the immune system
Langerhans cells are the major antigen presenting cells in the skin [11] and represent a major component of the dendritic cell network that plays a key role in the development of immune responses including CHS. UV irradiation has been shown to have multiple deleterious effects on Langerhans cells and mice sensitized through UV-irradiated skin fail to generate CHS response to contact allergens [8]. UV irradiation results in a depletion of Langerhans cells in the skin [8]. This may be due to the emigration of Langerhans cells from the skin to the draining lymph nodes since Langerhans cells harboring UV-induced DNA damage can be detected in the draining lymph nodes [28]. In addition, UV exposure impairs the ability of Langerhans cells to present antigens, thus further compromising the dendritic cell network [8]. UV radiation suppresses the expression of major histocompatibility (MHC) class II surface molecules on Langerhans cells and their adenosine triphosphatase activity [29]. Both of these markers are used to identify Langerhans cells in the epidermis. Inhibition of the expression of the adhesion molecule ICAM-1 by UV radiation may be responsible for impaired clustering of Langerhans cells and T cells. Other antigen presenting cells, including human peripheral blood-derived dendritic cells and splenic dendritic cells, also exhibit a significant impairment in their ability to stimulate allogeneic T cells when exposed to UV both in vitro and in vivo. It has been reported that UVB-induced reactive oxygen species may contribute to the impairment of the function of antigen presenting cells [30]. Antigen presentation may also be impaired by the photoproduct cis-urocanic acid and by immunosuppressive cytokines, such as IL-10. Taken together, these observations suggest that the deleterious effects of UV irradiation on Langerhans cells play a key role in the induction of UV-induced immune suppression [11].
UV-induced DNA damage triggers immunosuppression
UV-induced DNA damage, predominantly in the form of cyclobutane pyrimidine dimers (CPDs), has been recognized as an important molecular trigger for the suppression of immune responses and initiation of UV-induced carcinogenesis [24, 31, 32]. UV-induced DNA damage is an important event in the migration of antigen presenting cells (i.e., Langerhans cells in the epidermis) from the skin to the draining lymph nodes. DNA damage in antigen presenting cells impairs their capacity to present antigen, which in turn results in a lack of sensitization [33]. CPD-containing antigen presenting cells have been found in the draining lymph nodes of UV-exposed mice [28, 34]. These antigen presenting cells were determined to be of epidermal origin and to exhibit an impaired ability to present antigen. Thus, UV-induced DNA damage is one of the earliest molecular events in the development of immune suppression. To directly test this concept, pyrimidine dimer formation has been repaired in mice by application of liposomes containing the bacteriophage excision repair enzyme, T4N5, to the skin of the UV-irradiated animals. This resulted in a reduction in the number of CPDs in epidermal DNA, and, subsequently, a reduction in the induction of immune suppression. The abrogation of UVB-induced immune suppression by T4N5-containing liposomes was associated with a reduction in the induction of suppressor T cells in the UV-irradiated mice [24].
The immunoregulatory cytokine, IL-12, also has been shown to remove, or repair, UV-induced DNA damage in the skin [35]. Depending on the severity of the DNA damage following UV exposure of the skin, keratinocytes in the skin can progress to either apoptosis or DNA repair pathways [28, 36]. If the DNA damage is irreparable, the cell cycle is arrested and the keratinocyte is transformed into a sunburn cell, which is an early morphologic indicator of epidermal cell apoptosis. To examine the role of IL-12 in removal of UVB-induced DNA damage, we compared the effects of acute low-dose UVB exposure on the numbers of CPD cells in the skin of IL-12-deficient or knock out (IL-12 KO) and in the skin of their wild-type counterparts, C3H/HeN mice. We observed that in wild-type mice, there were considerably lower numbers of UV-induced CPD-positive cells in the skin at 24 hours or 48 hours after UV exposure than immediately after exposure. In contrast, the numbers of CPD-positive cells in the skin of the IL-12 KO mice did not exhibit a significant reduction at 24 hours or 48 hours after UV exposure. This suggested that the endogenous DNA repair mechanism requires the presence of IL-12 in mice, which was further indicated by the finding that subcutaneous injection of recombinant IL-12 into the UV-irradiated skin of the IL-12 KO mice enhanced the repair of UVB-induced CPDs. These findings support the concept that IL-12 has the ability to repair UVB-induced DNA damage in vivo, at least in animal models [35,37].
Induction of T regulatory cells
The induction of hapten-specific tolerance by UV radiation appears to be mediated, at least in part, through the generation of T cells with suppressive or inhibitory immune activity. The UV-induced suppressor T cells mediate their suppressive effects by releasing immune regulatory factors, particularly IL-10 [11, 38]. Several different regulatory T cells with unique phenotypes have been identified as being involved in the various different models of UV-mediated tolerance (local, systemic, high and low dose) [38]. These cells may represent a separate subtype of regulatory T (T-reg) cells since they exhibit characteristics of naturally occurring regulatory T cells, e.g., expression of CD4 and CD25, but also of type 1 regulatory R (Tr1) cells, e.g., release of IL-10 [11,38]. Cells transferring suppression in the low-dose CHS model appear to belong to the CD4+CD25+ subtype, they express CTLA-4 [11,38], bind the lectin dectin-2 [39] and, in contrast to the classical CD4+CD25+ T cells, release high amounts of IL-10 upon antigen-specific activation [11,38].
IL-12 has been shown to prevent the suppression of CHS by UV, to prevent the development of regulatory T cells and even to break UV-induced tolerance by yet un-identified mechanisms [38,40,41]. Since a reduction in UV-induced DNA damage is associated with the inhibition of UV-induced immunosuppression, DNA damage is regarded as the major molecular trigger of UV-induced immunosuppression [24,42]. The prevention of UV-induced immunosuppression by IL-12 may be due to its capacity to reduce DNA damage via induction of DNA repair [35,37] since the preventive effect of IL-12 is not observed in mice that are deficient in DNA repair [28]. UV-induced DNA damage appears to be an important event in the UV-mediated induction of regulatory T cells. This assumption is based on the observation that a reduction in Langerhans cells containing DNA damage in the regional lymph nodes by IL-12 prevents the development of regulatory T cells [28]. Notably, UV-induced regulatory T cells also appear to play an important role in photocarcinogenesis.
UV-induced immune suppression in humans
The majority of information regarding UV-induced immune suppression is derived from studies of experimental animal models, mostly mice. There is, however, evidence that UV radiation also suppresses the induction of CHS in humans. In humans, the induction of delayed-type hypersensitivity [11,43] and CHS [11,44] is suppressed after a single, or short-term, exposure to UV radiation. In addition, immune suppressive cytokines, such as IL-10, are produced in human skin in response to UV exposure, although the identity of the cell that releases IL-10 may be different in humans and mice [45]. UV exposure suppresses human antigen-presenting cell function with the doses of UV irradiation that induce immune suppression in humans and mice being comparable [11]. Many of the mechanisms involved in UV-induced immune suppression in humans are similar to those described in experimental animals. UV-induced DNA damage triggers immune regulatory cytokine production and triggers immune suppression. Wolf et al. [46] observed that exposure of human skin to solar simulated light resulted in the up-regulation of IL-10 and TNF-α in the epidermis. Application of T4N5-containing liposomes to the skin of UV-irradiated individuals immediately after UV exposure inhibits cytokine production and Stege et al. [47] found that repair of CPDs in human skin using photolyase prevented UV-induced suppression of the CHS response. Moreover, sensitivity to sunburn appears to be associated with susceptibility to UV radiation-induced suppression of cutaneous cell-mediated immunity in humans [44].
Role of dietary agents in the prevention of UV-induced immune suppression
There has been great interest in the use of dietary agents that are derived from plants for the photoprotection of the skin, including their use to reduce the risk of melanoma and nonmelanoma skin cancers. Dietary botanicals, possessing anti-inflammatory, immunomodulatory and anti-oxidant properties are among the most promising group of compounds that can be exploited as ideal chemopreventive agents for skin diseases. Among the botanical agents that have been identified as having potential chemopreventive activities are retinoids, green tea polyphenols, grape seed proanthocyanidins, resveratrol, curcumin and silymarin [48]. Importantly we should recognize that nutrition now includes food components that do not provide nutrition in the traditional sense, in that they do not contribute calories, nor are metabolized to building blocks for proteins, carbohydrates or fats. Yet their inclusion in the diet appears to have important health benefits particularly with regard to age-related chronic diseases including cancers. Polyphenols, particularly the catechins and proanthocyanidins that are contained in certain leaves and in some seeds, appear to have important health benefits particularly with regard to age-related diseases. Dietary supplements containing these ingredients are widely available over-the-counter remedies for age-related diseases. Moreover, as far as skin is concerned, most skin care lotions or sunscreens are applied topically on the skin for health benefits. This indicates that skin has the ability to consume its nutrition through topical application of nutrients. Therefore, it is important to mention that nutrition for the skin can be provided through both oral and topical administration. Here, we will particularly discuss and summarize the effects of green tea polyphenols, grape seed proanthocyanidins and silymarin on the UV radiation-induced immune suppression, which is considered as a potential risk factor for the development of skin cancers.
Dietary agents prevent UV-induced immune suppression
As discussed above, the immunosuppressive effects of solar UV radiation (both UVA and UVB) are well established. Many of the adverse effects of solar UV radiation on human health, including exacerbation of infectious diseases and induction of skin cancer, are mediated at least in part by the ability of UV radiation to induce immune suppression (10,12,49). As UV-induced immunosuppression is considered as a risk factor for the induction of skin cancer, prevention of UV-induced immunosuppression represents a potential strategy for the management of skin cancers in humans. We have shown that topical administration of green tea polyphenols (GTPs), a mixture of polyphenols or epicatechin derivatives, to C3H/HeN mice resulted in a significant protection against local and systemic models of CHS in which 2,4-dinitrofluorobenzene was used as a contact sensitizer [50]. Similar effects were also noted when a water extract of green tea was given to mice as the sole source of drinking water. (−)-Epigallocatechin-3-gallate (EGCG) was found to be the most effective constituent of the GTPs and has been shown to prevent UVB-induced suppression of CHS when applied topically [51, Table 1]. The prevention of UVB-induced immunosuppression by EGCG treatment was found to be associated with a reduction in the number of infiltrating CD11b+ cells (a cell surface marker of macrophages and neutrophils) in UVB-irradiated skin [51]. The blocking of UV-induced infiltration of leukocytes using anti-CD11b antibody inhibited UV-induced immune suppression and tolerance induction in C3H/HeN mice [52]. Therefore, it appears that inhibition of UV-induced immunosuppression by EGCG is mediated, at least in part, through the inhibition of infiltrating activated macrophages and neutrophils in the UVB-irradiated skin.
Table 1.
Chemopreventive effects of selected dietary botanical agents on some biological effects in UV-irradiated animals
| Botanicals | Source | Molecular targets/Mechanisms | References |
|---|---|---|---|
| Green tea polyphenols | Leaves and bud (Camellia sinensis, Linn) |
Prevents immune suppression, DNA damage, increases stimulation of CD8+ cells, IL-12 induction, decreases IL-10, infiltration, photocarcinogenesis |
34, 48, 50,51,58 59, 60 |
| Proanthocyanidins | Grape seeds (Vitis vinifera) | Prevents immune suppression, increases IL-12, decreases IL-10 & photocarcinogenesis |
48, 54, 55 |
| Silymarin | Milk thistle (Silybum marianum) | Prevents immune suppression, increases IL-12 decreases IL-10, leukocyte infiltration & carcinogenesis |
34, 56, 57 |
In UVB-irradiated skin, IL-10 is secreted, primarily by activated macrophages, and acts to down-regulate CHS responses. Intraperitoneal administration of IL-10 to mice inhibited delayed type hypersensitivity responses, and intraperitoneal injection of anti-IL-10 antibody prevented UV-induced tolerance induction [53]. In accordance with these observations, by topical application of EGCG to the mouse skin resulted in a lower level of IL-10 in the UV-irradiated skin, as well as in the draining lymph nodes, than in mice which were not treated with EGCG. Treatment of mice with EGCG significantly reduced the number of IL-10 producing cells and this was accompanied with a reduction in infiltrating leukocytes [51]. This suggests a possible mechanism by which EGCG prevents UVB-induced immune suppression in mice.
Topical treatment with EGCG prior to UVB exposure also resulted in an enhancement in the levels of IL-12 in the skin and draining lymph nodes as compared to the levels of IL-12 in the skin and lymph nodes of non-EGCG treated but UVB-exposed mice [51]. Higher levels of IL-12 may contribute to stimulation of the anti-tumor immune response. Recently, Meeran et al. [34] have shown that topical treatment of EGCG prevented UV-induced suppression of CHS in wild-type mice as shown by a significant enhancement of the CHS response (ear swelling). In contrast, UV-exposed IL-12-deficient or knockout (KO) mice remained unresponsive to dinitrofluorobenzene, a sensitizer, despite the application of EGCG on the mouse skin. This suggests that the immunopreventive effect of EGCG against UV-induced suppression of the CHS response requires IL-12. Further, intraperitoneal injection of C3H/HeN mice with anti-IL-12 antibody significantly reduced the preventive effect of EGCG on UV-induced suppression of CHS. These studies provide convincing evidence that prevention of UV-induced immunosuppression by EGCG is mediated, at least in part, through IL-12 induction.
Similar chemopreventive effects on UV-induced immunosuppression have been observed in studies of other botanicals. We have observed that supplementation of a control AIN76A diet with grape seed proanthocyanidins (GSPs) markedly inhibited photocarcinogenesis in mice [54]. It also was found that dietary GSPs prevent UVB-induced suppression of the CHS response in a local model of immunosuppression and had moderate inhibitory effects in a systemic model of CHS [55]. Dietary GSPs reduced UVB-induced increases in the levels of IL-10 in the skin and enhanced the production of IL-12 in C3H/HeN mice. Intraperitoneal injection of GSP-fed mice with a neutralizing anti-IL-12 antibody abrogated the protective effects of the GSPs against UVB-induced suppression of the CHS [55]. Silymarin, a product of milk thistle, is another botanical agent that has been tested for its ability to prevent UVB-induced immunosuppression. Topical treatment with silymarin significantly inhibited photocarcinogenesis in SKH-1 hairless mice [56]. More recently, we have tested the effect of silymarin on UVB-induced immunosuppression using local and systemic models of CHS in C3H/HeN mice [57]. We found that topical treatment of mice with silymarin inhibited UVB-induced immunosuppression in mice, and that the mechanism of action appeared to be similar to that described above for GTPs and GSPs. Taken together, these observations indicate that certain botanicals with anti-oxidant and anti-inflammatory properties can protect the skin from the adverse biological effects of UV radiation and that they share very similar mechanisms of action.
Inhibition of UV-induced immunosuppression is mediated through IL-12-dependent DNA repair
UV-induced DNA damage in the form of CPDs is an important molecular trigger for UV-induced immunosuppression and photocarcinogenesis [28,35] and that a reduction in the levels of UV-induced CPDs can prevent UV-induced immunosuppression [24,34]. It has been shown that IL-12 has the ability to remove or repair UV-induced CPDs [35]. We and others [28,34] have shown that the prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair and that it acts through the induction of a nucleotide excision repair mechanism. The effect of topically administered EGCG on UV-induced CPDs in IL-12 KO mice, as compared with their wild-type counterparts, has been determined. Twenty-four hours after UV-irradiation, the numbers of CPD-positive cells were significantly lower in the EGCG-treated wild-type mice than in the wild-type mice that were not treated with EGCG but were exposed to UVB. In contrast, the UVB-induced DNA damage in the IL-12 KO mice that had been treated with EGCG did not differ from that in the IL-12 KO mice that had not been treated with EGCG [34]. These observations suggest that EGCG-induced IL-12 contributes to the repair of UV-damaged DNA and that this leads to the prevention of UV-induced immune suppression and photocarcinogenesis [34,58].
UV-induced DNA damage has been identified as an important molecular trigger for the migration of antigen presenting cells (Langerhans cells) from the skin to the draining lymph nodes. DNA damage in antigen presenting cells impairs their capacity to present antigen, which in turn results in a lack of sensitization [33]. CPD-containing antigen presenting cells have been found in the draining lymph nodes of UV-exposed mice. The cells were identified as being of epidermal origin and found to exhibit an impaired antigen presentation capacity. Immunohistochemical analysis of CPD-positive cells in draining lymph nodes obtained 36 hours after UV irradiation revealed significantly higher numbers of CPD-positive cells in UV-exposed wild-type mice and IL-12 KO mice as compared to unexposed wild-type mice; however, the numbers of CPD-positive cells in the draining lymph nodes of the UV-exposed IL-12 KO mice were approximately 4-fold higher than in the draining lymph nodes of their UV-exposed wild-type counterparts. The lower percentage of CPD-positive cells in the lymph nodes of UV-exposed wild-type mice than UV-exposed IL-12 KO mice may be attributable to the presence of endogenous IL-12 in the wild-type mice at levels that are capable of partial removal of the damaged DNA in the migrating cells. Treatment of mice with EGCG resulted in a significant reduction in the numbers of CPD-positive cells in the draining lymph nodes of UV-exposed wild-type mice as compared to the numbers in the lymph nodes of UV-exposed wild-type mice that did not receive EGCG. In contrast, there was no significant difference in the number of CPD-positive cells in the draining lymph nodes of EGCG-treated and non-EGCG-treated UV-exposed IL-12 KO mice [34]. This observation provides further evidence that the reduction in the numbers of CPD-positive cells in the draining lymph nodes of wild-type mice after EGCG treatment may be due to EGCG-induced IL-12-mediated repair of CPDs in the cells.
Green tea polyphenols stimulate cytotoxic T cells in skin tumors
IL-12 has been shown to stimulate the production of IFNγ and stimulate the development of cytotoxic T cells (CD8+ T cells), which are tumoricidal. These effects of IL-12 may result in inhibition of tumor growth or regression of the tumors. Administration of GTPs in the drinking water inhibits UV-induced skin tumor development in SKH-1 hairless mice [59]. It was also observed that the numbers of CD8+ T cells in the tumors of the UVB-exposed mice that were treated with GTPs were higher than in the tumors of the UVB-exposed mice that were not treated with GTPs. This study suggests that GTPs can inhibit tumor growth by more than one mechanism. CD8+ T cells are the effector cells in the cytotoxic response of the host to UV-induced skin tumor cells. They play an important role in protection against tumor immunity, at least in photocarcinogenesis. It is possible that EGCG-induced IL-12 plays a role in stimulation of CD8-positive cells. Similar observations were noted when the mice were treated topically with EGCG and subjected to a photocarcinogenesis protocol [60].
Conclusion and future prospects of dietary agents in the prevention of photo-immunosuppression
The mechanistic studies which have been summarized in this review article indicate the potential beneficial effects of dietary agents in the prevention of UV-induced immune suppression and subsequent prevention of photocarcinogenesis in experimental animals. The botanical agents discussed proved to be effective in the animal models whether administered in the drinking water, as dietary supplements, or topically, depending on the nature of the agent. The supplementation of the use of sunscreens with these dietary agents may provide an effective strategy for the prevention of melanoma and nonmelanoma skin cancers in humans. The dietary botanical agents discussed are considered to be non-toxic and pharmacologically safe for human consumption. Clinical trials are needed to validate the preventive and therapeutic medicinal value of these dietary agents, either alone or in combination with existing therapies for melanoma and nonmelanoma skin cancers, in high-risk human populations.
Acknowledgments
The work reported from the author's laboratory was supported by the funds from National Institutes of Health (CA104428, CA105368, CA089738, ES11421, AT002536), Cancer Research and Prevention Foundation and VA Merit Review Award. The content of this article does not necessarily reflect the views or policies of the funding sources. The author apologizes for not discussing and citing several important publications because of the limitations of space and of the number of references.
Abbreviations used
- UV
ultraviolet
- CHS
contact hypersensitivity
- IL
interleukin
- GTPs
green tea polyphenols
- GSPs
grape seed proanthocyanidins
- CPD
cyclobutane pyrimidine dimers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, Chen GJ. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J. Am. Acad. Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 2.Urbach F. Evidence of epidemiology of UV-induced carcinogenesis in man. Natl. Cancer Inst. Monogr. 1978;50:5–10. [PubMed] [Google Scholar]
- 3.Van der Leun JC. In: Human Health: United Nations Environmental Program report on the Environmental Effects of ozone Depletion. Van der Leun JC, Tevini M, editors. Environmental Protection Agency; Washington, DC: 1989. [Google Scholar]
- 4.Jessup JM, Hanna N, Palaszynski E, Kripke ML. Mechanisms of depressed reactivity to dinitrochlorobenzene and ultraviolet-induced tumors during ultraviolet carcinogenesis in BALB/c mice. Cell Immunol. 1978;38:105–115. doi: 10.1016/0008-8749(78)90036-9. [DOI] [PubMed] [Google Scholar]
- 5.Howie SEM, Norval M, Maingay J. Exposure to low dose UVB light suppresses delayed type hypersensitivity to herpes simplex virus in mice. J. Invest. Dermatol. 1986;86:125–128. doi: 10.1111/1523-1747.ep12284128. [DOI] [PubMed] [Google Scholar]
- 6.Jeevan A, Kripke ML. Effect of a single exposure to ultraviolet radiation on Mycobacterium bovis bacillus Calmette–Guerin infection in mice. J. Immunol. 1989;143:2837–2843. [PubMed] [Google Scholar]
- 7.Denkins Y, Fidler IJ, Kripke ML. Exposure of mice to UVB radiation suppresses delayed hypersensitivity to Candida albicans. Photochem. Photobiol. 1989;49:615–619. doi: 10.1111/j.1751-1097.1989.tb08432.x. [DOI] [PubMed] [Google Scholar]
- 8.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J. Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 9.Schwarz T, Urbanska A, Gschnait F, Luger TA. Inhibition of the induction of contact hypersensitivity by a UV-mediated epidermal cytokine. J. Invest. Dermatol. 1986;87:289–291. doi: 10.1111/1523-1747.ep12696708. [DOI] [PubMed] [Google Scholar]
- 10.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev. Med. Interne. 1998;19:247–254. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest. Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 13.Kinlen L, Sheil A, Peta J, Doll R. Collaborative United Kingdom-Australia study of cancer in patients treated with immunosuppressive drugs. Br. J. Med. 1979;ii:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnet FM. Immunological surveillance. Pergamon Press; Oxford: 1970. [Google Scholar]
- 15.Ondrus D, Pribylincova V, Breza J, Bujdak P, Miklosi M, Reznicek J, Zvara V. The incidence of tumors in renal transplant recipients with long-term immunosuppressive therapy. Int. Urol. Nephrol. 1999;31:417–422. doi: 10.1023/a:1007194607496. [DOI] [PubMed] [Google Scholar]
- 16.Cowen EW, Billingsley EM. Awareness of skin cancer by kidney transplant patients. J. Am. Acad. Dermatol. 1999;40:697–701. doi: 10.1016/s0190-9622(99)70149-0. [DOI] [PubMed] [Google Scholar]
- 17.Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–262. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- 18.Fortina AB, Caforio AL, Piaserico S, Alaibac M, Tona F, Feltrin G, Livi U, Peserico A. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J. Heart Lung Transplant. 2000;19:249–255. doi: 10.1016/s1053-2498(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 19.Jensen P, Moller B, Hansen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 2000;42:307–308. doi: 10.1016/s0190-9622(00)90154-3. [DOI] [PubMed] [Google Scholar]
- 20.Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, Fauchald P, Simonsen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999;40:177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 21.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br. J. Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- 22.DiGiovanna JJ. Posttransplantation skin cancer: scope of the problem, management and role for systemic retinoid chemoprevention. Transplant. Proc. 1998;30:2771–2775. doi: 10.1016/s0041-1345(98)00806-9. [DOI] [PubMed] [Google Scholar]
- 23.Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiated systemic immunosuppression in UV-irradiated mice. Proc. Natl. Acad. Sci. USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Pinnell SR, Streilein JW. Antioxidants can reverse the deleterious effects of ultraviolet B radiation on cutaneous immunity. J. Invest. Dermatol. 1995;104:600. doi: 10.1111/1523-1747.ep12276349. (abstract) [DOI] [PubMed] [Google Scholar]
- 26.Dummer W, Becker JC, Schwaaf A, Leverkus M, Moll T, Brocker EB. Elevated serum levels of IL-10 in patients with metastatic malignant melanoma. Melanoma Res. 1995;5:67–68. doi: 10.1097/00008390-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J. Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 28.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J. Exp. Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aberer W, Schuler G, Stingl G, Honigsmann H, Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. J. Invest. Dermatol. 1981;76:202–210. doi: 10.1111/1523-1747.ep12525745. [DOI] [PubMed] [Google Scholar]
- 30.Caceres-Dittmar G, Ariizumi K, Xu S, Tapia FJ, Bergstresser PR, Takashima A. Hydrogen peroxide mediates UV-induced impairment of antigen presentation in a murine epidermal-derived dendritic cell line. Photochem. Photobiol. 1995;62:176–183. doi: 10.1111/j.1751-1097.1995.tb05255.x. [DOI] [PubMed] [Google Scholar]
- 31.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of molecular targets for the suppression of contact hypersensitivity by ultraviolet radiation. J. Exp. Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarosh D, Alas LG, Yee V, Oberyszyn A, Kibitel JT, Mitchell D, Rosenstein R, Spinowitz A, Citron M. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992;52:4227–4231. [PubMed] [Google Scholar]
- 33.Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, Kripke ML. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc. Natl. Acad. Sci. USA. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat. Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 37.Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of ultraviolet Radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol. Cancer Ther. 2006;5:825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz T. Mechanisms of UV-induced immunosuppression. Keio J. Med. 2005;54:165–171. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 39.Aragane Y, Maeda A, Schwarz A, Tezuka T, Ariizumi K, Schwarz T. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J. Immunol. 2003;171:3801–3807. doi: 10.4049/jimmunol.171.7.3801. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz A, Grabbe S, Aragane Y, Sandkuhl K, Riemann H, Luger TA, Kubin M, Trinchieri G, Schwarz T. Interleukin-12 prevents ultraviolet B-induced local immunosuppression and overcomes UVB-induced tolerance. J. Invest. Dermatol. 1996;106:1187–1191. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J. Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- 42.Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc. Natl. Acad. Sci. U S A. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyal D, Courbiere C, Le Corre Y, de Lacharriere O, Hourseau C. Immunosuppression induced by chronic solar simulated irradiation in humans and its prevention by sunscreens. Eur. J. Dermatol. 1997;7:223–225. [Google Scholar]
- 44.Damian DL, Halliday GM, Barnetson RS. Broad-spectrum sunscreens provide greater protection against ultraviolet-radiation-induced suppression of contact hypersensitivity to a recall antigen in humans. J. Invest. Dermatol. 1997;109:146–151. doi: 10.1111/1523-1747.ep12319200. [DOI] [PubMed] [Google Scholar]
- 45.Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J. Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 46.Wolf P, Maier H, Mullegger RR, Chadwick CA, Hofmann-Wellenhof R, Soyer HP, Hofer A, Smolle J, Horn M, Cerroni L, Yarosh D, Klein J, Bucana C, Dunner K, Jr, Potten CS, Honigsmann H, Kerl H, Kripke ML. Topical treatment with liposomes containing T4 endonuclease V protects human skin in vivo from ultraviolet-induced upregulation of interleukin-10 and tumor necrosis factor-alpha. J. Invest. Dermatol. 2000;114:149–156. doi: 10.1046/j.1523-1747.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- 47.Stege H, Roza L, Vink AA, Grewe M, Ruzicka T, Grether-Beck S, Krutmann J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. U S A. 2000;97:1790–1795. doi: 10.1073/pnas.030528897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem. Photobiol. Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 49.Chapman RS, Cooper KD, De Fabo EC, Frederick JE, Gelatt KN, Hammond SP, Hersey P, Koren HS, Ley RD, Noonan F. Solar ultraviolet radiation and the risk of infectious disease. Photochem. Photobiol. 1995;61:223–247. doi: 10.1111/j.1751-1097.1995.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 50.Katiyar SK, Elmets CA, Agarwal R, Mukhtar H. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. Photochem. Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 51.Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 52.Hammerberg C, Duraiswamy N, Cooper KD. Reversal of immunosuppression inducible through ultraviolet-exposed skin by in vivo anti-CD11b treatment. J. Immunol. 1996;157:5254–5261. [PubMed] [Google Scholar]
- 53.Schwarz A, Grabbe S, Riemann H, Aragane Y, Simon M, Manon S, Andrade S, Luger TA, Zlotnik A, Schwarz T. In vivo effects of interleukin-10 on contact hypersensitivity and delayed-type hypersensitivity reactions. J. Invest. Dermatol. 1994;103:211–216. doi: 10.1111/1523-1747.ep12393073. [DOI] [PubMed] [Google Scholar]
- 54.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 55.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 56.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of Silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 57.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol. Cancer Ther. 2006;5:1660–1668. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 58.Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 59.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J. Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 60.Mantena SK, Roy AM, Katiyar SK. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem. Photobiol. 2005;81:1174–1179. doi: 10.1562/2005-04-11-RA-487. [DOI] [PubMed] [Google Scholar]