Abstract
In this review we describe some of the remarkable and intricate mechanisms through which the calcium ion (Ca2+) contributes to detection, transduction and synaptic transfer of light stimuli in rod and cone photoreceptors. The function of Ca2+ is highly compartmentalized. In the outer segment, Ca2+ controls photoreceptor light adaptation by independently adjusting the gain of phototransduction at several stages in the transduction chain. In the inner segment and synaptic terminal, Ca2+ regulates cells’ metabolism, glutamate release, cytoskeletal dynamics, gene expression and cell death. We discuss the mechanisms of Ca2+ entry, buffering, sequestration, release from internal stores and Ca2+ extrusion from both outer and inner segments, showing that these two compartments have little in common with respect to Ca2+ homeostasis. We also investigate the various roles played by Ca2+ as an integrator of intracellular signaling pathways, and emphasize the central role played by Ca2+ as a second messenger in neuromodulation of photoreceptor signaling by extracellular ligands such as dopamine, adenosine and somatostatin. Finally, we review the intimate link between dysfunction in photoreceptor Ca2+ homeostasis and pathologies leading to retinal dysfunction and blindness.
Keywords: Retina, Photoreceptor, Rod, Cone, Calcium, Plasma Membrane Calcium Atpase, Na-Ca Exchange, Calcium Store, Calcium Buffering, Calcium Channel, Calcium Extrusion, Ryanodine, Inner Segment, Dystrophy, Synaptic Transmission, Review
2. INTRODUCTION
The calcium ion (Ca2+) is a major messenger for coordinating activity of eukaryote cells. Its extensive distribution and functioning as a control ion is common to a huge range of eukaryote cell types from animal, plant or fungal sources which diverged billions of years ago. The signaling potential of Ca2+ is linked to the ability of cells to maintain a large concentration gradient between the cytosol (with basal Ca2+ levels typically ~ 50 nM) and the extracellular media (with Ca2+ ~ 1.5 mM). Because of this 10,000-fold difference in [Ca2+]i between the cytosol and the extracellular space, Ca2+ influx across the plasma membrane and Ca2+ release from intracellular stores may produce large fluctuations of free cytosolic Ca2+ (1). Another important feature of Ca2+ homeostasis is that [Ca2+]i elevations in the cell can be highly localized. We now know that the diffusion of Ca2+ within the cytosol is much slower than that of other intracellular messengers (2, 3). Ca2+ is thus able to regulate a variety of different functions in different parts of the cell. Such selective action of Ca2+ is facilitated by Ca2+-binding proteins which are often localized to specific sites mediating individual functions (4). To understand Ca2+ signaling, it is therefore essential to map the spatial distribution of Ca2+ effector proteins such as Ca2+ channels, Ca2+ pumps, cytoplasmic buffers and intracellular Ca2+ stores. This is especially true for primary sensory neurons, such as olfactory cells, hair cells, electroreceptors and photoreceptors which are composed of different functional compartments.
Anatomically, primary sensory neurons are notable for their compartmentalization into input (sensory) and output (synaptic) regions. Different sets of lipid and protein molecules are expressed in the two regions. The sensory regions are enriched in protein cascades designed to intercept and transduce specific environmental signals while the output regions contain the synaptic release machinery. Sensory systems such as vision and hearing require extraordinary rates of information transfer and the finest sensory discrimination (5). These requirements guided the evolution of highly specialized mechanisms for signal detection and transmission by primary sensory neurons. Stimuli such as photons, pressure, odors or electric fields are transduced into changes in the potential across the plasma membrane, which in turn controls neurotransmitter release. Ca2+ plays an essential role in both sensory transduction and in synaptic transmission and is regulated by distinct and separate signaling cascades in each region.
In this review we chose vertebrate photoreceptors as a model for the role of Ca2+ in regulating sensory processing at the cellular level. We will describe some of the remarkable and intricate roles that Ca2+ plays as an integrator of light adaptation as well as the final common path for regulating transmitter release by focusing on mechanisms through which Ca2+ regulates the sensory input.
3. THE PHOTORECEPTOR LIGHT RESPONSE
Photoreceptors can be divided into two general classes: (1) rods, which reliably signal single photon absorptions, are relatively slow in response to light stimulation, exhibit a high sensitivity to light and thus function as mediators of nocturnal vision, and (2) cones, which are less sensitive, faster and more noisy, mediate color vision and operate optimally in bright daylight. The basic components of the phototransduction cascade in rods and cones are very similar. As discussed in more detail below, many, if not most, known kinetic and sensitivity differences between rod and cone phototransduction machineries may be accounted for by the differences in Ca2+ regulation in rod and cone outer segments (OSs) (6–8; see below).
In the 1970s Tsuneyoshi Tomita and his coworkers revolutionized sensory physiology by observing that, in darkness, the resting membrane potential of photoreceptors is relatively depolarized at −35 to −40 mV. They also found that light hyperpolarized the membrane potential to ~ −70 mV (9). Photoreceptors therefore respond to stimulation in the opposite way from most CNS neurons, which tend to depolarize when activated. Another unexpected finding by Tomita was that photoreceptors do not signal with action potentials – instead, changes in the intensity of the light stimulus are signaled by photoreceptors as graded variations in membrane potential. The graded nature of photoreceptor signaling allows maximization of the rate of information transfer at the photoreceptor synapse (5, 10).
Figure 1 shows a schematic view of a rod photoreceptor. The cell consists of two distinct compartments – (1) an “outer segment” (OS) which is uniquely designed to carry out transduction of the photon energy into an electrical signal, and (2) an “inner segment” (IS), hosting the cells’ metabolic and synaptic machinery. Rod OSs are filled with disks (Figure 1), rather mysterious intracellular organelles which contain a high density of the visual pigment rhodopsin. Cone OSs possess a slightly different visual pigment which is inserted directly into folds of their plasma membrane. A thin nonmotile cilium links the OS to the IS. The IS is sometimes thought to constitute a delimited region of the photoreceptor between the OS and the photoreceptor cell body. However, based on the remarkable uniformity of the plasma membrane and cytoplasmic constituents of the non-OS region (see below Section 5) and the fact that identical voltage responses to light stimulation can be recorded throughout the non-OS part of rods and cones, this region can be seen as forming a functionally integrated compartment (the inner segment) composed of several highly morphologically and functionally distinct subcompartments. These subregions are responsible for crucial cellular functions such as gene expression, protein synthesis, energy metabolism and transmitter release. Such compartmentalization is a characteristic feature of vertebrate primary sensory cells (hair cells (11), olfactory cells (12–13)).
Figure 1.
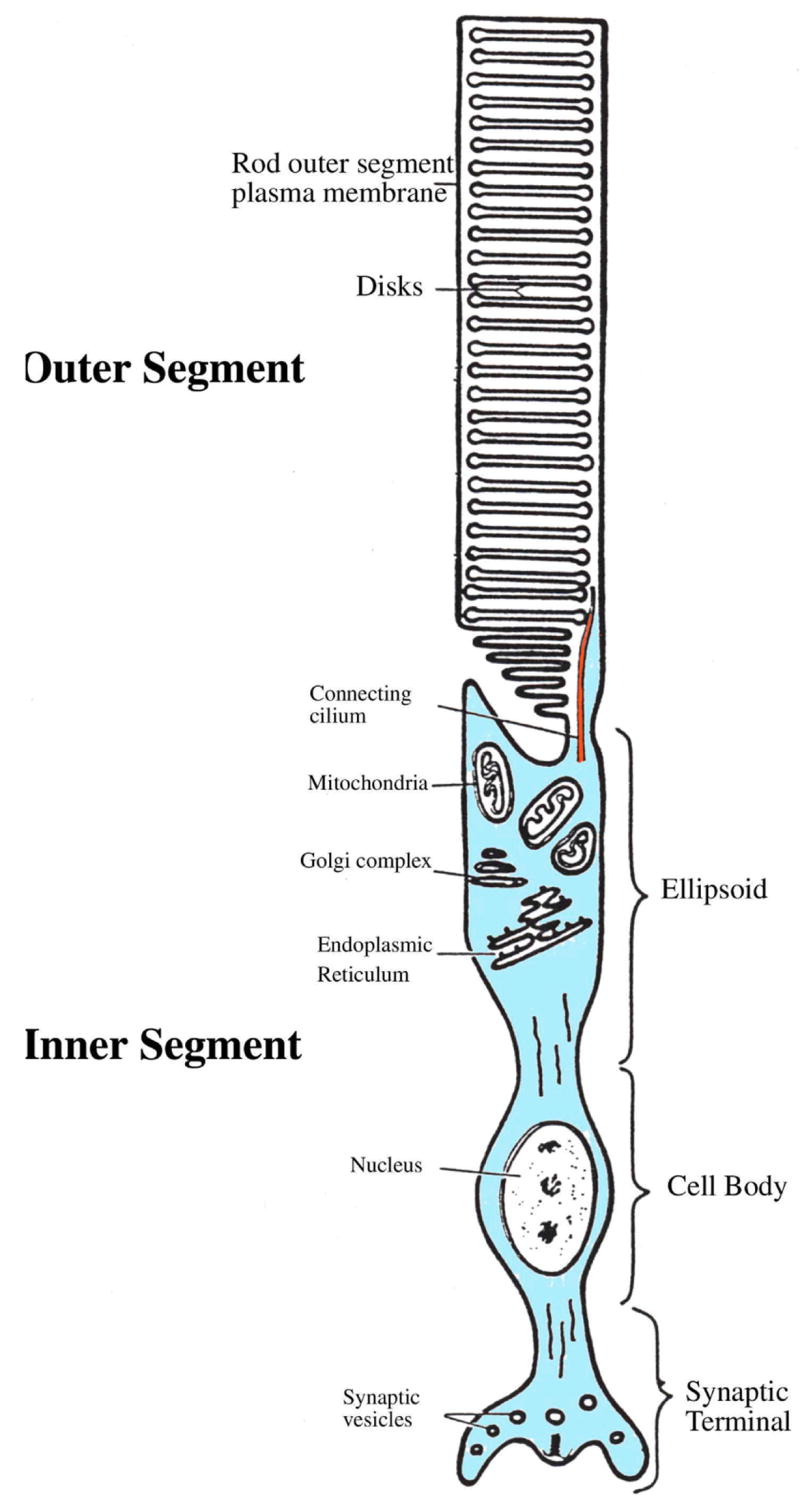
Schematic structure of a rod photoreceptor. The cell is composed of an outer segment which is connected to the inner segment via a thin connecting cilium. The outer segment is filled with disks which contain the visual pigment rhodopsin. The inner segment is composed of an ellipsoid (containing the mitochondria and the endoplasmic reticulum), the cell body and the synaptic terminal. Adapted from (189).
The OSs and ISs are composed of different sets of membrane lipids and proteins. As a result, intracellular signaling pathways and the time course of the light response are very different in both regions of the photoreceptor (see below). OSs and ISs also differ with respect to Ca2+ regulation. Although in darkness both regions are flooded with calcium (14–15), recent experiments have shown that the mechanisms which control the steady-state [Ca2+]i in each compartment (i.e., entry, extrusion and buffering of Ca2+) consist of separate sets of proteins in both regions of the photoreceptor (Sections 4.3 and 5.3). These allow [Ca2+]i to be regulated quasi-independently in each region.
[Ca2+]i in darkness is thought to range from ~300 to ~ 500 nM. These Ca2+ levels are high enough to regulate the phototransduction machinery in the outer segment and transmitter release in the inner segment (see below) but are at the same time low enough to prevent Ca2+-mediated cytotoxicity. [Ca2+]i increases above the 500 nM threshold trigger apoptosis known to underlie many retinal and CNS diseases (16–17). Because high [Ca2+]i levels are neurotoxic (18–19) photoreceptors had to evolve efficient mechanisms for Ca2+ extrusion, buffering and Ca2+ sequestration into internal stores (7, 20–22). The kinetics of Ca2+ removal also determines the kinetics of exocytosis (22) and light responses in postsynaptic horizontal and bipolar cells. Thus light-evoked [Ca2+]i decrease in the OS and IS (14–15, 23) constitutes a main pathway by which information about light intensity is used to adjust both the sensitivity and kinetics of the phototranduction cascade as well as the sensitivity of glutamate release (23–27; see below Sections 4.1 and 6).
4. CALCIUM AND THE OUTER SEGMENT
A sketch of the biochemical machinery involved in the phototransduction process is shown in Figure 2 [for detailed recent reviews on phototransduction see (24, 26, 28)]. In darkness, the photoreceptor is continually depolarized by an influx of Na+, Mg2+ and Ca2+ through cation-selective cGMP-gated (CNG) channels. CNG channels are kept open by cGMP, the concentration of which is regulated via a dynamic equilibrium between synthesis (by a guanylyl cyclase; GC) and hydrolysis (by a phosphodiesterase; PDE). This equilibrium shifts when the visual pigment (rhodopsin in rods, iodopsin in cones) absorbs a photon, starting the phototransduction cascade (Figure 2A). The activated visual pigment activates a heterotrimeric G protein (transducin, G t) which in turn activates the PDE. The PDE cleaves cGMP into 5′-GMP. As a result, [cGMP]i falls, the CNG channels close and the influx of cations is blocked. With influx blocked, Ca2+ continues to be extruded from the OS by a Na+/Ca2+, K+ exchanger (NCKX), expressed at high density in the OS membrane, resulting in a drop in [Ca2+]i. In time, extrusion decreases and comes to be in equilibrium with the influx, resulting in a steady-state [Ca2+]i which determines the functional state of the cell, i.e., its sensitivity to light.
Figure 2.
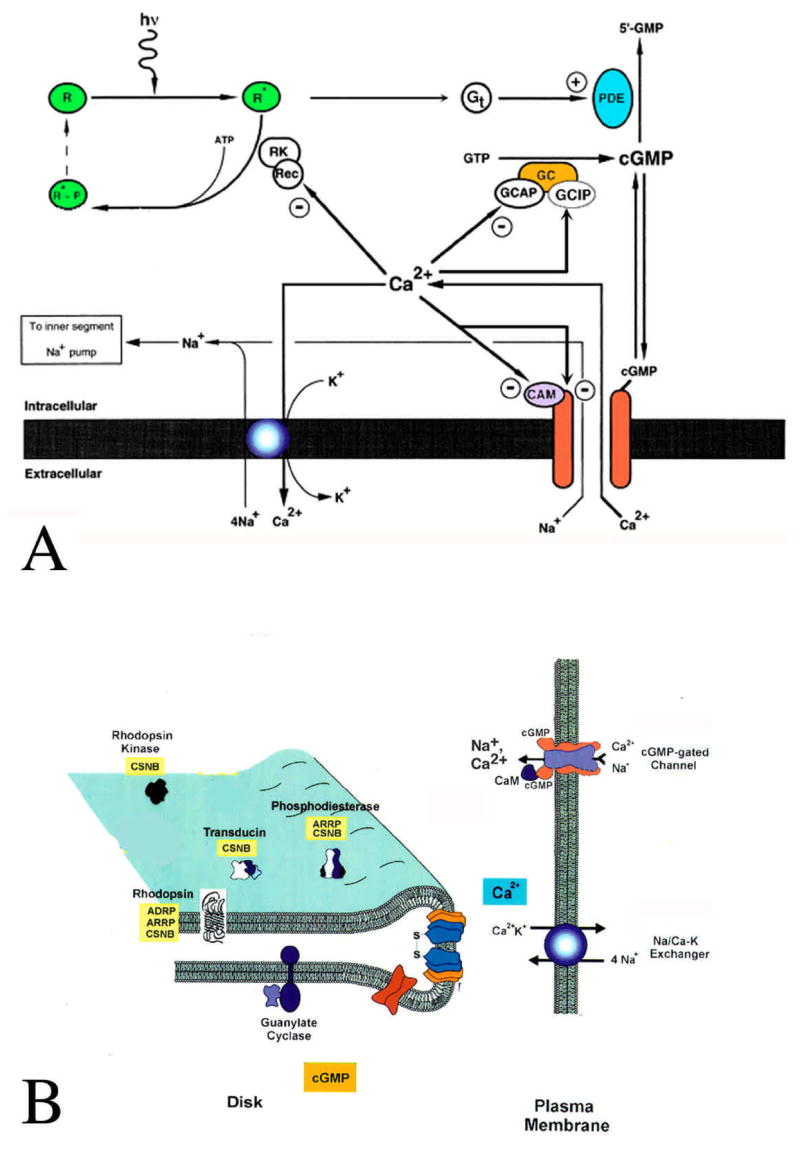
A: Schematic of calcium regulation in the OS. Ca2+ enters the OS through CNG channels and is extruded via NKCX exchangers. [Ca2+]i stimulates guanylyl cyclase (GC) via the GC activating protein (GCAP) and inhibits it via a GC inhibitory protein (GCIP). Ca2+ also inhibits the rhodopsin kinase via its action on recoverin (Rec). Moreover, Ca2+ suppresses further Ca2+ influx via its action on CNG channels, which can be direct, or via calmodulin (CAM). Adapted from (190). B: A topological model of outer segment constituents. Also indicated are retinal diseases linked to mutations in these proteins (See Section 7). ADRP autosomal dominant retinitis pigmentosa; ARRP autosomal recessive retinitis pigmentosa; CSNB congenital stationary night blindness. Adapted from (189).
4.1. The role of Ca2+ in light adaptation
Light adaptation is a regulatory process which is critical for maintaining responsiveness of the visual system to light over a wide range of illumination conditions. During light adaptation the transduction signal elicited by light is lower in sensitivity and faster in time compared to dark-adapted cells. An important stage of this process occurs in the OS and is orchestrated by Ca2+. As shown in Figure 2A, Ca2+ controls adaptation of the phototransduction machinery to background illumination at virtually every level of the phototransduction cascade (the only component of the cascade which is not directly modulated by Ca2+ is the PDE).
Unlike other guanylyl cyclases, the activity of the photoreceptor guanylyl cyclase (the synthesizing enzyme of cGMP) is controlled by Ca2+. cGMP synthesis increases as Ca2+ levels decrease with a Kd of about 70 nM (29). The modulation of GC occurs via the Ca2+ binding protein guanylyl cyclase activating protein (GCAP-1 and GCAP-2; 30). GCAPs are EF-hand Ca2+-binding proteins, each of which binds 2–3 Ca2+. In its Ca2+-bound form, GCAP is inactive (an interesting exception from most other EF-hand proteins, such as calmodulin, which are activated by Ca2+), thus the rate of synthesis of cGMP is relatively low. At high [Ca2+]i, GC is additionally suppressed by a guanylyl cyclase inhibitory protein (GCIP; 30–31). As [Ca2+]i decreases during sustained illumination, GCAPs become activated and stimulate the GC leading to an increase in [cGMP]i. Such an increase occurs in the continued presence of the background illumination. As a result of increased cGMP synthesis, CNG channels reopen, [cGMP]i increases and more intense light is required to achieve a channel closure comparable to that achieved in the dark-adapted state. Interestingly, a mutation in human GCAP-1 causes this protein to lose its inhibitory activity at high [Ca2+]i and has been linked to the autosomal dominant cone dystrophy, a blindness-causing disease (see below, Section 7).
Ca2+ has an essential role in modulation of sensitivity of the visual pigment itself. For example, the ability of rhodopsin to activate transducin is decreased through an inactivation process mediated by rhodopsin kinase. The phosphorylation reaction mediated by the kinase is in turn is inhibited by the Ca2+-binding protein recoverin (32). A decrease in [Ca2+]i, caused by continued illumination, disinhibits the kinase and results in a shorter life-time of the activated rhodopsin (33) and PDE (24). Indeed, when the [Ca2+]i was varied between 10 nM and 1 μM the activity of PDE was changed by 30-fold (24).
In addition to its effects on synthesis and degradation of cGMP, Ca2+ exerts a direct control over the cation influx through the CNG channel. The single-channel conductance of the rod CNG channel pore may be reduced 200-fold by selective binding of Ca2+ to acidic residues in the pore region (34). This effect has been reported for high, nonphysiological levels [Ca2+]i. At lower, physiological [Ca2+]i, Ca2+ regulates CNG channel permeability via the Ca2+ effector calmodulin (CaM). Calmodulin binds to the β-subunit of the CNG channel, shifting the dose response curve to higher Ca2+ concentrations (35). Consequently, at the high [Ca2+]i that occurs in dark adapted photoreceptors, the affinity of the channel for cGMP is low. As [Ca2+]i decreases during sustained illumination, Ca2+ and CaM dissociate from the channel, and the affinity of the CNG channel for cGMP is increased (35). This effect is much more pronounced for cone CNG channels than rod (192; see Section 4.2). Thus, in light adapted cells, the number of open CNG channels at a given light intensity can be much higher compared to the same stimulus applied when cells are dark-adapted. As the photoreceptors adapt to light, the amplitude of the photoresponse and the light sensitivity decrease. Both of these adaptive responses result from Ca2+ action on the visual pigment and the phosphodiesterase (24). Even more pronounced Ca2+-mediated modulation of a sensory transduction cascade occurs in the olfactory CNG channel (36).
The role of Ca2+ in light adaptation has been confirmed conclusively. When cells are superfused with Ca2+ -free solutions, or [Ca2+]i is kept constant with high concentrations of Ca2+ buffers such as BAPTA and/or by blocking calcium extrusion from the OS, light adaptation is blocked (27, 37). Interestingly, constant [Ca2+]i had virtually no effect on the rising phase of the photoresponse, indicating that Ca2+ has little effect on the initial steps in the phototransduction cascade (26–27, 37).
Having been independently measured, the properties of all phototransduction pathways were incorporated into a quantitative model in order to predict the relative contribution of different Ca2+-controlled pathways on light adaptation of the rod photoreceptor (24). The model shows that, at low light levels, the effect of Ca2+ on cGMP synthesis predominates by regulating the sensitivity of the cell whereas, at high light levels, modulation by Ca2+ of the kinase is more important and functions to extend the operating range of the photoreceptor. These early experiments demonstrated that changes in [Ca2+]OS serve as the intracellular signal for light adaptation. Ca2+ therefore acts as a global integrator by coordinating the activity of many different signaling pathways within the OS.
4.2. Ca2+ homeostasis differs between outer segments of rods and cones
Light flashes which generate equal photocurrents tend to cause a larger change in cytoplasmic Ca2+ in cones than in rods. During light stimulation, Ca2+ concentration in the cone OS will vary over a 75-fold dynamic range in red-sensitive cones, a value more than three times greater than in rods of the same species (9). The percentage of the dark current carried by Ca2+ is about twice as high in cones (35%) than in rods (~20%; 38–39). Moreover, Juan Korenbrot and his colleagues have shown that in cones, but not in rods, the cGMP sensitivity of the CNG channels changes with physiological concentrations of [Ca2+]i (38–39). Ca2+ modulation in cones has a midpoint at ~290 nM [Ca2+]i, whereas in rods the midpoint is ~50 nM, i.e., at the borderline of the physiological range which occurs in light-adapted cells only (15, 26, 192). In addition to more Ca2+ entering into the cone OS relative to the rod OS, cone OSs are also more efficient at Ca2+ extrusion. Extrusion of Ca2+ is 4–8 times faster in cones compared to rods (7–9). This is consistent with a much faster and efficient light adaptation in cones compared to rods. Interestingly, despite dramatic differences in rates of Ca2+ influx and clearance from rod and cone OSs, the Ca2+-dependence of the GC and phosphorylation of the visual pigment are not appreciably different (33).
4.3. Ca2+ extrusion and buffering in the outer segment
Ca2+ is extruded from the outer segment by Na+/Ca2+, K+ exchange. Na+/Ca2+, K+ exchangers (NCKXs), cloned from photoreceptor OSs, belong to a unique transporter family, distinct from the more generic Na+/Ca2+ exchangers (NCXs) found in the majority of other neurons. Photoreceptors express the NCKX1 isoform (40–41). The cloning of NCKX1 from rat eye (42) revealed the presence of alternatively spliced isoforms in this species, which differ in the arrangement of four exons at the N terminus of the large intracellular loop. Another isoform was recently isolated from cones (43). The NCKX1 transporters are expressed exclusively in the OS, with no trace found in the IS (40–41). The NCKX transporter exchanges 4Na+ for one Ca2+ and one K+ (44–45). Owing to the additional free energy of transport contributed by the K+ gradient, the theoretical thermodynamic equilibrium concentration in the OS Ca2+ should be ~500 times lower compared to other cells which express the NCX exchanger. However, studies have shown that the true equilibrium (~ 0.2 nM) is never reached, because the exchanger is inactivated at low [Ca2+]i. Ca2+ extrusion will continue to a baseline concentration of 20–50 nM but goes no further (46). The affinity (Km) of the NCKX exchanger for Ca2+ ranges from 1 to 2 μM (46–47). NCKX has a high capacity for Ca2+ transport: Na+/Ca2+, K+ exchange caused a decline in [Ca2+]i at a rate of 30 μM/s. This rate translates into flux of 250 pmol cm−2 s−1 (2500 times higher than in the squid axon; 48). Interestingly, it has been recently discovered that the Na+/Ca2+, K+ exchanger binds the α subunit of the cGMP-gated channel, forming a stable complex (49).
[Ca2+]i in the OS is also regulated by intracellular mobile and immobile buffers. In an intriguing study, Leibovic and Bandarchi (50) found that there is a gradient of buffered Ca2+ along the long axis of the OS and that Ca2+ extrusion near the tip of the OS is almost three times higher compared to that at the base. The low longitudinal diffusion coefficient for Ca2+ in the OS (~2 μm2s−1; 4) is consistent with the diffusional barrier presented by the intracellular disks. The diffusion coefficient for radial diffusion (~15 μm2s−1; 3) is, on the other hand, consistent with binding of Ca2+ to immobile buffering systems.
Intracellular Ca2+ buffers and sensors are thought to bind as much as 99% of Ca2+ which enters the cell through CNG channels (47, 51). Their action seems to be essential for cell survival, because mutations in genes encoding different photoreceptor Ca2+-binding proteins are linked to several human pathologies leading to blindness (52). During the light response, Ca2+ buffering slows the relaxation in free OS [Ca2+], and may therefore affect the onset of light adaptation. Indeed, the faster time constant of light-induced changes in cone [Ca2+]i compared to the time course of [Ca2+]i changes in rods (9) could be interpreted to indicate that the Ca2+ buffer capacity of rod outer segments significantly exceeds that in cone outer segments. Unfortunately, relatively little is known about the intrinsic buffering capacity of photoreceptors and the physiological roles played by Ca2+ buffers in intact OSs of rods and cones. The available data from Lagnado et al. (47) suggest the buffering capacity of salamander rod OSs to be ~ 0.24 mM. Other estimates of OS Ca2+ buffering power range from 0.1 to 5 mM (53). The early experiments in salamander rods suggested that the OS cytoplasm contains a high and a low affinity buffer, respectively. The high Ca2+ affinity buffer could be washed out with a prolonged perfusion (47); therefore it is likely that this buffer corresponds to a small, soluble Ca2+-binding protein such as parvalbumin, calbindin, calretinin, recoverin, visinin, calmodulin and/or GCAP proteins which have all been reported to be present in the OS (52). The high affinity buffering of Ca2+ would be especially important in light-adapted cells in which [Ca2+]i is low and in which small [Ca2+]i changes would have significant effect on function of the phototransduction cascade. The second Ca2+ buffering mechanism identified by Lagnado et al. (47) consisted of a low affinity high capacity buffer. This system may absorb much of Ca2+ entering the OS in the darkness.
In addition to plasma membrane Ca2+ influx and extrusion, [Ca2+]i in the OS may also be regulated by Ca2+ release and uptake from internal compartments, such as the stack of disks which fill the rod OS (54–57). Early studies suggested that the disks can accumulate significant amounts of Ca2+. The mechanism that sequesters Ca2+ into the disks is still unknown. Preliminary evidence suggests sequestration may be due to the superfamily of Ca2+ ATPases (58); it will be of great interest to determine whether these putative Ca2+ ATPases belong to one of the principal two Ca2+ ATPase families, PMCAs or SERCAs (see below), or whether they belong to a novel as yet undescribed transporter family. Nonetheless, Ca2+ stored in disks can apparently be released in response to light stimuli (55). Light flashes were also shown to release IP3, a well-known trigger of Ca2+ release from intracellular Ca2+ compartments (1), from bovine OS membranes (59–61). These experiments suggest that rod OS disks function similarly to intracellular Ca2+ stores found in many other neurons. IP3 is generated by phospholipase C (PLC) which hydrolyses the membrane phospholipid phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (1). Substantial PLC activity is reportedly characteristic of rod disks (62) as well as of the cone OS membrane (63). The PLC expressed in OSs is apparently under control of both light (59–60) and Ca2+ (64). Moreover, immunocytochemical and EM studies have shown that photoreceptors express IP3 receptor mRNA (65). IP3 receptor protein was shown to be expressed in rod disks as well as in the plasma membrane of cones (65–66). Moreover, activation of IP3 receptor was shown to release Ca2+ from disks in bovine rod OSs (46, 57). Given that IP3 receptors tend to be expressed in intracellular compartments (1), their localization to the cone plasma membranes (65) is surprising. The functional significance of IP3 receptors in the OS remains to be elucidated.
Genetic elimination of PLC from mammalian rods results in a decrease in the light response of these cells. Measurements of light responses from PLCβ4 knockout mice showed that the a-wave component of the ERG (thought to reflect the compound light response of retinal photoreceptors) is four times smaller than the wild type control (67). Paradoxically, recordings from single rods exhibited little difference from their wild type littermates (67), suggesting that the major role of PLC may be in controlling synaptic function rather than phototransduction per se. At the moment, therefore, the roles of PLC and Ca2+ stores in OS disks are still unresolved. In particular, it remains to be determined whether sequestration and release of Ca2+ from rod disks occurs under physiological circumstances and what role, if any, do these signaling pathways play in OS signaling. For example, it would be interesting to determine whether photopic vision in IP3R1 knockout mice, which exhibit severe ataxia and epileptic seizures, is compromised. Possibly, an important clue for the role of Ca2+ stores in OS function, may arise out of the extensive work on invertebrate phototransduction. For example, both IP3 and ryanodine receptors/channels may play important roles in invertebrate photoreceptor transduction processes (68–69).
In conclusion, steady-state [Ca2+]i in the OS is determined by the balance between the influx of Ca2+through the CNG channels and its extrusion from the cytoplasm by the Na+/Ca2+, K+ exchanger. In the dark, the cytoplasmic Ca2+ concentration in both rods and cones is ~ 300–500 nM (8, 14–15). When CNG channels close following illumination, the influx of Ca2+ stops but the extrusion continues, leading to a decrease in [Ca2+]i the magnitude of which depends on the fraction of channels closed. In saturating light [Ca2+]i can decrease to 20–50 nM in rods and < 10 nM in cones (8, 14–15, 20). Photoreceptor OSs also contain significant internal Ca2+ stores whose function in the phototransduction and/or light adaptation is as yet unknown.
5. Ca2+ REGULATION IN THE INNER SEGMENT
The inner segment (IS) is the output compartment of rods and cones. It plays an important role in photoreceptor signaling by filtering and modifying the electrical signal generated in the outer segment (10, 70). The IS also functions as the command center for metabolism, transcription, protein synthesis and synaptic signaling in rods and cones. Despite these critical roles played by the IS, it has received much less attention compared to the OS. Understanding how the IS and its subcompartments coordinate and regulate the wide multiplicity of cellular functions and events is a major task awaiting retinal research.
Anatomically, the IS is composed of several distinct regions: the cell body (the site of gene expression), the “ellipsoid” region (the center of cells’ energy metabolism), the myoid (contains cytoskeletal elements which regulate contractility of the IS in teleost fish) and synaptic terminal (where exocytosis occurs) (see Figure 1). Coordinating the function of these regions are fluxes of ions across the plasma membrane and several arrays of second messenger mechanisms, which include cAMP, cGMP, NO, IP3 and Ca2+. As opposed to the OS which only possesses a single class of ion channel (the cGMP-sensitive photochannel), several different classes of ion channels are localized to the inner segment plasma membrane. These include voltage-activated Ca2+, K+ and Cl− conductances and the inward rectifier I h (reviewed in 70). Cl− and K+ currents, activated by Ca2+, further limit the depolarization produced by cation influx in the OS (53, 71). The membrane potential across IS plasma membrane therefore represents a dynamic equilibrium between ICa and a variety of outward conductances, including calcium-activated IClCa and IKCa. The IS plasma membranes also contains high densities of other ion transporters absent from the OS. These include Na+/K+ ATPases, Ca2+ ATPases and Na+/H+ exchangers (72–73) as well as still unindentified chloride transporters.
The metabolic and transcriptional activities of photoreceptors are vastly different in light and in darkness (74–75). Light-dependent integration of metabolism and signaling occurs via second messengers such as Ca2+ and NO. Many key Ca2+-dependent cellular enzymes (including Ca2+-dependent protein kinases, phosphatases, lipases, the nitric oxide synthase and nuclear transcription factors) are localized to subcompartments within the IS such as the cell nucleus, the ER and the mitochondria. Within the IS, Ca2+ may activate CaM kinases and the protein kinase A, which could phosphorylate important targets within the synaptic terminal, such as phosducin, protein 14-3-3 and Ca2+ ATPases.
OSs and ISs possess different sets of plasmalemmal mechanisms which govern Ca2+ influx and extrusion (6, 20, 24, 38, 41, 65). Moreover, they differ with respect to cytoplasmic compartments: the endoplasmic reticulum (ER) and mitochondria, two important regulators of [Ca2+]i which are abundantly present within the IS, are absent from the OS. The differences in ion influx and extrusion mechanisms between OSs and ISs are especially illustrative when one compares transport of Na+ and Ca2+ ions. Transport and diffusion of Ca2+ between OS and IS appears to be limited. The signaling action of Ca2+ may be restricted to the level of nanodomains and microdomains rather than tens of microns. Ca2+ imaging studies suggest that as [Ca2+]i changes in one compartment, [Ca2+]i changes in the other compartment are relatively small (20, but see 76). Moreover, biophysical properties of Ca2+ diffusion and the spatial distribution of Ca2+ influx/extrusion mechanisms further suggest that, with respect to Ca2+ fluxes, both compartments are relatively insulated from one another. For example, the cilium, which restricts movements of cytoplasmic and plasmalemmal constituents between OS and IS (72), may hinder diffusion of free Ca2+. The cilium is packed with specialized Ca2+ binding proteins such as centrin, calmodulin, kinesin II and myosin VII (e.g., 77) and may contain specialized Ca2+ extrusion machinery (DK, Emanuel Strehler and DRC, manuscript in preparation). In contrast, the early work by Hagins demonstrated that Na+ diffuses freely between the OS and the IS; the large majority of Na+ entering the OS via the CNG channels is extruded via Na+/K+ ATPases localized to the IS.
5.1. L-type calcium channels are the main mode of Ca2+ entry from extracellular space
It is now firmly established that Ca2+ enters the ISs mainly through voltage-gated Ca2+ channels. Ca2+ influx though these Ca2+ channels is required for vision –mutation of the Ca2+ channel gene results in blindness, most likely because release of the neurotransmitter glutamate is impaired in the absence of Ca2+ influx (78). Putative Ca2+ channels were first observed with freeze-fracture studies in mammalian and reptilian photoreceptors. The pioneering studies by Elio Raviola (79–80) showed clusters of particles inserted into the plasma membrane at the active zone in photoreceptor synaptic terminals; these investigators were the first to suggest that these particles corresponded to calcium channels. The localization of voltage-gated Ca2+ channels to photoreceptors was later demonstrated directly by intracellular recording from photoreceptors in intact retinae (81). The early intracellular recordings by Fain and coworkers (81) were later extended by many other studies, using patch electrodes (71, 82–84), intracellular recording (85) and glutamate measurements (86). Ca2+ influx through voltage-gated Ca2+ channels was shown to be both necessary and sufficient for triggering glutamate release (86–87). Recent immunocytochemical and Ca2+ imaging studies suggest that voltage-gated Ca2+ channels in rods and cones are found at highest density in synaptic terminals (20, 88).
The operating voltage range of rods and cones is compressed between ~−40 mV in the darkness to ~ −65 mV in saturating light. Ca2+ influx can control transmitter release over the full range of these potentials (23). The requirement for Ca2+ to sustain transmitter release and the need for tonic depolarization in darkness implies, however, a potentially lethal physiological trap. By definition, the high voltage-gated Ca2+ current is an inward, self-regenerative current activated at depolarized potentials. Activation of the inner segment ICa might therefore be expected to trigger a self-regenerative cycle of Ca2+ influx and depolarization which would result in impaired photoreceptor function and, possibly, cell death (see below, Section 7). Indeed, when photoreceptors are depolarized beyond their resting potentials by replacing extracellular Ca2+ with Ba2+ or Sr2+, which traverse Ca2+ channels but do not inactivate them, the cells respond with oscillations caused by regenerative activation of Ca2+ channels (81, 85). This problem is illustrated in Figure 3. When the rod photoreceptor is pushed into the depolarized range by Ba2+ substitution, its membrane potential will oscillate at a frequency of ~ 3–4 Hz. The oscillation in the photoreceptor voltage causes a profound change in synaptic transmission. By recording simultaneously from postsynaptic horizontal cells it was possible to show that photoreceptor oscillations are transmitted across the synapse (85). This experiment suggests that, in order to maintain normal photoreceptor function it is crucial to curtail Ca2+ influx by keeping the resting membrane potential of the photoreceptor IS just below the threshold for regenerative activation of Ca2+ channels. We now know that the Ca2+ influx is controlled via many mechanisms, including Ca2+ channel inactivation via Ca2+ release from internal stores and activation of outwardly rectifying Ca+-activated Cl− and K+ channels. These channels are often colocalized with the Ca2+ channels (89), so that their activation state can rapidly follow the changes of [Ca2+] associated with Ca2+ influx. The outward current flowing through Cl− and K+ channels thus acts to clamp the membrane potential via fluxes of Cl− and K+ and sets the upper upper limit to which local [Ca2+]i can rise. This prevents both Ca2+ spiking and Ca2+-mediated toxicity.
Figure 3.
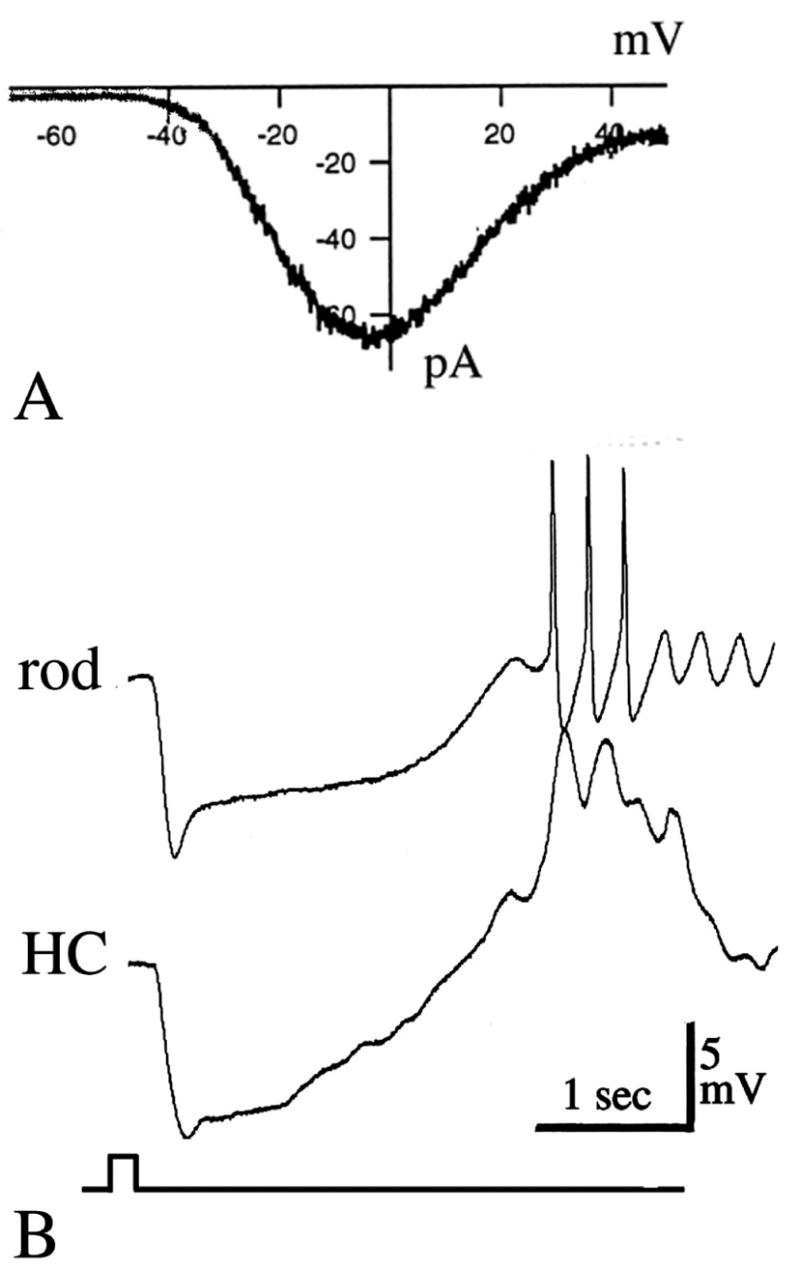
Signal transmission across the photoreceptor synapse is determined by the properties of voltage-activated Ca2+ current in photoreceptors. A: Voltage-gated Ca2+ current from salamander rod photoreceptor recorded in whole-cell patch configuration. The cell was voltage-clamped at −65 mV and depolarized with a voltage ramp from −70 to + 50 mV. The Ca2+ current activates at around −55 mV and reaches its peak at −10 mV (ref. 21). B: The membrane potential of rod photoreceptors oscillates following removal of Ca2+ channel inactivation. Simultaneous intracellular recording of light responses from a rod and a horizontal cell (HC) in the Xenopus retina. [Ca2+]o was substituted by [Ba2+]o. Under these conditions the rod depolarized by ~5mV and the membrane potential started to oscillate at ~ 3Hz. Note the concomitant change in the waveform of the light response of the horizontal cell (ref. 85).
5.1.1. Properties of voltage-gated Ca2+ channels in the inner segment
As in other neurons, the ion pores of photoreceptor Ca2+ channels are blocked by high extracellular levels of divalent cations such as Cd2+ and Co2+ and by various pharmacological antagonists (25, 84, 86–87, 90). Immunocytochemistry and electrophysiology in amphibian and mammalian photoreceptors suggest that voltage gated Ca2+ channels in the ISs belong to the L-type class of high voltage-gated Ca2+ channels (82, 86–87, 91–92). For example, these channels were found to be inhibited by dihydropyridine blockers such as nifedipine and nimodipine (22, 84, 86). However, closer analysis reveals that these channels are distinct from the “generic” L-type Ca2+ channel (84, 86, 91). Ca2+ channels found in rods and cones tend to be less sensitive to dihydropyridine antagonists such as nifedipine (84, 93). Cone Ca2+ channels can also be blocked by high concentrations of omega-conotoxin (84), generally considered as a N-type Ca2+ channel antagonist.
The voltage-gated Ca2+ current (ICa) in rods and cones operates in a sustained manner over the voltage excursion produced by light. The ICa in photoreceptor inner segments does not inactivate at holding potentials at which the L-type I Ca of other neurons is largely inactivated (83–84, 94) but it can be inactivated by Ca2+ itself (DK, DRC, unpublished observations). The activation threshold for ICa in amphibian photoreceptors is around −55 mV (21–23, 53, 83) and in mammalian photoreceptors the threshold may be as low as −60 mV (91, 93). These values are much lower compared to a “typical” L-type Ca2+ channel (95). Due to this voltage-sensitivity of photoreceptor voltage-gated Ca2+ channels, the IS cytosol experiences a large influx of Ca2+ at the photoreceptor resting potential (~−40 mV) (22–23).
Voltage-gated Ca2+ channels are protein complexes consisting of a pore-forming alpha1 subunit, whose gating is modulated by additional beta and gamma/delta subunits. Several different alpha1 subunits have been cloned; 1C, D, F and S all form 1,4-dihydropyridine (DHP)-sensitive L-type calcium channels, whereas 1A, B, and E, respectively, form P/Q, N, and possibly R channels (101). Recently, a novel subtype of the alpha subunit was isolated from human rods (alpha1F; 96). These subunits appear to be localized at the active zones of rod photoreceptors (97). These are likely to support synaptic transmission at the rod synapse, as a mutation in the gene for this rod Ca2+ channel has a selective effect on rod neurotransmission by causing a severe form of the stationary blindness (78). Since rod IS is composed of several different functional compartments, it will be of interest to examine whether different Ca2+ channel subunits are expressed in different regions of the IS. Unlike rods, which apparently possess the alpha1F subunit, cone IS Ca2+ channels may be of the alpha1D subtype. Unlike alpha1F, the 1D subtype has been found in many other classes of neuron. Red-sensitive cones in the tree shrew retina are immunopositive for the alpha1D antibody whereas blue-sensitive cones are not and may possess another currently unknown subtype of Ca2+ channel (98–99).
5.1.2. Modulation of voltage-gated Ca2+ channels in the photoreceptor inner segment
Because of the key role that L-type Ca2+ channels play in sustaining glutamate release at the photoreceptor synapse, their modulation is likely to emerge as one of the important regulators of visual function. The IS Ca2+ channels are modulated by a number of different mechanisms, which include both membrane-delimited and second-messenger pathways. These involve Ca2+ acting via calmodulin, phosphorylation via cGMP- and cAMP-dependent pathways, facilitation by voltage and inhibition through the activation of heterotrimeric G proteins. G protein-mediated modulation of these channels is probably responsible for the presynaptic inhibition of synaptic transmission mediated by a wide variety of 7 transmembrane-domain receptors (such as D2 dopamine receptors and somatostatin receptors) (100, 102–108). Modulation of the Ca2+ current is therefore likely to affect synaptic gain. The gain function (which depends on the slope of the Ca2+ current vs the membrane potential) for the photoreceptor synapse is the steepest around the resting potential (i.e., around −40 mV; (see 23, 101)). This, therefore, is where neuromodulators should be the most effective. Among neuromodulators, shown to control I Ca at the photoreceptor synapse are the following:
5.1.2.1. Calcium regulation of ICa
Extra- and intracellular Ca2+ regulates I Ca via different mechanisms. Extracellular divalent cations, including calcium, cobalt and cadmium, screen charges on the extracellular surface of the membranes thereby shifting the activation range of I Ca to more positive potentials with increasing concentrations (123). This screening action explains the paradoxical finding that lowering external calcium can unexpectedly increase synaptic signaling from photoreceptors to second order cells (124). Calcium-induced inactivation of photorecpetor L-type Ca2+ channels can also regulate I Ca from the cytoplasmic side (DK, unpublished observations). This action can be by direct binding of Ca2+ to a modulatory binding site on the L-type channel or via calcium activation of calmodulin. Little has been reported on the extent by which I Ca and intracellular calcium in photoreceptors can be regulated by either of these two mechanisms. Preliminary work in our own lab has demonstrated that release of Ca2+ from intracellular stores can control the magnitude of ICa (21) as well as that Ca2+ influx itself causes inactivation of ICa (unpublished observations). This suggests that the sustained influx of calcium, which is maximal in the dark, can control subsequent influx via a feedback mechanism. Thus calcium concentration is maintained in a state of dynamic equilibrium.
5.1.2.2. Dopamine
Rods and cones immunostain for D2/D4 dopamine receptors (102). Retinal dopamine, known to be upregulated in light (103–104), exerts multiple effects on photoreceptors including the retraction and elongation of fish rods and cones, respectively; suppression of rod-driven light responses to second order cells and electrical coupling between rods and cones (103–105). Dopamine also modulates Ica in photoreceptors. D2 receptor agonists, but not D1 agonists, inhibited Ica in a class of red-sensitive, large single cones but facilitated ICa in all other classes of cone and in rods (106). These results suggested a pathway by which D2 receptors couple negatively to adenylyl cyclase, the synthesizing enzyme for cAMP, to regulate calcium channels in the IS. However, there is a still unresolved discrepancy between the actions of dopamine on photoreceptors. The studies of synaptic transmission strongly suggest that signaling at the cone synapse is potentiated by dopamine whereas signaling at the rod synapse is decreased (104, 107). In contrast, ICa recordings (106) suggested dopamine decreases the calcium current in some cones but increases it in rods. One explanation for the discrepancy is that Ca2+-dependent pathways in (106) were suppressed by substituting Ca2+ with Ba2+. In the presence of Ca2+, dopamine and D2 agonists may act to reduce [Ca2+]i (107).
5.1.2.3. Somatostatin
Akopian et al. (108) found that both rods and cones in salamander retina immunostained with an antibody to the sst(2A) receptor. Whole-cell recordings from individual cells revealed that somatostatin reduced L-type calcium currents in rods but increased them in cones. Calcium imaging showed that somatostatin reduced potassium-evoked rises in intracellular calcium in rods but enhanced them in cones. The action of somatostatin on Ica was blocked by pertussis toxin, implicating a G-protein pathway coupling between the somatostain receptors and the L-type calcium channels.
5.1.2.4. Adenosine
In the retina, production of adenosine is elevated in darkness, possibly as a result of the dramatic turnover of ATP in the IS (74, 109). Adenosine is released by depolarized photoreceptors (109) and may be taken up into photoreceptors by adenosine transporters. Autoradiography and in situ hybridization suggest that A1 and A2 adenosine receptors are expressed in photoreceptors (110–111). Activation of these receptors was recently suggested to regulate retinomotor movements in lower vertebrates (112). One possible mechanism of action would be an adenosine A2 mediated inhibition of Ca2+ influx via the L-type calcium channels in cone (83) and rod inner segments (113).
5.1.2.5. Protons
Extracellular alkalinization and acidification regulates how calcium channels are activated by voltage. Barnes et al. (114) showed that between pH 7 and 8 the activation of I Ca shifts by ~ −12 mV. At resting potential, an outside acidification would reduce calcium influx through ICa. The authors confirmed functional consequences of the pH modulation by demonstrating that light-evoked synaptic currents in neurons postsynaptic to the photoreceptors were diminished at lower pH. A 2.3-fold change in postsynaptic signals occurred with a pH decrease of 0.23 pH units. In addition to the sensitivity of I Ca to extracellular pH, studies of L-type calcium channels in other retinal neurons suggest that intracellular pH can modulate photoreceptor Ca2+ channels as well. Takahashi et al. (115) showed that intracellular alkalinization and acidification of catfish horizontal cells enhanced and reduced, respectively, L-type calcium currents. It is very plausible to assume that changes in pH within photoreceptors due to metabolic activity, ATP hydrolysis, regulation of proton extrusion or transport of calcium via PMCA (20) could in turn regulate calcium influx. DeVries has recently shown that protons, released from synaptic vesicles during exocytosis, may feed back to suppress Ca2+ influx across the voltage-gated Ca2+ channels (115a and to move the activation voltage of the Ca current to coincide with the cone resting potential (114, 115a). The action of protons is potentiated by the absence of carbonyc anhydrase from the cleft at the photoreceptor synapse.
5.1.2.6. mGluR modulation
Metabotropic glutamate receptors are known to regulate calcium in neurons. Recently, mGluR8, a class III metabotropic receptor was immunolocalized to mammalian photoreceptors (116). Calcium imaging in isolated photoreceptors showed that glutamate and class III metabotropic agonists lowered resting calcium levels. This action was blocked by class III antagonists and was not mimicked by agonists for other types of metabotropic or ionotropic glutamate receptors. These findings raise the possibility that glutamate released from photoreceptor terminals can act through feedback to the presynaptic mGluR8 receptors to down-regulate the level of glutamate release. Intracellular signaling pathways for mGluR8-mediated actions have not yet been identified.
5.1.2.7. Chloride ion modulation
An investigation of ICa in salamander photoreceptors revealed that low extracellular chloride reduces current through Ca2+ channels (117) and hyperpolarizes second order retinal neurons, consistent with lowered intracellular calcium diminishing the release of glutamate from the rods and cones. Lowering extracellular calcium caused a concomitant reduction of intracellular chloride. Patch recordings demonstrated that intracellular chloride modulated the open probability of ICa in photoreceptors (117). However, little is known whether intracellular chloride is likely to change enough in vivo to regulate intracellular calcium in photoreceptors.
5.1.2.8. Nitric oxide modulation of ICa
Nitric oxide (NO) is a short-lived gas generated by nitric oxide synthase (NOS). NO freely diffuses across lipid membranes and is known to exert modulatory actions on ionic channels of many cell types including neurons. The neuronal isoform of the nitric synthase, the key enzyme in NO synthesis (nNOS) is immunolocalized in or near the synaptic terminals of rods and cones, providing an immediate local source of NO (118–119). Electrophysiological examination of ICa in tiger salamander rods demonstrated that application of an NO-generating compound increased the current (120). This suggests that stimulation of NOS could lead to an increase of calcium within rods, and perhaps in cones as well. It remains to be shown whether NO levels are high enough in vivo to affect changes in ICa. NOS is localized in the ellipsoid region of the IS and shows dramatic dependence on free [Ca2+]i (EC50 ~ 0.84 μM; 121). NO synthesis will therefore depend directly on Ca2+ influx into the IS and is likely to have little effect on cGMP synthesis in outer segments. NO is likely to influence synaptic transmission via its effect on the soluble form of guanylyl cyclase and [cGMP]i levels in the cell body and the synaptic terminal (121–122). NO released from photoreceptor ISs could also diffuse to ON bipolar cells and activate their guanylyl cyclase within a diffusion distance of ~100 microns (121).
5.1.2.9. Cannabinoids
Photoreceptors in both mammalian and non-mammalian species bind antibodies directed against the CB1-cannabinoid receptor. (125–126). Although there are no reports of cannabinoids regulating intracellular calcium or ICa in photoreceptors, cannabinoid agonists reduced ICa in retinal bipolar cells, implicating an inhibitory effect on calcium-dependent synaptic transmission.
5.1.2.10. Insulin
Both inner and outer segments of photoreceptors express receptors for insulin. Insulin itself causes a decrease in both the a- and b-waves of the electroretinogram (127). Stella et al. (128) have recently shown that insulin also inhibits influx of Ca2+ into ISs of rods via the insulin receptor-mediated activation of tyrosine kinases.
5.1.2.11. Retinoids
Docosahexanoic (DHA) and arachidonic acid are major phospholipid constitutents of the photoreceptor membrane. Phospholipids are cleaved by the phospholipase 2 (PLA2) which, when activated by light, causes release of both DHA and arachidonic acid (129–130). Exposure to retinoids causes a large suppression of photoreceptor I Ca at micromolar concentrations (131).
5.1.2.12. Ephaptic stimulation
Verweij et al. (132) proposed that the feedback exerted by horizontal cells upon cones is mediated by a modulation of cone I Ca. Further work from the same group suggested that the activation voltages of ICa in photoreceptors are regulated by the extracellular current density generated by current flow across the hemi-gap junctions in the postsynaptic horizontal cells (133).
5.1.2.13. GABA
GABA is released by postsynaptic horizontal cells. Blocking of GABAA receptors with specific antagonists disrupted normal development of cone synapses (134). Activation of GABAA receptors in developing rabbit cones may increase [Ca2+]i (135) thus it is possible that GABAergic control of [Ca2+]i may regulate normal development of cone ISs.
5.2. Cyclic nucleotide-gated channels in the inner segment
Rieke and Schwartz (136) and Savchenko et al. (122) described a cGMP-dependent (CGN) current in salamander cone ISs. This current is voltage- and cAMP-insensitive (136) and its magnitude depends on [cGMP]i only. Although the physiological function of this current is not clear, it has been suggested that it controls exocytosis of synaptic vesicles at hyperpolarized levels of membrane potential at which there is a large driving force for Ca2+ influx (122, 136). Thus glutamate release from photoreceptor terminals may continue as voltage-gated Ca2+ channels close during the light-evoked hyperpolarization (122, 136). In this way, cones could use the cGMP-dependent channel to extend the operating range of the synapse beyond −45 mV. However, Maricq and Korenbrot (53) failed to observe the CNG current in lizard cone pedicles. It therefore remains to be seen if CNG channels are a ubiquitous feature of Ca2+ homeostasis in the IS.
5.3. Ca2+ extrusion from the inner segment
Because the photoreceptor light response consists of a hyperpolarization and a decrease in Ca2+-dependent glutamate release (23, 25), the properties of Ca2+ extrusion from the photoreceptor inner segment define the kinetics of the visual signal in second order horizontal and bipolar cells. Transmitter release from photoreceptors can be tuned to changes in presynaptic membrane potential as small as 10 μV (137). Such small, light-evoked hyperpolarizations are likely to be associated with minute decreases in [Ca2+]i (22). In order for the high-sensitivity coupling between [Ca2+]i and membrane potential to occur, the mechanism for extrusion of Ca2+ from ISs must possess a high affinity for Ca2+. This high affinity Ca2+ extrusion mechanism has been recently determined to be a plasma membrane Ca2+ ATPase (PMCA) (20, 73, 138). Figure 4A shows simultaneous imaging of [Ca2+]i from an OS and an IS of a rod photoreceptor. During depolarization, [Ca2+]i in both regions of the cell was raised by opening of voltage-gated Ca2+ channels, and by reversing the direction of the NCKX exchanger in the OS. As illustrated in Figure 4A, panel b, Ca2+ extrusion from the OS was blocked by removing Na+ from the superfusing solution, consistent with a dominant role for this Ca2+ extrusion mechanism in the OS (40, 46). Na+ removal had no effect on Ca2+ extrusion from the IS. In the Na+-free solution, [Ca2+]i in the IS decayed with a time constant of several seconds, demonstrating that Ca2+ extrusion from this compartment does not depend on [Na+]o (Figure 4A, panel a).
Figure 4.

A: Calcium extrusion is regulated independently in photoreceptor IS (a) and OS (b). Simultaneous [Ca2+]i measurement from salamander rods. a, b: Switch from control saline (2 mM KCl) to high KCl superfusate (90 mM KCl) raised [Ca2+]i in both segments. Immediately following high KCl, the superfusate was switched to saline in which [Na+]o was replaced by [Li+]o (0 mM NaCl, 2 mM KCl, 97 mM LiCl). The IS [Ca2+]i returned to the baseline (panel a), whereas the OS [Ca2+]i remained at a plateau in LiCl saline (panel b). [Ca2+] in the OS returned to baseline following reintroduction of Na+ (20). B: Confocal fluorescence labeling indicates photoreceptor terminals express the isoform 1 of the PMCAs. a: PMCA1 is prominently expressed in photoreceptor synaptic terminals. The labeling is moderate in bipolar cell bodies in the distal INL and weak in horizontal cells. b: Calbindin antibody strongly labels horizontal cells. Prominent somas with dendritic knobs, extending into photoreceptor terminals (arrowheads), are observed. c: Superposition of images from a and b. PMCA1 is expressed in photoreceptor terminals but is excluded from calbindin-immunopositive amacrine cells. Scale bar = 10 μm. d–f: High power fluorescence micrograph of the same section as shown in a–c with magnification focused on the OPL. Each dendritic knob emanating from horizontal cells is associated with exactly one photoreceptor terminal immunolabeled by PMCA1. OPL, outer plexiform layer; INL inner nuclear layer. Scale bar = 2.5 μm. (from 73).
The PMCA family consists of four members with 70 – 80% homology (139). The four isoforms possess different Ca2+ affinities and different consensus sites for PKA and PKC-mediated phosphorylation and are differentially distributed within brain tissues (139–140). A detailed analysis of PMCA isoform expression in mammalian retina revealed that photoreceptor ISs express isoform 1 of the PMCA family; which is most pronounced in the synaptic terminal (73). Figure 4B illustrates synaptic terminals of rod photoreceptors in the mouse retina immunostained with antibody directed against PMCA1 and against calbindin, a known marker for postsynaptic horizontal cells. As seen in Figure 4B, the photorecepor terminals are strongly immunopositive for PMCA1, suggesting an important role for this isoform in regulating synaptic transmission at the photoreceptor synapse. Immunocytochemical data demonstrated a higher density of PMCAs in the synaptic region compared to the rest of the IS (73). The large contribution of PMCAs to Ca2+ homeostasis in the synaptic terminal was confirmed by Ca2+ imaging experiments, which revealed that depolarization-evoked Ca2+ decreases are much larger and faster in the synaptic terminal than in the soma and the ellipsoid (20).
What physiological advantage do PMCAs confer to IS function? First, due to the tonically attached calmodulin (139), PMCAs possess an affinity for Ca2+ which is at least 10 times higher than the affinity of the NCK exchanger (46, 139). Second, unlike the NCKXs, Ca2+ extrusion via PMCAs is independent of the membrane potential. Therefore, PMCAs will be able to translate the magnitude of [Ca2+]i signal into proportional changes in the rate of Ca2+ extrusion and maintain the linearity of synaptic transmission at the photoreceptor synapse (23).
Intriguingly, PMCA1 in synaptic terminals of mammalian photoreceptors colocalizes with PSD-95, a membrane-associated guanylyl kinase protein (MAGUK) known to organize the neurotransmitter receptors and ion channels within the postsynaptic density (73). PDZ domain-containing proteins, such as PSD-95, are abundantly expressed in photoreceptor terminals (141–142) and could act to organize PMCAs within presynaptic macromolecular complexes.
Little evidence for the presence of Na+/Ca2+ or Na+/Ca2+, K+ exchangers was found in the IS (20, 41). This provides another characteristic which distinguishes Ca2+ homeostasis in photoreceptor terminals from synaptic terminals of the majority of other neurons where both Ca2+ extrusion systems are generally found (e.g., 143–144). PMCA-mediated Ca2+ extrusion is voltage-independent, as opposed to the action of the Na+/Ca2+ exchangers. We suggest that because Na+/Ca2+ exchange reverses at depolarized potentials, causing an influx of Ca2+ and transmitter release (e.g., 145), it is fundamentally incompatible with regulation of synaptic transmission at the photoreceptor synapse.
5.4. Intracellular Ca2+ stores in the inner segment
Most neurons store Ca2+ in intracellular organelles such as the endoplasmic reticulum (ER) and the mitochondria. ER can function as a Ca2+ source and as a sink (1, 146), depending on levels of resting [Ca2+]i, distribution of Ca2+ release mechanisms within the ER itself and configuration of Ca2+ release and sequestration mechanisms within the cytoplasm. Ca2+, stored within the ER, is released by two different families of intracellular Ca2+ channels, each of which consists of three members. One family of intracellular Ca2+ release channels is blocked by the alkaloid ryanodine (ryanodine receptors, RyR), whereas the second family includes channels that bind inositol 1,4,5-triphosphate (IP3 receptors; IP3R) (147). Each channel gene can generate a number of alternatively spliced variants which display distinctive regulatory properties, providing an additional level of complexity (148).
Photoreceptor inner segments possess several compartments capable of accumulating and releasing Ca2+, including ER (149), the Golgi apparatus (155) and the mitochondria. ER and its specializations are found in all parts of the inner segment, including the soma and the synaptic terminal (149–150). Ca2+ is sequestered into both rough and smooth ER (151–153). The density of the ER structures is highest in the subellipsoid space (the myoid), from which other organelles are effectively excluded. The myoid ER is involved in synthesis of OS and IS proteins (149). It is thought to be continuous with the smooth ER which has access into synaptic terminals (149, 151). ER often appears in the form of the so called subsurface cisternae, which are localized within 30 nm of the plasma membrane. Subsurface cisternae have been described in teleost cones (154) as well as in amphibian photoreceptors (DK and DRC, manuscript submitted). Release of Ca2+ from subsurface cisternae has been shown to modulate function of many types of ion channels in the plasma membrane (1).
Ca2+ release from internal stores sensitive to caffeine and ryanodine was described in salamander rod inner segments (21). The magnitude of caffeine-evoked Ca2+ release in rods depended critically on the steady-state level of global IS [Ca2+]. When [Ca2+]i was around 300–600 nM (the free Ca2+ concentration measured in darkness) caffeine-sensitive stores accumulated Ca2+ to several hundred nM. Caffeine triggered large transient increases in [Ca2+]i in depolarized cells but evoked only small amounts of Ca2+ release from hyperpolarized cells. This suggests that the function of Ca2+ stores will depend on the adaptational state of the cell. The role of the stores will be more significant in darkness (when baseline [Ca2+]i is high) compared to the background light (when [Ca2+]i is low). Caffeine-induced elevations in [Ca2+]i were observed throughout the rod IS but were not seen in the OS. This is similar to olfactory cells, where release of Ca2+ from stores occurs in the soma (the output compartment) but not the cilia (the signal transduction compartment) (13). The RyR isoform in photoreceptors remains to be identified.
Ca2+ release from internal Ca2+ stores may have a complex effect on intracellular Ca2+ homeostasis. Figure 5A illustrates a simultaneous [Ca2+]i recording from two salamander rod photoreceptors depolarized to ~−35 mV. Exposure to caffeine caused a large Ca2+ elevation in both cells; the elevation was followed by a prolonged [Ca2+]i undershoot. In between the two caffeine applications, one of the cells exhibited a spontaneous Ca2+ elevation, which also was accompanied by a [Ca2+]i undershoot. The observation that Ca2+ release from Ca2+ stores may trigger an elevation as well as a decrease in [Ca2+]i suggests that Ca2+ stores can act as amplifiers as well as suppressors of the steady-state [Ca2+]i. A schema showing the complex interaction between RyR localized to the ER and voltage-gated Ca2+ channels localized to plasma membrane of rods is shown in Figure 5B.
Figure 5.
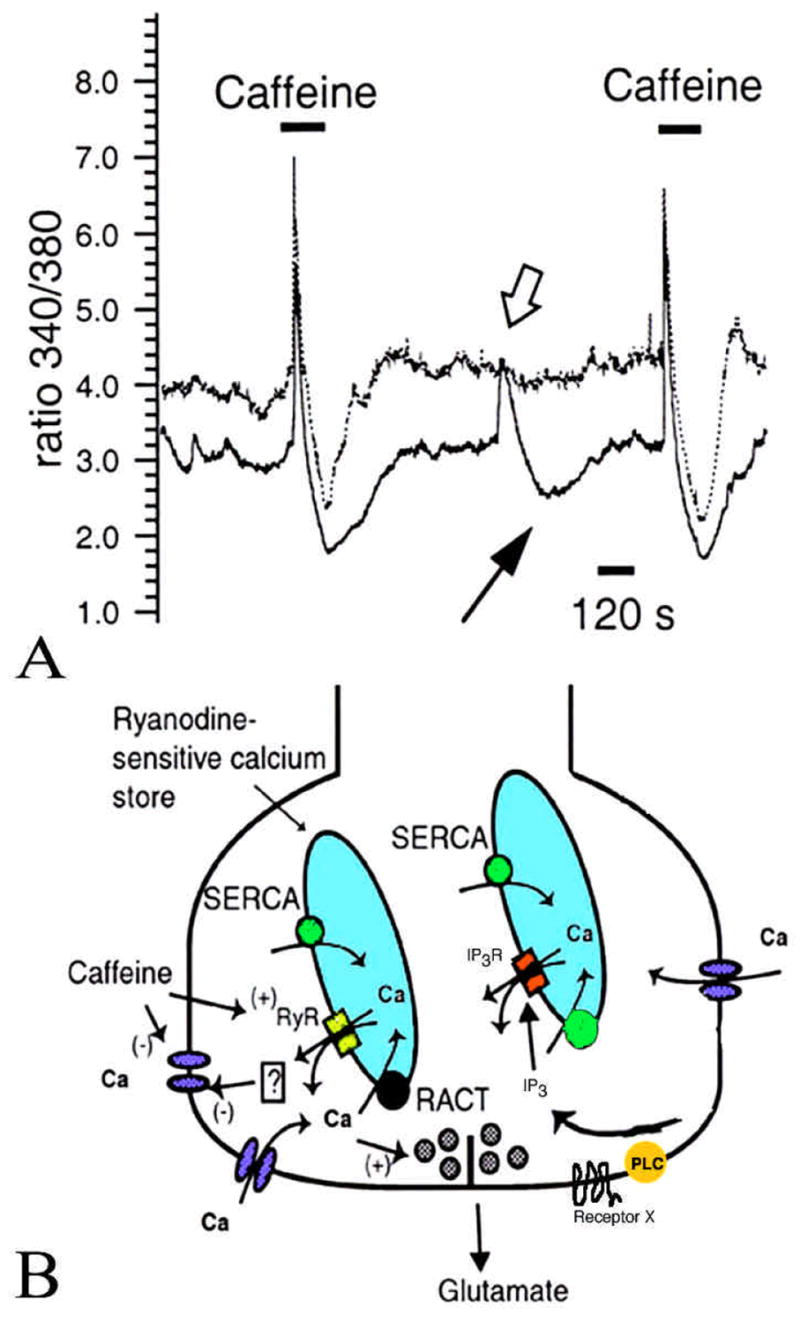
A: Caffeine evokes Ca2+ release from intracellular stores. Two rod photoreceptors were loaded with a Ca2+ indicator dye and depolarized with KCl to raise [Ca2+]i by ~ 300 nM. Exposure to 10 mM caffeine resulted in a complex [Ca2+]i response seen in both cells. The response had two parts: a [Ca2+]i increase followed by a prolonged [Ca2+]i undershoot. Note that in cell 2, a spontaneous [Ca2+]i increase occurred (open arrow) and was also followed by a [Ca2+]i undershoot (arrow). B: A model of calcium store mechanism in rod photoreceptor IS. Ca2+ enters the cell through voltage-gated Ca2+ channels and binds ryanodine receptors localized in the ER, triggering more Ca2+ release. Ca2+, released from the stores, subsequently acts to suppress further Ca2+ influx via an inactivation process. The stores are filled by two separate mechanisms – “sarcoplasmic-endoplasmic reticulum Ca2+ ATPases” (SERCAs) and a “release-activated calcium transport” (RACT) mechanism which may participate in the [Ca2+]i undershoot. The IS also possess IP3-sensitive Ca2+ stores, activated by IP3 and phospholipase C (PLC). The plasma membrane receptors which activate the PLC are still unknown (modified after 21).
Phospholipase C (PLC) which catalyzes the hydrolysis of phosphatidylinositol biphosphate PIP2 to produce the intracellular messengers IP3 and DAG, is activated by the binding of many hormones, neuromodulators and growth factors to specific receptors on the plasma membrane. Peng et al. (156) found that IP3 receptors are expressed at high density in synaptic terminals of both rods and cones. In contrast, isoform 1 of the IP3R has been reported to be expressed mostly in OSs of red and green-sensitive cones (65). PLC can be activated by Ca2+ influx through voltage-gated Ca2+ channels (e.g., 157). Thus depolarization-evoked [Ca2+]i increases may stimulate synthesis of inositol triphosphates in cone ISs (158). Moreover, the activation of IP3Rs themselves is enhanced by 0.5 – 1.0 μM [Ca2+]i (159). Thus, at physiological [Ca2+]i (~500 nM), IP3Rs will be activated, causing a steady release of Ca2+ from internal stores.
The functional role of IP3R in the inner segment is currently unknown. IP3 synthesis could be activated by one of the many neuromodulators whose receptors have been characterized in the photoreceptor IS – including the D2/D4 dopamine receptor (105), metabotropic glutamate receptors (116) or adenosine A1 and A2 receptors (112). A role of IP3R in control of transmitter release has been suggested by Peng et al. (156) and has recently been supported by the finding that synaptic transmission in PLCβ4 knockouts is substantially decreased compared to control animals (67).
ER and the mitochondria not only function as a source of Ca2+ by releasing Ca2+ into the cytosol but also act as a Ca2+ buffering system (a “sink”), which can sequester Ca2+ from of the cytoplasm. Functional Ca2+ stores exhibit a large (> three orders of magnitude) Ca2+ concentration gradient between their lumen and the cytosol. This is achieved via the sarcoplasmic-endoplasmic reticulum calcium ATPase (SERCA pump). Ca2+ accumulation by photoreceptor ER is ATP-dependent (151), consistent with the action of SERCAs. Growing evidence supports the view that the contribution of SERCAs is not limited to refilling the intracellular Ca2+ stores, but that they participate in modulating the spatiotemporal pattern of the Ca2+ rise (160). The steady-state [Ca2+]i in the IS will be determined by the combined action of PMCAs and SERCAs.
The mitochondria are increasingly viewed as an important element in Ca2+ homeostasis in normally functioning cells (161–162). These cytoplasmic organelles are prominent in vertebrate photoreceptors. The mitochondria have a substantial capacity for Ca2+ uptake and can significantly buffer Ca2+. Their concentration in the ellipsoid region is amongst the highest in any known vertebrate cell. These mitochondria may accumulate large amounts of Ca2+ during pathological conditions (17).
6. THE PHOTORECEPTOR TERMINAL: CALCIUM ION REGULATION AND GRADED RELEASE
Photoreceptors release the neurotransmitter glutamate from large synaptic terminals characterized by a number of specialized features. The defining feature is the abundance of synaptic vesicles – synaptic terminals are typically filled with hundreds of thousands of vesicles (163–164). Also present are dense core vesicles, elongated cisterns of the endoplasmic reticulum and microtubules (149). Photoreceptor terminals are not static structures. Raviola and coworkers (80) have shown that the synaptic terminal of turtle cones, which is highly invaginated in the darkness, flattens out after exposure to light (163).
Electron microscopic studies indicate that the presynaptic terminal contains from 1 (rods) to ~ 50 (cones) active zones, each associated with a cluster of synaptic vesicles and an electron-dense lamellar structure known as the synaptic ribbon (164). The ribbon is located perpendicular to the active zone and is surrounded by a halo of synaptic vesicles. A recently identified component of the ribbon has an intriguing similarity to CtBP2, a transcriptional repressor related to 2-hydroxyacyl dehydrogenases (165). The authors therefore suggest that the ribbon may function in redox and enzymatic reactions associated with priming and transport of synaptic vesicles. Active zones of ribbon synapses are longer than those of conventional synapses and are thus able to accommodate more docked vesicles (~130). It is generally thought that the ribbons function as a conveyor belt specialized for fast and efficient delivery of synaptic vesicles to the active zone, where exocytosis occurs. In cat, the ribbon tethers ~ 600 synaptic vesicles. An elevation in [Ca2+]i triggers release of the entire pool of tethered vesicles. It has to be stressed, however, that neither the mechanism of action of the synaptic ribbon nor its structure have been determined, and these remain two of the key questions in understanding transmitter release at the photoreceptor synapse and at graded ribbon synapses in general.
Release of synaptic vesicles at the photoreceptor synapse is likely to occur at a high rate – measurements from dissociated photoreceptors range from ~30 vesicles per cone synapse (166, 167) to more than 400 vesicles/sec per rod synapse (22). This is much higher than in conventional synapses, where rates of ~20 vesicles/sec have been reported (168). As shown in Figure 6, release of glutamate from rod photoreceptors can be approximated as a linear function of presynaptic [Ca2+]i (23). Light responses from rods were matched to glutamate released from these cells. Figure 6 shows that the relation between rod voltage and glutamate release corresponds to the activation function of the L-type Ca2+ current of the rod. This finding suggests that the relationship between [Ca2+]i and transmitter release may be linear over a large range of presynaptic potentials and [Ca2+]i. Similar linearity has been suggested for the relationship between [Ca2+]i and exocytosis in salamander rods (22).
Figure 6.

A: Relation between voltage and glutamate release from rod photoreceptors. Combined data from two separate experiments. The membrane potential change in a rod evoked by light stimulation and recorded with an intracellular electrode is depicted by the green line. Change in relative glutamate release is depicted by red squares. B: Relation of rod voltage and glutamate release to calcium current. The data points ± SE were obtained from plots in A and fit with a Boltzman function. (modified after 21).
Glutamate release should also depend on the efficacy of presynaptic Ca2+ buffering. The passive properties of Ca2+ diffusion and buffering in the cytoplasm of the synaptic terminal of rods and cones have not been studied. One question relates to the efficiency of Ca2+ extrusion from the terminal. Spatial arrangement of Ca2+ channels relative to each other and to the relevant Ca2+-binding proteins such as Ca2+ ATPases and Ca2+ buffers will determine the characteristics of transmitter release. Ca2+ channels and PMCAs, which are necessary to control local [Ca2+]i, are both likely to be localized close to the active zone (73, 80). It has been suggested that Ca2+ is extruded from PMCAs localized at the neck of the terminal itself, relatively far from the active zone (98, 138). [Ca2+]i and exocytosis rates would in this case be dominated by a longitudinal Ca2+ gradient. Alternatively, if Ca2+ extrusion and influx apparati are colocalized, release is likely to depend on Ca2+ microdomains.
Measurements of [Ca2+]i in photoreceptor ISs and synaptic terminals suggested that the dynamic range of the synaptic [Ca2+]i is ~10 fold, ranging from ~ 500 nM in darkness to ~ 50 nM in bright light (20–22). The photoreceptors have therefore evolved mechanisms to control transmitter release with very low levels of [Ca2+]i, at least 100 –fold lower compared to most conventional synapses (169–171; but also see 172). In spiking synapses, vesicle exocytosis occurs only for a brief period related to the arrival of the spike (169) and this is ensured by the high cooperativity between the Ca2+ entry and transmitter release (173).
It has long been thought that the uniqueness of dark release from photoreceptor terminals requires some adaptation of the protein machinery, such as the ribbon itself. It was therefore a surprise to discover that ribbon synapses in mammalian retinae contain the same proteins found in conventional synapses, including synaptophysin, syntaxin, synaptotagmin and SNAP-23/25 (174). Apparently, presynaptic depolarization and the resulting Ca2+ influx activate a similar chain of molecular events in both conventional and ribbon synapses. In both cases, Ca2+ influx triggers exocytosis of small, clear vesicles docked at the active zone. However, the ribbon synapses of rods and cones do differ from conventional synapses in the rest of the central nervous system in other, more structural, features: 1) calcium influx is via sustained L-type channels rather than more transient N-, P/Q- or R- type calcium channels (88) 2) many more vesicles (~130) are docked at the active zone than in conventional synapses (~50; ref. 168) and still more are stored at the ribbon itself (~770 in cat; 168). 3) some photoreceptor terminals lack synapsins I and II and rabphilin, the vesicle proteins thought to guide vesicles towards the active zone (174–175).
In summary, photoreceptor terminals exhibit a number of morphological and physiological specializations required to sustain high rates of tonic neurotransmitter release. The precise mechanisms used to couple changes in [Ca2+]i to changes in the release rate remain to be identified. This includes the structure of the synaptic ribbon, the molecular components that constitute active zones at central and basal synapses and the Ca2+ influx and extrusion mechanisms that maintain the steady-state [Ca2+]i.
7. CALCIUM AND PHOTORECEPTOR PATHOLOGY
Sustained increases in intracellular [Ca2+]i may occur during inherited retinal degenerations, retinal diseases, injuries and chemical exposure and may play a key role in apoptotic cell death in humans and animals. In photoreceptors, elevated [Ca2+]i could be responsible for photoreceptor cell death in the rd (retinal degeneration) mouse, autosomal cone dystrophy and retinitis pigmentosa (an inherited degeneration of rod photoreceptors) (16, 176), as well as in cancer-associated retinopathy (177), in lead-exposed rats (17), ischemia (178) and during light-induced retinal damage (18). Calcium channel blockers apparently provide some degree of rod photoreceptor rescue in the rd mouse and the RCS rat (two model systems for retinitis pigmentosa), as well as in ischemia (178) and cancer retinopathy (19, 177, 179). It has been suggested that photoreceptor rescue occurs as a result of reduced influx of Ca2+, lower intracellular levels of Ca2+ and consequent stabilization of Ca2+ homeostasis (19). Mutations in the rd-like (PDE β subunit) gene are also found in some human subjects with retinitis pigmentosa, suggesting this mechanism may also occur in humans.
Apoptosis of retinal neurons in diabetic retinae during development is blocked by insulin, which may act by reducing Ca2+ influx through L-type voltage-gated Ca2+ channels in the IS (128, 180). On the other hand, Ca2+ influx is necessary to sustain normal physiological and anatomical activity at the photoreceptor synapse. Mutations in the α1F subunit of the L-type Ca2+ channel have been shown to cause a form of stationary night blindness, possibly by impairing influx of Ca2+ required to sustain tonic glutamate release from photoreceptor terminals in darkness (78, 96). Impaired calcium regulation in the inner segment has also been implicated in diseases such as Duchenne muscular dystrophy (181). Typically, high levels of dystrophin are observed in photoreceptor terminals (182). In individuals with Duchenne’s dystrophy synaptic transmission at the rod synapse is suppressed, as evidenced by ERG recordings (181). These individuals are consequently blind at night. Although the exact nature of photoreceptor dysfunction in retinae of Duchenne patients is not known, dystrophin has been suggested to play a role in controlling Ca2+ influx through ion channels in the plasma membrane. An increased leak of Ca2+ from diseased cells has been suggested to result in a loss of Ca2+ homeostasis and cell death as well as in aberrant expression of intracellular Ca2+ release mechanisms, such as the receptors for the intracellular Ca2+ messenger IP3 (183).
Another mechanism leading to cell death might be related to dysfunctional Ca2+ buffering. Ca2+-binding proteins are directly responsible for at least two degenerative diseases in photoreceptors. First, cancer-associated retinopathy is an autoimmune disease initiated by aberrant expression of the Ca2+-binding protein recoverin in some tumors outside the retina. Its expression leads to an immune response against photoreceptor cells and leads to visual impairment or complete blindness (184). The loss of vision often precedes development of the cancer and may serve as a diagnostic tool (52). Second, perturbation in Ca2+ homeostasis caused by mutant Ca2+-binding protein GCAP1 result in autosomal dominant cone dystrophy and photoreceptor degeneration (52). This triggers a constitutive activation of GC at high [Ca2+]i, and causes a sustained increase in [cGMP]. High levels of cGMP have been directly linked to photoreceptor degeneration (176). Overexpression of the Ca2+-binding protein calbindin protects some cells from signals that increase [Ca2+]i and trigger apoptosis.
ER and mitochondria are implicated in Ca2+-mediated apoptosis in photoreceptors (17–18, 185). Normal function of Ca2+ stores is apparently required for photoreceptor survival. When Ca2+ sequestration via SERCA pumps was blocked with thapsigargin, rapid apoptosis of photoreceptors was observed (185). The duration of high amplitude Ca2+ signals may be controlled by specific characteristics of PMCA and SERCA pumps which remove Ca2+ from the cytosol (160). At the moment, the specific mechanisms which may underlie pathological Ca2+ release/sequestration from internal compartments in the inner segment are not known. For example, it will be interesting to determine whether treatment of retinae with agents that suppress ER-mediated Ca2+ release via RyRs also protects photoreceptors against Ca2+ excitotoxicity, as found in other neuronal tissues (e.g., 186–187). Neuroprotective strategies may also involve blockers of IP3 receptors and voltage-gated Ca2+ channels (18–19, 177–179, 188).
8. CONCLUSIONS
In this review we have attempted to illustrate the multiplicity of processes that regulate [Ca2+]i in vertebrate photoreceptors. Although Ca2+ has been known to perform specialized functions in different parts of cells (including neurons), Ca2+ signaling in photoreceptors stands out because of the elegance with which Ca2+ simultaneously regulates the transduction of the photon signal as well as synaptic signaling. The accessibility of signaling cascades in photoreceptors has proven to be advantageous for furthering our understanding of stimulus transduction and cellular signaling in general. For example, Ca2+ regulation in photoreceptors shares many features in common with olfactory cells and auditory hair cells, including sensory transduction and adaptation. We showed that the biophysical properties of the Ca2+ ion in combination with photoreceptor morphology participate in maintaining the polarized and compartmentalized function of rods and cones. An example of the sophisticated cellular machinery run by Ca2+ in its role as an integrator is the processes of light adaptation. Conversely, Ca2+ can act to differentiate and seclude cellular processes within different cellular regions via selective localization of Ca2+ influx, extrusion and buffering mechanisms. Finally, we showed that normal function of Ca2+ signaling pathways has a strong impact on visual signaling and is therefore likely to be fundamental for maintaining the quality of human life experience. In the years to come we need to understand more how OS function is integrated with the IS processes to transmute the OS signal, transmit it across the synapse and thus provide the foundation on which vision is built.
Acknowledgments
We thank Drs. René Rentería and Tania Rebrik for critical reading of the manucript. The preparation and writing of this manuscript was supported by the NIH (EY 01869 and NS16033 to D.R.C and EY 13870 to D.K.) and the Wheeler Center for the Neurobiology of Addiction (D.K. and D.R.C.). Additional support was provided by a NEI core grant to UCSF and an unrestricted grant from Research to Prevent Blindness foundation.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Allbritton NL, Meyer T, Stryer LF. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani K, Chen C, Koutalos Y. Calcium diffusion coefficient in rod photoreceptor outer segments. Biophys J. 2002;82:728–39. doi: 10.1016/S0006-3495(02)75435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;27:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 5.Juusola M, French AS, Uusitalo RO, Weckstrom M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19:292–297. doi: 10.1016/S0166-2236(96)10028-X. [DOI] [PubMed] [Google Scholar]
- 6.Nakatani K, Yau KW. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol. 1989;409:525–548. doi: 10.1113/jphysiol.1989.sp017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JL, Picones A, Korenbrot JI. Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr Opin Neurobiol. 1994;4:488–495. doi: 10.1016/0959-4388(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 8.Sampath AP, Matthews HR, Cornwall MC, Bandarchi J, Fain GL. Light-dependent changes in outer segment free-Ca2+ concentration in salamander cone photoreceptors. J Gen Physiol. 1999;113:267–77. doi: 10.1085/jgp.113.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyoda J, Hashimoto H, Anno H, Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970;10:1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- 10.Laughlin SB. The role of sensory adaptation in the retina. J Exp Biol. 1989;146:39–62. doi: 10.1242/jeb.146.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci. 2001;21:5066–5078. doi: 10.1523/JNEUROSCI.21-14-05066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses. 2000;25:473–481. doi: 10.1093/chemse/25.4.473. [DOI] [PubMed] [Google Scholar]
- 14.Ratto GM, Payne R, Owen WG, Tsien RY. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988;8:3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray-Keller M, Detwiler PB. Ca2+-dependence of dark- and light-adapted flash responses in rod photoreceptors. Neuron. 1996;17:323–331. doi: 10.1016/s0896-6273(00)80163-4. [DOI] [PubMed] [Google Scholar]
- 16.Chang GQ, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 17.He L, Poblenz AT, Medrano CJ, Fox DA. Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem. 2000;275:12175–12184. doi: 10.1074/jbc.275.16.12175. [DOI] [PubMed] [Google Scholar]
- 18.Edward DP, Lam TT, Shahinfar S, Li J, Tso MO. Amelioration of light-induced retinal degeneration by a calcium overload blocker. Flunarizine. Arch Ophthalmol. 1991;109:554–562. doi: 10.1001/archopht.1991.01080040122042. [DOI] [PubMed] [Google Scholar]
- 19.Frasson M, Sahel JA, Fabre M, Simonutti M, Dreyfus H, Picaud S. Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat Med. 1999;5:1183–1187. doi: 10.1038/13508. [DOI] [PubMed] [Google Scholar]
- 20.Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krizaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J Neurosci. 1999;19:7249–7261. doi: 10.1523/JNEUROSCI.19-17-07249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol. 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witkovsky P, Schmitz Y, Akopian A, Krizaj D, Tranchina D. Gain of rod to horizontal cell synaptic transfer: relation to glutamate release and a dihydropyridine-sensitive calcium current. J Neurosci. 1997;17:7297–7306. doi: 10.1523/JNEUROSCI.17-19-07297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz Y, Witkovsky P. Glutamate release by the intact light-responsive photoreceptor layer of the Xenopus retina. J Neurosci Methods. 1996;68:55–60. doi: 10.1016/0165-0270(96)00070-2. [DOI] [PubMed] [Google Scholar]
- 26.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–149. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 27.Matthews HR, Murphy RL, Fain GL, Lamb TD. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988;334:67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- 28.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 29.Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylyl cyclase by calcium ions. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Sokal I, JD Bronson, Palczewski K, Baehr W. Identification of functional regions of guanylyl cyclase-activating protein 1 (GCAP1) using GCAP1/GCIP chimeras. Biol Chem. 2001;382:1179–1188. doi: 10.1515/BC.2001.148. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Fariss RN, Zhang K, Otto-Bruc A, Haeseleer F, Bronson D, Qin N, Yamazaki A, Subbaraya I, Milam AH, Palczewski K, Baehr W. Guanylyl-cyclase-inhibitory protein is a frog retinal Ca2+-binding protein related to mammalian guanylyl-cyclase-activating proteins. Eur J Biochem. 1998;252:591–599. doi: 10.1046/j.1432-1327.1998.2520591.x. [DOI] [PubMed] [Google Scholar]
- 32.Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylyl cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura S. Rhodopsin phosphorylation as a mechanism of cGMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- 34.Eismann E, Muller F, Heinemann SH, Kaupp UB. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci U S A. 1994;91:1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Chen TY, Ahamed B, Li J, Yau KW. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 37.Nakatani K, Yau KW. Calcium and light adaptation in retinal rods and cones. Nature. 1988;334:69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- 38.Picones A, Korenbrot J. Permeability and interaction of Ca2+ with cGMP-gated channels differ in retinal rod and cone photoreceptors. Biophys J. 1997;69:120–127. doi: 10.1016/S0006-3495(95)79881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohyama T, Hackos DH, Frings S, Hagen V, Kaupp UB, Jorenbrot JI. Fraction of dark current carried by Ca2+ through cGMP-gated ion channels of intact rod and cone photoreceptors. J Gen Physiol. 2000;116:735–753. doi: 10.1085/jgp.116.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase W, Friese W, Gordon RD, Muller H, Cook NJ. Immunological characterization and localization of the Na+/Ca(2+-exchanger in bovine retina. J Neurosci. 1990;10:1486–1494. doi: 10.1523/JNEUROSCI.10-05-01486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim TS, Reid DM, Molday RS. Structure-function relationships and localization of the Na/Ca-K exchanger in rod photoreceptors. J Biol Chem. 1998;273:16561–16567. doi: 10.1074/jbc.273.26.16561. [DOI] [PubMed] [Google Scholar]
- 42.Poon S, Leach S, Li XF, Tucker JE, Schnetkamp PPM, Lytton J. Am J Physiol. 2000;278:C651–C660. doi: 10.1152/ajpcell.2000.278.4.C651. [DOI] [PubMed] [Google Scholar]
- 43.Prinsen CF, Szerencsei RT, Schnetkamp PP. Molecular cloning and functional expression of the potassium-dependent sodium-calcium exchanger from human and chicken retinal cone photoreceptors. J Neurosci. 2000;20:1424–1434. doi: 10.1523/JNEUROSCI.20-04-01424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnetkamp PP, Basu DK, Szerencsei RT. Na+-Ca2+ exchange in bovine rod outer segments requires and transports K+ Am J Physiol. 1989;257:C153–C157. doi: 10.1152/ajpcell.1989.257.1.C153. [DOI] [PubMed] [Google Scholar]
- 45.Szerencsei RT, Prinsen CF, Schnetkamp PP. Stoichiometry of the retinal cone Na/Ca-K exchanger heterologously expressed in insect cells: comparison with the bovine heart Na/Ca exchanger. Biochemistry. 2001;40:6009–6015. doi: 10.1021/bi0102353. [DOI] [PubMed] [Google Scholar]
- 46.Schnetkamp PP. How does the retinal rod Na-Ca K exchanger regulate cytosolic free Ca2+? J Biol Chem. 1995;270:13231–13239. doi: 10.1074/jbc.270.22.13231. [DOI] [PubMed] [Google Scholar]
- 47.Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiPolo R, Rojas HR, Beaugé L. Vanadate inhibits uncoupled Ca efflux but not Na-Ca exchange in squid axons. Nature. 1979;281:229–230. doi: 10.1038/281228a0. [DOI] [PubMed] [Google Scholar]
- 49.Schwarzer A, Schauf H, Bauer PJ. Binding of the cGMP-gated channel to the Na/Ca-K exchanger in rod photoreceptors. J Biol Chem. 2000;275:13448–13454. doi: 10.1074/jbc.275.18.13448. [DOI] [PubMed] [Google Scholar]
- 50.Leibovic KN, Bandarchi J. Phototransduction and calcium exchange along the length of the retinal rod outer segment. Neuroreport. 1997;8:1295–1300. doi: 10.1097/00001756-199703240-00047. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy ST, Younger JP, Owen WG. Dynamic, spatially nonuniform calcium regulation in frog rods exposed to light. J Neurophysiol. 1996;76:1991–2004. doi: 10.1152/jn.1996.76.3.1991. [DOI] [PubMed] [Google Scholar]
- 52.Palczewski K, Polans AS, Baehr W, Ames JB. Ca(2+)-binding proteins in the retina: structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–50. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 53.Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron. 1988;1:503–15. doi: 10.1016/0896-6273(88)90181-x. [DOI] [PubMed] [Google Scholar]
- 54.Liebman PA. Light-dependent Ca++ content of rod outer segment disc membranes. Invest Ophthalmol. 1974;13:700–701. [PubMed] [Google Scholar]
- 55.Fain GL, Schroder WH. Light-induced calcium release and re-uptake in toad rods. J Neurosci. 1990;10:2238–2249. doi: 10.1523/JNEUROSCI.10-07-02238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnetkamp PP, Bownds MD. Na+- and cGMP-induced Ca2+ fluxes in frog rod photoreceptors. J Gen Physiol. 1987;89:481–500. doi: 10.1085/jgp.89.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnetkamp PP, Szerencsei RT. Intracellular Ca2+ sequestration and release in intact bovine retinal rod outer segments. Role in inactivation of Na-Ca+K exchange. J Biol Chem. 1993;268:12449–12457. [PubMed] [Google Scholar]
- 58.Davis WL, Hagler HK, Farmer GR, Martin JH, Bridges G. Electron microscopic cytochemical localization of Ca-ATPase in the rod outer segments of the toad Bufo marinus. Anat Rec. 1988;221:761–768. doi: 10.1002/ar.1092210312. [DOI] [PubMed] [Google Scholar]
- 59.Ghalayini A, Anderson RE. Phosphatidylinositol 4,5-bisphosphate: light-mediated breakdown in the vertebrate retina. Biochem Biophys Res Commun. 1984;124:503–506. doi: 10.1016/0006-291x(84)91582-1. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi F, Amakawa T. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem Biophys Res Commun. 1985;128:954–959. doi: 10.1016/0006-291x(85)90139-1. [DOI] [PubMed] [Google Scholar]
- 61.Brown JE, Blazynski C, Cohen AI. Light induces a rapid and transient increase in inositol-trisphosphate in toad rod outer segments. Biochem Biophys Res Commun. 1987;146:1392–1396. doi: 10.1016/0006-291x(87)90804-7. [DOI] [PubMed] [Google Scholar]
- 62.Ghalayini AJ, Tarver AP, Mackin WM, Koutz CA, Anderson RE. Identification and immunolocalization of phospholipase C in bovine rod outer segments. J Neurochem. 1991;57:1405–1412. doi: 10.1111/j.1471-4159.1991.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira PA, Pak WL. Bovine phospholipase C highly homologous to the norpA protein of Drosophila is expressed specifically in cones. J Biol Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- 64.Gehm BD, Pinke RM, Laquerre S, Chafouleas JG, Schultz DA, Pepperl DJ, McConnell DG. Activation of bovine rod outer segment phosphatidylinositol-4,5-bisphosphate phospholipase C by calmodulin antagonists does not depend on calmodulin. Biochemistry. 1991;30:11302–11306. doi: 10.1021/bi00111a016. [DOI] [PubMed] [Google Scholar]
- 65.Wang TL, Sterling P, Vardi N. Localization of type I inositol 1,4,5-triphosphate receptor in the outer segments of mammalian cones. J Neurosci. 1993;19:4221–4228. doi: 10.1523/JNEUROSCI.19-11-04221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Day NS, Koutz CA, Anderson RE. Inositol-1,4,5-trisphosphate receptors in the vertebrate retina. Curr Eye Res. 1993;12:981–992. doi: 10.3109/02713689309029224. [DOI] [PubMed] [Google Scholar]
- 67.Jiang H, Lyubarsky A, Dodd R, Vardi N, Pugh E, Baylor D, Simon MI, Wu D. Phospholipase C β4 is involved in modulating the visual response in mice. Proc Natl Acad Sci U S A. 1996;93:14598–14601. doi: 10.1073/pnas.93.25.14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ukhanov K, Mills SJ, Potter BV, Walz B. InsP(3)-induced Ca(2+) release in permeabilized invertebrate photoreceptors: a link between phototransduction and Ca(2+) stores. Cell Calcium. 2001;29:335–345. doi: 10.1054/ceca.2001.0195. [DOI] [PubMed] [Google Scholar]
- 69.Chorna-Ornan I, Joel-Almagor T, Ben-Ami HC, Frechter S, Gillo B, Selinger Z, Gill DL, Minke B. A common mechanism underlies vertebrate calcium signaling and Drosophila phototransduction. J Neurosci. 2001;21:2622–2629. doi: 10.1523/JNEUROSCI.21-08-02622.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnes S. After transduction: response shaping and control of transmission by ion channels of the photoreceptor inner segments. Neuroscience. 1994;58:447–459. doi: 10.1016/0306-4522(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 71.Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spencer M, Detwiler PB, Bunt-Milam AH. Distribution of membrane proteins in mechanically dissociated retinal rods. Invest Ophthalmol Vis Sci. 1988;29:1012–1020. [PubMed] [Google Scholar]
- 73.Krizaj D, DeMarco S, Johnson J, Strehler EE, Copenhagen DR. Cell-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. J Comp Neurol. 2002 doi: 10.1002/cne.10281. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haugh-Scheidt LM, Linsenmeier RA, Griff ER. Oxygen consumption in the isolated toad retina. Exp Eye Res. 1995;61:63–72. doi: 10.1016/s0014-4835(95)80059-x. [DOI] [PubMed] [Google Scholar]
- 75.Laughlin SB. Energy as a constraint on the coding and processing of sensory information. Curr Opin Neurobiol. 2001;11:475–80. doi: 10.1016/s0959-4388(00)00237-3. [DOI] [PubMed] [Google Scholar]
- 76.Matthews HR, Fain GL. A light-dependent increase in free Ca2+ concentration in the salamander rod outer segment. J Physiol. 2001;532:305–321. doi: 10.1111/j.1469-7793.2001.0305f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marszalek JR, Liu X, Roberts EA, Chui JD, Marth Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 78.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 79.Raviola E, Gilula GB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaeffer SF, Raviola E, Heuser JE. Membrane specializations in the outer plexiform layer of the turtle retina. J Comp Neurol. 1982;204:253–67. doi: 10.1002/cne.902040305. [DOI] [PubMed] [Google Scholar]
- 81.Fain GL, Quandt FN, Gerschenfeld HM. Calcium-dependent regenerative responses in rods. Nature. 1977;269:707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- 82.Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989;94:719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilkinson MF, Barnes S. The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akopian A, Krizaj D, Witkovsky P. Both high-and low voltage-activated calcium currents contribute to the light-evoked responses of luminosity horizontal cells in the Xenopus retina. Brain Res. 1999;762:121–130. doi: 10.1016/s0006-8993(97)00374-0. [DOI] [PubMed] [Google Scholar]
- 86.Schmitz Y, Witkovsky P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- 87.Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- 88.Nachman-Clewner M, Jules RSt, Townes-Anderson E. L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity. J Comp Neurol. 1999;415:1–16. [PubMed] [Google Scholar]
- 89.Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994;14:3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dowling JE, Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973;242:101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- 91.Taylor WR, Morgans C. Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci. 1998;15:541–552. doi: 10.1017/s0952523898153142. [DOI] [PubMed] [Google Scholar]
- 92.Kourennyi DE, Barnes S. Depolarization-induced calcium channel facilitation in rod photoreceptors is independent of G proteins and phosphorylation. J Neurophysiol. 2000;84:133–138. doi: 10.1152/jn.2000.84.1.133. [DOI] [PubMed] [Google Scholar]
- 93.Yagi T, Macleish PR. Ionic conductances of monkey solitary cone inner segments. J Neurophysiol. 1994;71:656–665. doi: 10.1152/jn.1994.71.2.656. [DOI] [PubMed] [Google Scholar]
- 94.Lasater EM, Witkovsky P. The calcium current of turtle cone photoreceptor axon terminals. Neurosci Res Suppl. 1991;15:S165–173. [PubMed] [Google Scholar]
- 95.Cox DH, Dunlap K. Pharmacological discrimination of N-type from L-type calcium current and its selective modulation by transmitters. J Neurosci. 1992;12:906–914. doi: 10.1523/JNEUROSCI.12-03-00906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 97.Firth SI, I, Morgan G, Boelen MK, Morgans C. Localization of voltage-sensitive L-type calcium channels in the chicken retina. Clin Experiment Ophthalmol. 2001;29:183–187. doi: 10.1046/j.1442-9071.2001.00401.x. [DOI] [PubMed] [Google Scholar]
- 98.Morgans CW. Neurotransmitter release at ribbon synapses in the retina. Immunol Cell Biol. 2000;78:442–446. doi: 10.1046/j.1440-1711.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- 99.Morgans CW. Calcium channel heterogeneity among cone photoreceptors in the tree shrew retina. Eur J Neurosci. 1999;11:2989–2993. doi: 10.1046/j.1460-9568.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 100.Dolphin AC. L-type calcium channel modulation. Adv Second Messenger Phosphoprotein Res. 1999;33:153–77. doi: 10.1016/s1040-7952(99)80009-3. [DOI] [PubMed] [Google Scholar]
- 101.Belgum JH, Copenhagen DR. Synaptic transfer of rod signals to horizontal and bipolar cells in the retina of the toad. J Physiol. 1988;396:225–245. doi: 10.1113/jphysiol.1988.sp016960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muresan Z, Besharse JC. D2-like dopamine receptors in amphibian retina: localization with fluorescent ligands. J Comp Neurol. 1993;331:149–60. doi: 10.1002/cne.903310202. [DOI] [PubMed] [Google Scholar]
- 103.Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas: I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 46:1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 104.Krizaj D, Witkovsky P. Effects of submicromolar concentrations of dopamine on photoreceptor to horizontal cell communication. Brain Res. 1993;627:122–128. doi: 10.1016/0006-8993(93)90755-c. [DOI] [PubMed] [Google Scholar]
- 105.Krizaj D, Gabriel R, Owen GW, Witkovsky P. Dopamine D2 receptor-modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- 106.Stella SL, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 107.Krizaj D. Mesopic state: cellular mechanisms involved in pre- and post-synaptic mixing of rod and cone signals. Microsc Res Tech. 2000;50:347–59. doi: 10.1002/1097-0029(20000901)50:5<347::AID-JEMT4>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akopian A, Johnson J, Gabriel R, Brecha N, Witkovsky P. Somatostatin modulates voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors of the salamander retina. J Neurosci. 2000;20:929–936. doi: 10.1523/JNEUROSCI.20-03-00929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blazynski C, Perez MT. Adenosine in vertebrate retina: localization, receptor characterization, and function. Cell Mol Neurobiol. 1991;11:463–484. doi: 10.1007/BF00734810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB. Localization of adenosine receptor messenger RNAs in the rat eye. Exp Eye Res. 1997;65:595–602. doi: 10.1006/exer.1996.0352. [DOI] [PubMed] [Google Scholar]
- 111.de Carvalho RP, Braas KM, Adler R, Snyder SH. Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Brain Res Dev Brain Res. 1992;70:87–95. doi: 10.1016/0165-3806(92)90106-7. [DOI] [PubMed] [Google Scholar]
- 112.Rey HL, Burnside B. Adenosine stimulates cone photoreceptor myoid elongation via an adenosine A2-like receptor. J Neurochem. 1999;72:2345–2355. doi: 10.1046/j.1471-4159.1999.0722345.x. [DOI] [PubMed] [Google Scholar]
- 113.Stella SL, Bryson EJ, Thoreson WB. A2 adenosine receptors inhibit calcium influx through L-type calcium channels in rod photoreceptors of the salamander retina. J Neurophysiol. 2002;87:351–360. doi: 10.1152/jn.00010.2001. [DOI] [PubMed] [Google Scholar]
- 114.Barnes S, Merchant V, Mahmud FM. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci U S A. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takahashi K, Dixon DB, Copenhagen DR. Modulation of a sustained calcium current by intracellular pH in horizontal cells of fish retina. J Gen Physiol. 1993;101:695–714. doi: 10.1085/jgp.101.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koulen P, Kihn R, Wassle H, Brandstatter JH. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc Natl Acad Sci U S A. 1999;96:9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thoreson WB, Nitzan R, Miller RF. Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: chloride imaging and cell-attached patch-clamp recordings. Vis Neurosci. 2000;17:197–206. doi: 10.1017/s0952523800172025. [DOI] [PubMed] [Google Scholar]
- 118.Liepe BA, Stone C, Koistinaho J, Copenhagen DR. Nitric oxide synthase in Muller cells and neurons of salamander and fish retina. J Neurosci. 1994;14:7641–7654. doi: 10.1523/JNEUROSCI.14-12-07641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haverkamp S, Eldred WD. Localization of nNOS in photoreceptor, bipolar and horizontal cells in turtle and rat retinas. Neuroreport. 1998;9:2231–2235. doi: 10.1097/00001756-199807130-00015. [DOI] [PubMed] [Google Scholar]
- 120.Kurenny DE, Moroz LL, Turner RW, Sharkey KA, Barnes S. Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron. 1994;13:315–324. doi: 10.1016/0896-6273(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 121.Koch KW, Lambrecht HG, Haberecht M, Redburn D, Schmidt HH. Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO J. 1994;13:3312–20. doi: 10.1002/j.1460-2075.1994.tb06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Savchenko A, Barnes S, Kramer RH. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390:694–698. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baldridge WH, Kurennyi DE, Barnes S. Calcium-sensitive calcium influx in photoreceptor inner segments. J Neurophysiol. 1998;79:3012–3018. doi: 10.1152/jn.1998.79.6.3012. [DOI] [PubMed] [Google Scholar]
- 124.Piccolino M, Vellani V, Rakotobe LA, Pignatelli A, Barnes S, McNaughton P. Manipulation of synaptic sign and strength with divalent cations in the vertebrate retina: pushing the limits of tonic, chemical neurotransmission. Eur J Neurosci. 1999;11:4134–4138. doi: 10.1046/j.1460-9568.1999.00842.x. [DOI] [PubMed] [Google Scholar]
- 125.Yazulla S, Studholme KM, McIntosh HH, Fan SF. Cannabinoid receptors on goldfish retinal bipolar cells: electron-microscope immunocytochemistry and whole-cell recordings. Vis Neurosci. 2000;17:391–401. doi: 10.1017/s0952523800173079. [DOI] [PubMed] [Google Scholar]
- 126.Lu Q, Straiker A, Lu Q, Maguire G. Expression of CB2 cannabinoid receptor mRNA in adult rat retina. Vis Neurosci. 2000;17:91–95. doi: 10.1017/s0952523800171093. [DOI] [PubMed] [Google Scholar]
- 127.Gosbell AD, Favilla I, Jablonski P. The location of insulin receptors in bovine retina and isolated retinal cells. Clin Experiment Ophthalmol. 2002;30:124–130. doi: 10.1046/j.1442-6404.2002.00499.x. [DOI] [PubMed] [Google Scholar]
- 128.Stella SL, Bryson EJ, Thoreson WB. Insulin inhibits voltage-dependent calcium influx into rod photoreceptors. Neuroreport. 2001;12:947–951. doi: 10.1097/00001756-200104170-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jelsema CL, Axelrod J. Stimulation of phospholipase A2 activity in bovine rod outer segments by the beta gamma subunits of transducin and its inhibition by the alpha subunit. Proc Natl Acad Sci U S A. 1987;84:3623–3627. doi: 10.1073/pnas.84.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reinboth JJ, Clausen M, Reme CE. Light elicits the release of docosahexaenoic acid from membrane phospholipids in the rat retina in vitro. Exp Eye Res. 1996;63:277–284. doi: 10.1006/exer.1996.0116. [DOI] [PubMed] [Google Scholar]
- 131.Vellani V, Reynolds AM, McNaughton PA. Modulation of the synaptic Ca2+ current in salamander photoreceptors by polyunsaturated fatty acids and retinoids. J Physiol. 2000;529:333–344. doi: 10.1111/j.1469-7793.2000.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- 133.Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma U, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 134.Huang BO, Redburn DA. GABA-induced increases in [Ca2+]i in retinal neurons of postnatal rabbits. Vis Neurosci. 1996;13:441–447. doi: 10.1017/s0952523800008117. [DOI] [PubMed] [Google Scholar]
- 135.Huang B, Mitchell CK, Redburn-Johnson DA. GABA and GABA(A) receptor antagonists alter developing cone photoreceptor development in neonatal rabbit retina. Vis Neurosci. 2000;17:925–935. doi: 10.1017/s0952523800176126. [DOI] [PubMed] [Google Scholar]
- 136.Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 137.Copenhagen DR, Hemilä S, Reuter T. Signal transmission through the dark-adapted retina of the toad (Bufo marinus). Gain, convergence, and signal/noise. J Gen Physiol. 1990;95:717–732. doi: 10.1085/jgp.95.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morgans CW, El Far O, Bernstson A, Wassle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. J Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guerini D, Carafoli EE. The calcium pumps. In: Carafoli E, Klee C, editors. Calcium as a cellular regulator. Oxford University Press; NY: 1998. pp. 249–278. [Google Scholar]
- 140.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 141.Koulen P, Fletcher EL, Craven SE, Bredt DS, Wassle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J Neurosci. 1998;18:10136–10149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vu TQ, Payne JA, Copenhagen DR. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J Neurosci. 2000;20:1414–1423. doi: 10.1523/JNEUROSCI.20-04-01414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reuter H, Porzig H. Localization and functional significance of the Na+/Ca2+ exchanger in presynaptic boutons of hippocampal cells in culture. Neuron. 1995;15:1077–1084. doi: 10.1016/0896-6273(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 144.Juhaszova M, Church P, Blaustein MP, Stanley EF. Location of calcium transporters at presynaptic terminals. Eur J Neurosci. 2000;12:839–846. doi: 10.1046/j.1460-9568.2000.00974.x. [DOI] [PubMed] [Google Scholar]
- 145.Wakade AR, Przywara DA, Bhave SV, Chowdhury PS, Bhave A, Wakade TD. Massive exocytosis triggered by sodium-calcium exchange in sympathetic neurons is attenuated by co-culture with cardiac cells. Neuroscience. 1993;55:813–821. doi: 10.1016/0306-4522(93)90443-j. [DOI] [PubMed] [Google Scholar]
- 146.Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci. 1994;14:4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- 149.Mercurio AM, Holtzman E. Smooth endoplasmic reticulum and other agranular reticulum in frog retinal photoreceptors. J Neurocytol. 1982;11:263–293. doi: 10.1007/BF01258247. [DOI] [PubMed] [Google Scholar]
- 150.Ripps H, Chappell RL. Ultrastructural and electrophysiological changes associated with K(+)-evoked release of neurotransmitter at the synaptic terminals of skate photoreceptors. Vis Neurosci. 1991;7:597–609. doi: 10.1017/s0952523800010385. [DOI] [PubMed] [Google Scholar]
- 151.Ungar F, Piscopo I, Letizia J, Holtzman E. Uptake of calcium by the endoplasmic reticulum of the frog photoreceptor. J Cell Biol. 1984;98:1645–1655. doi: 10.1083/jcb.98.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Somlyo APB, Walz Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985;358:183–95. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Freihofer D, Kortje KH, Rahmann H. Ultrastructural localization of endogenous calcium in the teleost retina. Histochem J. 1990;22:63–72. doi: 10.1007/BF01885783. [DOI] [PubMed] [Google Scholar]
- 154.Berger ER. Subsurface membranes in paired cone photoreceptor inner segments of adult and neonatal Lebistes retinae. J Ultrastruct Res. 1967;17:220–232. doi: 10.1016/s0022-5320(67)80044-3. [DOI] [PubMed] [Google Scholar]
- 155.Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH., Jr Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet Jan. 2000;24(1):61–5. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 156.Peng YW, Sharp AH, Snyder SH, Yau KW. Localization of the inositol 1,4,5-trisphosphate receptor in synaptic terminals in the vertebrate retina. Neuron. 1991;6:525–31. doi: 10.1016/0896-6273(91)90055-5. [DOI] [PubMed] [Google Scholar]
- 157.Masgrau R, Servitja JM, Young KW, Pardo R, Sarri E, Nahorski SR, Picatoste F. Characterization of the metabotropic glutamate receptors mediating phospholipase C activation and calcium release in cerebellar granule cells: calcium-dependence of the phospholipase C response. Eur J Neurosci. 2001;13:248–256. doi: 10.1046/j.0953-816x.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 158.Gan J, Iuvone PM. Depolarization and activation of dihydropyridine-sensitive Ca2+ channels stimulate inositol phosphate accumulation in photoreceptor-enriched chick retinal cell cultures. J Neurochem. 1997;68:2300–2307. doi: 10.1046/j.1471-4159.1997.68062300.x. [DOI] [PubMed] [Google Scholar]
- 159.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 160.Brini M, Bano D, Manni S, Rizzuto R, Carafoli E. Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca(2+) signalling. EMBO J. 2000;19:4926–4935. doi: 10.1093/emboj/19.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 162.Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;15:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adly MA, Spiwoks-Becker I, Vollrath VL. Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Invest Ophthalmol Vis Sci. 1999;40:2165–2172. [PubMed] [Google Scholar]
- 164.Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265:471–89. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- 165.Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 166.Ashmore JF, Copenhagen DR. An analysis of transmission from cones to hyperpolarizing bipolar cells in the retina of the turtle. J Physiol. 1983;340:569–597. doi: 10.1113/jphysiol.1983.sp014781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rao R, Buchsbaum G, Sterling P. Rate of quantal transmitter release at the mammalian rod synapse. Biophys J. 1994;67:57–63. doi: 10.1016/S0006-3495(94)80454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stevens CF, Tsujimoto TC. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc Natl Acad Sci U S A. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Simon SM, Llinas RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yazejian B, Sun XP, Grinnell AD. Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels. Nat Neurosci. 2000;3:566–571. doi: 10.1038/75737. [DOI] [PubMed] [Google Scholar]
- 171.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 172.Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 173.Augustine GJ, Charlton MP, Smith SJ. Calcium entry into voltage-clamped presynaptic terminals of squid. J Physiol. 1985;367:143–162. doi: 10.1113/jphysiol.1985.sp015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.von Kriegstein K, Schmitz F, Lonk E, Sudhof T. Distribution of synaptic vesicle protins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur J Neurosci. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 175.Mandell JW, Czernik AJ, De Camilli P, Greengard P, Townes-Anderson E. Differential expression of synapsins I and II among rat retinal synapses. J Neurosci. 1992;12:1736–1749. doi: 10.1523/JNEUROSCI.12-05-01736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ulshafer RH, Garcia CA, Hollyfield JC. Sensitivity of photoreceptors to elevated levels of cGMP in the human retina. Invest Ophthal Vis Sci. 1980;19:1236–1241. [PubMed] [Google Scholar]
- 177.Ohguro H, Ogawa K, Maeda T, Maruyama I, Maeda A, Takano Y, Nakazawa M. Retinal dysfunction in cancer-associated retinopathy is improved by Ca(2+) antagonist administration and dark adaptation. Invest Ophthalmol Vis Sci. 2001;42:2589–2595. [PubMed] [Google Scholar]
- 178.Crosson CE, Willis JA, Potter DE. Effect of the calcium antagonist, nifedipine, on ischemic retinal dysfunction. J Ocul Pharmacol. 1990;6:293–299. doi: 10.1089/jop.1990.6.293. [DOI] [PubMed] [Google Scholar]
- 179.Yamazaki H, Ohguro H, Maeda T, Maruyama I, Takano Y, Metoki T, Nakazawa M, Sawada H, Dezawa M. Preservation of retinal morphology and functions in royal college surgeons rat by nilvadipine, a Ca(2+) antagonist. Invest Ophthalmol Vis Sci. 2002;43:919–926. [PubMed] [Google Scholar]
- 180.Diaz B, Serna J, De Pablo F, de la Rosa EJ. In vivo regulation of cell death by embryonic (pro)insulin and the insulin receptor during early retinal neurogenesis. Development. 2000;127:1641–1649. doi: 10.1242/dev.127.8.1641. [DOI] [PubMed] [Google Scholar]
- 181.Fitzgerald KM, Cibis GW, Giambrone SA, Harris DJ. Retinal signal transmission in Duchenne muscular dystrophy: evidence for dysfunction in the photoreceptor/depolarizing bipolar cell pathway. J Clin Invest. 1994;93:2425–2430. doi: 10.1172/JCI117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Blank M, Koulen P, Blake DJ, Kroger S. Dystrophin and beta-dystroglycan in photoreceptor terminals from normal and mdx3Cv mouse retinae. Eur J Neurosci. 1999;11:2121–2133. doi: 10.1046/j.1460-9568.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 183.Alderton JM, Steinhardt RA. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc Med. 2000;10:268–272. doi: 10.1016/s1050-1738(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 184.Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998;11:523–533. doi: 10.1006/jaut.1998.0221. [DOI] [PubMed] [Google Scholar]
- 185.Linden R, Rehen SK, Chiarini LB. Apoptosis in developing retinal tissue. Prog Retinal Eye Res. 1999;18:133–165. doi: 10.1016/s1350-9462(98)00020-2. [DOI] [PubMed] [Google Scholar]
- 186.Frandsen A, Schousboe A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. J Neurochem. 1991;56:1075–1078. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 187.Pelletier MR, Wadia JS, Mills LR, Carlen PL. Seizure-induced cell death produced by repeated tetanic stimulation in vitro: possible role of endoplasmic reticulum calcium stores. J Neurophysiol. 1999;81:3054–3064. doi: 10.1152/jn.1999.81.6.3054. [DOI] [PubMed] [Google Scholar]
- 188.Sahly I, Bar Nachum S, Suss-Toby E, Rom A, Peretz A, Kleiman J, Byk T, Selinger Z, Minke B. Calcium channel blockers inhibit retinal degeneration in the retinal-degeneration-B mutant of Drosophila. Proc Natl Acad Sci U S A. 1992;89:435–439. doi: 10.1073/pnas.89.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Molday RS. Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1998;39:2491–2513. [PubMed] [Google Scholar]
- 190.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]


