Abstract
The control of gene expression by the mitogen-activated protein (MAP) kinase extracellular signal-regulated kinase (ERK) requires its translocation into the nucleus. In Drosophila S2 cells nuclear accumulation of diphospho-ERK (dpERK) is greatly reduced by interfering double-stranded RNA against Drosophila importin-7 (DIM-7) or by the expression of integrin mutants, either during active cell spreading or after stimulation by insulin. In both cases, total ERK phosphorylation (on Westerns) is not significantly affected, and ERK accumulates in a perinuclear ring. Tyrosine phosphorylation of DIM-7 is reduced in cells expressing integrin mutants, indicating a mechanistic link between these components. DIM-7 and integrins localize to the same actin-containing peripheral regions in spreading cells, but DIM-7 is not concentrated in paxillin-positive focal contacts or stable focal adhesions. The Corkscrew (SHP-2) tyrosine phosphatase binds DIM-7, and Corkscrew is required for the cortical localization of DIM-7. These data suggest a model in which ERK phosphorylation must be spatially coupled to integrin-mediated DIM-7 activation to make a complex that can be imported efficiently. Moreover, dpERK nuclear import can be restored in DIM-7–deficient cells by Xenopus Importin-7, demonstrating that ERK import is an evolutionarily conserved function of this protein.
INTRODUCTION
The integrin cell surface receptors regulate numerous cellular processes, including growth, differentiation, apoptosis and migration (Hynes, 2002). Integrins are heterodimers made up of α and β subunits, each with short cytoplasmic tails and large extracellular domains. Integrins function as adhesion molecules and frequently form a physical connection between the extracellular matrix (ECM) and the actin cytoskeleton.
In addition to their function in cell adhesion, integrins are critical to many of the signaling pathways of cells (Giancotti and Ruoslahti, 1999; Giancotti and Tarone, 2003; Juliano et al., 2004). Of particular relevance to our studies, numerous examples have been documented in which integrins regulate the activity of mitogen-activated protein (MAP) kinases such as extracellular signal-regulated kinase (ERK) (Roux and Blenis, 2004), or in turn are regulated by these enzymes (Assoian and Schwartz, 2001; Howe et al., 2002). Integrins may directly mediate ERK activation, or in other cases, they may function to modulate the activities of growth factor receptors on ERK signaling.
ERK-induced gene expression requires the transport of ERK into the nucleus (Pouysségur et al., 2002). In the absence of stimulation, ERK is maintained in the cytoplasm through an interaction with its upstream activator mitogen-activated protein kinase kinase (MEK) (Fukuda et al., 1997). MEK phosphorylates both tyrosine and threonine residues in the activation loop of ERK (Pouysségur et al., 2002). After phosphorylation by MEK, diphospho-ERK (dpERK) probably dimerizes and enters the nucleus via an active transport mechanism (Görlich, 1998; Khokhlatchev et al., 1998; Adachi et al., 1999). The subcellular localization of dpERK after activation offers an additional level of regulation of ERK signaling (Pouysségur et al., 2002; Kumar et al., 2003; Smith et al., 2004; Marenda et al., 2005; Vrailas et al., 2006).
In general, cells in suspension respond weakly to growth factor stimulation compared with cells adhering to the ECM (Comoglio et al., 2003), and regulation of ERK nuclear import is one potential step where integrin and receptor tyrosine kinase (RTK) signals may be integrated. For example, after activation by MEK in NIH 3T3 cells maintained in suspension the majority of ERK remains in the cytoplasm, and ERK activation of the transcription factor Elk-1 is reduced compared with adherent cells (Aplin et al., 2001). A β4 integrin signaling domain has been shown to affect the nuclear translocation of MAP kinases and NF-κB although the large cytoplasmic domain of β4 is not homologous to that of other integrin β subunits (Nikolopoulos et al., 2005). The nuclear localization of other transcriptional regulators also has been shown to be altered by integrin function in mammalian cells, including the c-Abl tyrosine kinase in mouse fibroblasts and the transcriptional coactivator JAB1 in a variety of cell types (Lewis et al., 1996; Taagepera et al., 1998; Bianchi et al., 2000). Additional connections between integrins and nuclear import are suggested by studies on proteins that are typically considered to be downstream of integrins. For example, integrin-linked kinase (ILK) has recently been shown to regulate the nuclear import of a c-Jun coactivator protein (Quelo et al., 2004).
A potential link between integrins and nuclear import has been further suggested by studies of wing development in Drosophila. In Drosophila, integrins are required to maintain the attachment of the dorsal and ventral wing epithelia during adult morphogenesis, and this process depends on the differential expression αPS1 and αPS2 integrin subunits on the dorsal and ventral cells, respectively (Bokel and Brown, 2002; Brabant et al., 1996; Brower, 2003). Loss of integrin function leads to wing blisters, where the two surfaces separate after eclosion of the adult from the pupal case. Surprisingly, wing blisters can also occur when an α subunit is inappropriately expressed on the wrong side of the wing, and experiments with various mutants have demonstrated that this is a gain-of-function phenotype (Brabant et al., 1996; Baker et al., 2002). That is, the activity of an integrin in the wrong place during a specific morphogenetic event causes a subsequent loss of epithelial attachment. A genetic screen for dominant suppressors of this gain-of-function wing blister phenotype identified null mutations in a gene named moleskin (msk) (Baker et al., 2002).
The moleskin gene encodes Drosophila Importin-7 (DIM-7), which is a close homologue of vertebrate Importin-7 (Lorenzen et al., 2001), also known as Ran Binding Protein-7 (RanBP-7). Importin-7 is a member of the importin β superfamily of nuclear importers, which can bind directly to the nuclear pore complex (Görlich et al., 1997; Jäkel et al., 1999). Vertebrate Importin-7 has been shown to mediate nuclear import of ribosomal proteins, histone H1, the HIV-1 reverse transcription complex and the glucocorticoid receptor (Jäkel et al., 1999; Fassati et al., 2003; Freedman and Yamamoto, 2004). In Drosophila, DIM-7 is tyrosine phosphorylated in response to growth factor stimulation of RTKs, and it physically binds Drosophila ERK (Lorenzen et al., 2001). Additionally, DIM-7 binds the tyrosine phosphatase Corkscrew (CSW), the Drosophila homologue of SHP-2 (Perkins et al., 1996; Lorenzen et al., 2001). Corkscrew is generally required for ERK signaling via RTKs (Perkins et al., 1992, 1996; Allard et al., 1996; Neel et al., 2003), and in vertebrate cells SHP-2 has been associated with integrin signaling and regulation of integrin activity, although the molecular bases of these interactions remain unclear (Yu et al., 1997; Oh et al., 1999).
Until recently, it has not been clear how dpERK gains entry to the nucleus after activation. In addition to regulated nuclear import, the cellular localization of phosphorylated ERK dimers can be influenced by release from cytoplasmic anchors and regulated nuclear retention or export (Pouysségur et al., 2002), and in at least one case it has been suggested that ERK2 may not require any additional import proteins (Whitehurst et al., 2002). Genetic experiments with Drosophila embryos demonstrate that DIM-7 is largely responsible for the nuclear import of activated ERK in this system (Lorenzen et al., 2001). The suppression of integrin-related phenotypes in fly wings by moleskin mutations led us to examine a potential connection between integrins and the regulation of ERK import in a Drosophila cell culture system, and our results suggest that DIM-7 may represent a novel nexus of integrin and RTK signaling.
MATERIALS AND METHODS
Cell Culture and Transfection
Drosophila S2/M3 cells were maintained at 22°C in M3 medium (Shields and Sang M3 insect medium; Sigma-Aldrich, St. Louis, MO) supplemented with 12.5% fetal calf serum (FCS; Irvine Scientific, Santa Ana, CA) and 0.2 μM methotrexate for transformed lines. Integrin expressing cell lines used in DIM-7 colocalization experiments have been described previously (Jannuzi et al., 2002). All cells used in dpERK experiments were transiently transfected or stably transformed with plasmids containing integrin transgenes and a selectable marker (Jannuzi et al., 2002). Control cells were transfected with wild-type αPS2CβPS. Quantitative experiments with transients (e.g., Figures 2, 4, and 5) were carried out 5 to 6 d after transfection. For nuclear dpERK experiments integrin surface expression for each transfection was checked by flow cytometry.
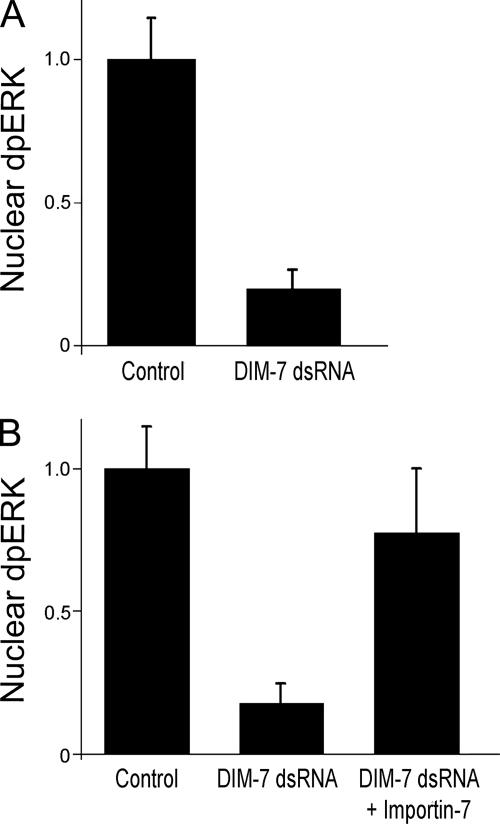
Figure 2.
DIM-7 is important for nuclear dpERK. (A) Spreading S2 cells expressing αPS2βPS integrins were stained for dpERK 30 min after plating on glass coverslips coated with RBB-Tiggrin. Nuclear dpERK was quantitated microscopically in control cells (treated with dsRNA against endogenous βPS) or cells treated with DIM-7 (mskA) dsRNA (control value normalized to 1). (B) Similar to (A), except cells were plated and serum starved overnight, and assayed 20 min after stimulation with insulin. Transfection with Xenopus Importin-7 largely rescues the inhibition of nuclear dpERK seen with dsRNA against DIM-7. In all cases, only cells that spread to similar extents were measured (see text). Error bars indicate standard deviations from averages for three experiments; each experiment represents a minimum of 10 cells from seven different fields. Representative images of treated cells can be found in Supplemental Figure 3.
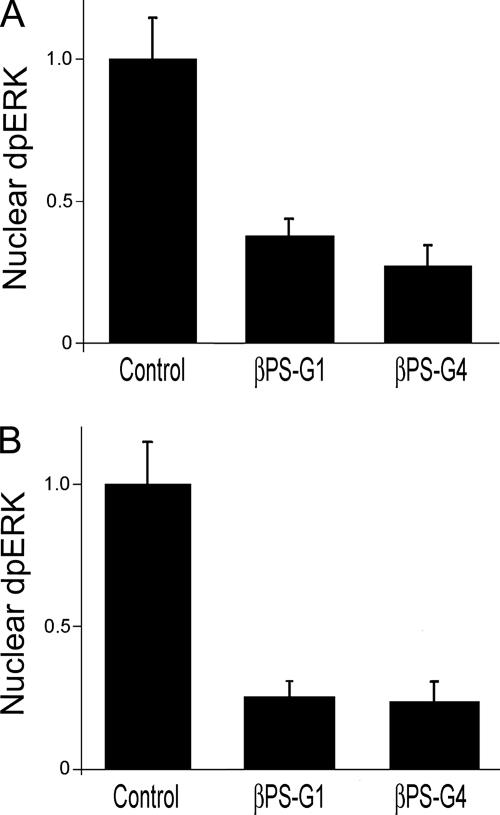
Figure 4.
Integrin mutations inhibit dpERK nuclear localization. Spreading (A) or insulin-stimulated (B) cells were stained for dpERK as described in Figure 2. Cells expressing mutations in integrin β subunits that alter cytoplasmic residues (G1) or ligand binding (G4) greatly reduce nuclear dpERK, even though these cells are spread to similar extents. (The βPS-G4 cells are spread independently of their integrins; see text.) Error bars indicate standard deviations from averages from three experiments; each experiment represents a minimum of 10 cells from seven different fields. Representative images of treated cells can be found in Supplemental Figure 3.
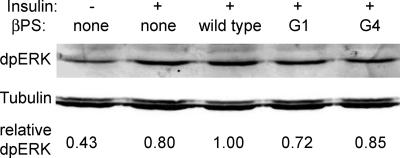
Figure 5.
Total phospho-ERK levels are similar in integrin mutants. Western blot showing levels of total dpERK 20 min after insulin stimulation (except lane 1) in cells expressing wild-type or mutant forms of βPS. Loading control is the same blot probed with antibodies against β-tubulin. As indicated by the quantitation below the bands (which reflects a ratio of dpERK to tubulin), dpERK is sometimes reduced in integrin mutants relative to cells expressing wild-type integrins, but integrin mutations are never seen to reduce total dpERK levels similarly to the reduction in nuclear dpERK at the same time point (Figure 4).
Double-stranded RNA (dsRNA) Treatment of S2 Cells
Stably transformed S2 cell lines were treated with dsRNA by using protocols described previously (Clemens et al., 2000; Jannuzi et al., 2002; Muda et al., 2002). To eliminate the endogenous βPS (myospheroid) gene, dsRNA against the 3′-untranslated sequence was used as described in Jannuzi et al. (2002). For the dpERK localization experiments, both the cells transfected with mutant and wild-type (control) integrins were treated with this myospheroid dsRNA, which therefore also serves as a control for nonspecific RNA interference (RNAi) effects. Primer sequences used to generate the dsRNAs are in the Supplemental Material, and examples of protein reductions seen in dsRNA-treated cells can be found in Supplemental Figure 1.
Cell Spreading and Immunofluorescence
Cells were spread on 10-well printed microscope slides (Carlson Scientific, Peotone, IL) coated with 500 ng/ml RBB-Tiggrin. RBB-Tiggrin is a bacterial fusion protein encoding 53 amino acids from the Drosophila extracellular matrix protein Tiggrin (Fogerty et al., 1994; Jannuzi et al., 2002). For experiments involving dpERK or total ERK localization, cells were washed twice with M3 medium with 2 mg/ml bovine serum albumin (M3 + BSA), and 10,000 cells were spread at 22°C in 50 μl of M3 + BSA. Cells treated with insulin were incubated on the slide for 2 h before replacing the medium with M3+BSA containing 10 μg/ml bovine insulin. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. Cells were then treated with 0.2% Triton X-100 in Tris-buffered saline (TBS; 150 mM NaCl and 50 mM Tris base, pH 7.5) for 2 min at room temperature, and washed three times for 1 min each with TBS. Slides were incubated with primary antibody overnight at 4°C. All antibody incubations were in M3 + 12.5% FCS except for the dpERK antibody, which was incubated in TBS + 2% BSA. Secondary Alexa antibodies (Invitrogen, Carlsbad, CA) were incubated for 2 h at room temperature. For phalloidin-stained cells, rhodamine-labeled phalloidin (Invitrogen) was incubated with the cells at 5 U/ml in TBS for 1 h at room temperature. Slides were then washed with TBS three times and mounted in VECTASHIELD (Vector Laboratories, Burlingame, CA).
The following antibodies were used for immunofluorescence: mouse anti-dpERK (1:250) and rabbit anti-ERK (1:2000) (Sigma-Aldrich), rabbit anti-DIM-7 (1:1000) (Lorenzen et al., 2001), mouse anti-βPS CF.6G11 (1:32,000) (Brower et al., 1984), and mouse anti-Paxillin (1:1000) (BD Biosciences, San Jose, CA).
Image Acquisition and Nuclear dpERK Quantification
For cytological quantification of nuclear dpERK, images were collected on a Zeiss Universal microscope by using an AxioCam MRM camera, and nuclear staining was measured using AxioVision version 4.1.1 software (Carl Zeiss, Jena, Germany). For each set of conditions in each experiment dpERK immunofluorescence was quantified from images taken at identical exposures. The intranuclear circle in which dpERK was measured was the same size for all nuclei, and the measurements therefore reflect dpERK concentration. However, because the nuclei are so similar in diameter (and the circle includes almost all of the nucleus), the values also closely reflect total dpERK. Each value represents measurements from a minimum of 10 nuclei from at least seven different fields. Only cells that were spread to similar degrees were scored. All values are normalized to wild type (=1.0). Other ERK images (Figures 1, 3, and 6) were collected on a Nikon Eclipse 90i microscope with a CoolSnap K4 camera running Simple PCI, version 6.0. Images of peripheral DIM-7 were collected using a 35-mm camera and 35-mm slide film. Confocal images were captured with a Nikon PCM 2000 confocal microscope running Simple PCI, version 4.0.6 software (Compix, Lake Oswego, OR). Optical sections were 8 μm in thickness.
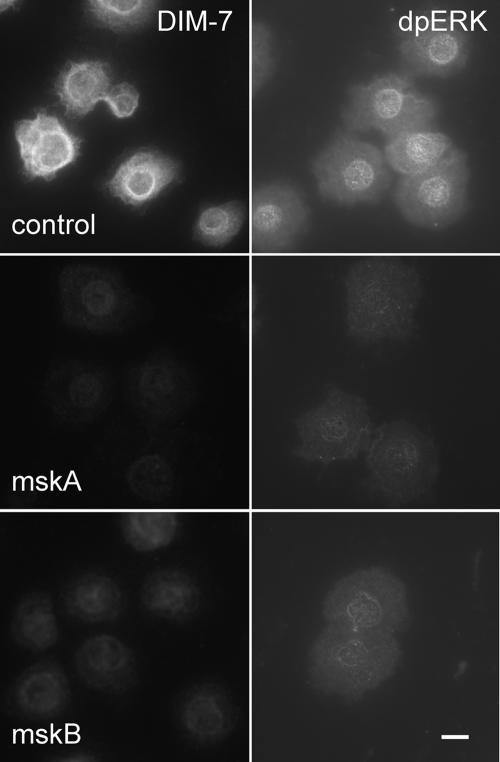
Figure 1.
DIM-7 is important for nuclear dpERK accumulation in spreading S2 cells. Integrin expressing, spreading cells were treated with control dsRNA (against the endogenous βPS) or one of two nonoverlapping dsRNAs that target DIM-7 (mskA and mskB, middle and bottom rows, respectively). The cells were fixed and stained with antibodies against DIM-7 (left column) or dpERK (right column). The DIM-7 dsRNAs reduce both DIM-7 and nuclear dpERK; mskA is slightly more effective in each assay, and was used in subsequent experiments. Bar, 10 μm.
Figure 3.
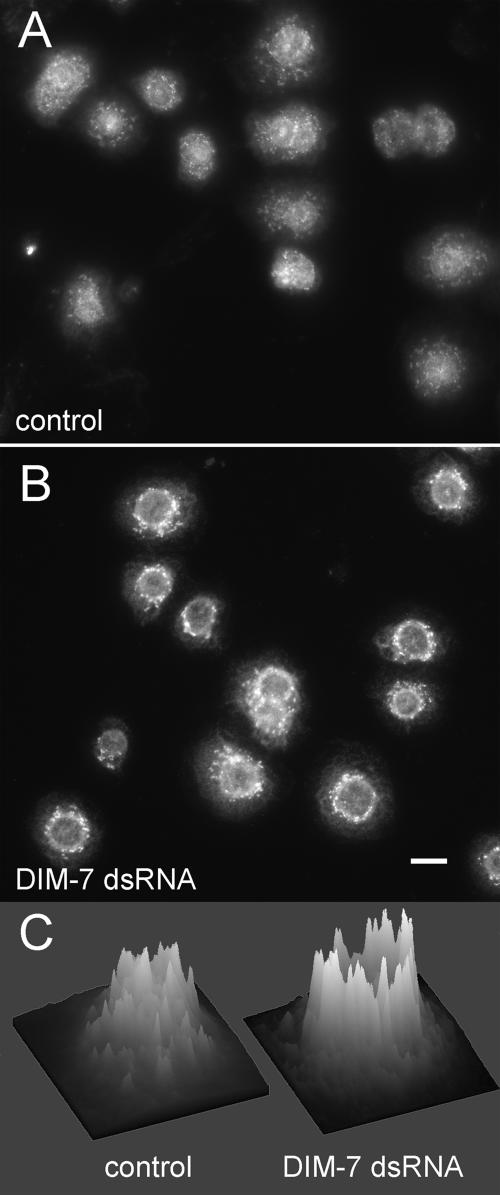
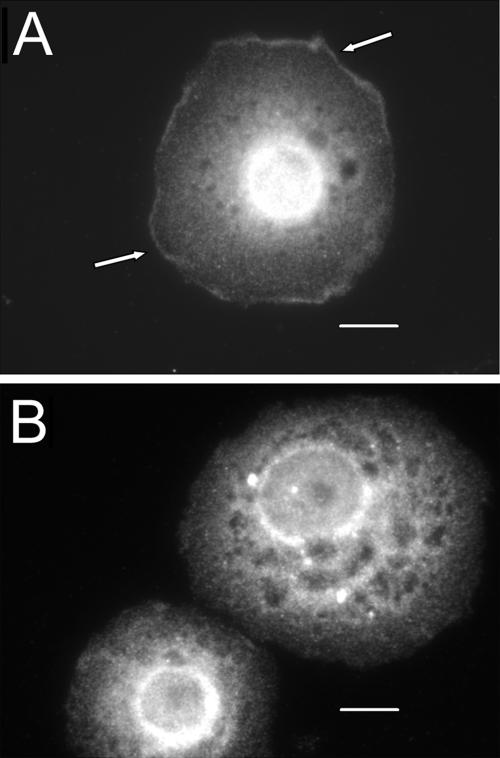
Distribution of total ERK in integrin-expressing, spreading S2 cells changes after DIM-7 reduction. αPS2βPS integrin-transfected cells were stained for total ERK (compared with dpERK staining in similarly treated cells in Figure 1). Cells in A were treated with only control dsRNA against endogenous βPS; cells in B were also grown in dsRNA targeting DIM-7. Most cytoplasmic ERK is present in aggregates. On reduction of DIM-7, the aggregates are brighter and concentrated around the nucleus, often creating a bright perinuclear ring. This is especially evident in intensity plots of individual cells, as illustrated in C. Similar plots for 8 cells from each panel can be found in the Supplemental Figure 6. Bar, 10 μm.
Figure 6.
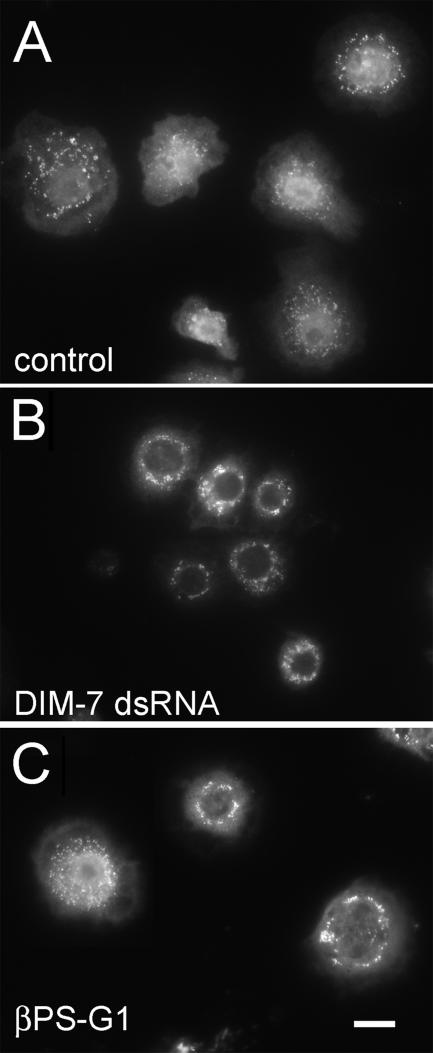
Total ERK in insulin treated, integrin expressing cells. As for spreading cells (Figure 3), total cytoplasmic ERK in control cells (expressing wild-type αPS2βPS) is found largely in aggregates (A), which tend to localize around the nucleus in cells treated with dsRNA against DIM-7 (B). Cells that express the cytoplasmic βPS-G1 mutant integrin with αPS2 (C) are more variable; most show similarities to DIM–depleted cells, although some, such as the cell on the left, look much like control cells. All cells are treated with dsRNA against the endogenous βPS gene. Bar, 10 μm.
Western Blotting
For experiments showing DIM-7 and CSW levels in dsRNA-treated cells, lysates containing 50,000 S2 cells per well were loaded on a 4–20% gradient gel. Blots were probed with rabbit anti-DIM-7 1129 (1:2000), and rabbit anti CSW F1088 (1:4000) (Lorenzen et al., 2001). Blots were then probed with horseradish peroxidase-conjugated anti-rabbit secondaries, and they were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL).
For experiments showing levels of total dpERK, transiently transfected S2 cells were serum starved for 24 h 4 d after transfection, stimulated with 5 μg/ml insulin and lysed after 20 min in a lysis buffer containing 20 mM Tris, pH 8, 1% Triton X-100, 10% glycerol, 140 mM NaCl, 2.5 mM sodium vanadate, 1 mM NaF, 1 mM EDTA, 1 mM EGTA, and 1% Sigma protease inhibitor cocktail (P-8340) for 30 min. Laemmli buffer was added to the total lysate, and samples were boiled and centrifuged before loading 10 μg per lane. Blots were probed with anti-dpERK monoclonal antibody (1:2000) (Sigma-Aldrich) and rabbit anti-β-tubulin (1:4000) (Zymed Laboratories, South San Francisco, CA). Secondary antibodies used were Alexa Fluor 680 (1:2000) (Invitrogen) and IRDye 800 (1:20,000) (Rockland, Gilbertsville, PA). Blots were then visualized on an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE).
For immunoprecipitation experiments showing levels of DIM-7 tyrosine phosphorylation in dsRNA-treated cells, 1 × 106 S2 cells (stably transformed to express integrins) were plated in six-well dishes in Drosophila serum-free medium (Invitrogen). Fifteen to 45 μg of dsRNA was added for each of the candidate genes of interest, and cells were incubated for 3 h. Then, 2 ml of complete medium with serum was added, and the cells were incubated for 2 d for protein depletion. Cells were then serum starved for 24 h and lysed 10 min after the addition of 5 μg/ml insulin in a lysis buffer containing 20 mM Tris, pH 8, 1% Triton X-100, 10% glycerol, 140 mM NaCl, 2.5 mM NaVO4, 1 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, and 2 μg/ml benzamidine for 30 min. Nuclei and cell debris were cleared by centrifugation at 20,000 × g for 10 min at 4°C.
About 500 μg of total protein was incubated with anti-DIM-7 1129 (1:200) overnight at 4°C. Sepharose-protein A/G beads were added and incubated for 1 h, and immunoprecipitates were washed three times with cold lysis buffer. Proteins in the immunoprecipitate complex were separated on an 8% polyacrylamide gel electrophoresis gel and probed with anti-DIM-7 1129 (1:1000) or anti-phospho-tyrosine (1:1000) (pTyr-100; Cell Signaling Technology, Danvers, MA).
RESULTS
DIM-7 Is Required for Nuclear Accumulation of dpERK
In the tracheal placodes of Drosophila embryos DIM-7 (encoded by the gene moleskin) is important for nuclear localization of activated ERK in response to either EGF or FGF signaling, and DIM-7 coimmunprecipitates with activated ERK in insulin stimulated S2 cells (Lorenzen et al., 2001). To examine DIM-7 requirements for nuclear accumulation of dpERK in more detail, Drosophila S2 cells transfected to express αPS2βPS integrins were treated with DIM-7 (moleskin) dsRNA, and the cells were spread on slides coated with a fragment of the integrin ligand Tiggrin (RBB-Tiggrin). (dsRNA targeting the endogenous myospheroid, or βPS, gene was added to all cells, and it serves as a control for nonspecific RNAi effects.) Nuclear dpERK was then assayed by immunofluorescence, in two different experimental paradigms. In actively spreading cells, nuclear dpERK peaks at ∼30 min after plating, with staining returning to basal levels after 1 h. Alternatively, previously spread cells are stimulated with insulin, in which case dpERK staining is maximal at ∼20 min (Supplemental Figure 2). The effects of DIM-7 reduction are similar in either paradigm. Cells treated with either of two different dsRNAs that reduce expression of DIM-7 (mskA or mskB) show a significant reduction in maximum nuclear dpERK compared with cells treated with a control dsRNA (Figure 1). Although both experimental dsRNAs reduce DIM-7 and nuclear dpERK immunofluorescence, the mskA dsRNA seems somewhat more effective, and this was chosen for subsequent studies. We quantitated the nuclear dpERK immunofluorescence, selecting only cells that were spread on the substratum. As shown in Figure 2, reducing DIM-7 results in a four- to fivefold decrease in nuclear dpERK staining in either experimental paradigm (during active cell spreading or after insulin stimulation).
Xenopus Importin-7 Can Mediate ERK Nuclear Localization
The vertebrate orthologue of DIM-7, Importin-7 (RanBP-7), is >50% identical to DIM-7, and this identity extends over the entire length of the primary sequence (Lorenzen et al., 2001). To determine whether the ERK import function of DIM-7 is shared with vertebrate Importin-7 family members, we tested the ability of Xenopus Importin-7 to rescue DIM-7 dsRNA-treated S2 cells. When stimulated with insulin, these DIM-7–depleted cells expressing Xenopus Importin-7 (and integrins) show levels of nuclear dpERK more similar to those seen in wild-type cells, by using either insulin stimulation (Figure 2) or the cell spreading paradigm (data not shown). This rescue also indicates that the earlier decrease in nuclear dpERK is not due to the unlikely possibility of off-target effects that are shared by the two DIM-7 dsRNAs.
Cytoplasmic ERK Distribution
In these experiments, we quantitated dpERK staining microscopically, restricting our analyses to the nuclei (see Materials and Methods). Importins are not expected to affect ERK phosphorylation directly, and on Western blots we see approximately similar dpERK levels in control cells and in cells with reduced DIM-7 after insulin treatment or during cell spreading (Supplemental Figure 4). Although we see a dramatic decrease in nuclear dpERK microscopically, we do not see a corresponding increase in cytoplasmic dpERK. This paradox could result from the fact that the dpERK antibody binds to a phosphorylation site that is recognized by other proteins, and the exposure of this epitope may be different in the nucleus and cytoplasm. In light of this, we also examined the distribution of total ERK in these cells.
Cytoplasmic ERK immunofluorescence in spreading S2 cells is most prevalent in aggregates or filamentous structures (Figure 3). Western blots indicate that overall ERK levels are unchanged in DIM-7 dsRNA-treated cells (Supplemental Figure 5); however, staining of the cytoplasmic aggregates is more intense after DIM-7 reduction, and the ERK is more concentrated around the nucleus. In fact, a distinct nuclear outline of ERK immunofluorescence is typically prominent in DIM-7 dsRNA cells. This is especially clear when the staining intensity is graphically displayed. (Figure 3C shows representative cells; a larger sample can be found in the Supplemental Figure 6.) Although they seem to contain most of the total cellular ERK, the extranuclear aggregates do not bind the dpERK antibody (Figure 1). Together, these data suggest that after treatment with DIM-7 dsRNA import of ERK is inhibited, resulting in a “traffic jam” around the nucleus. This results in greater concentration of cytoplasmic ERK into perinuclear complexes, and the aggregation of ERK may contribute to the masking of the phospho-ERK epitope. Regardless, the concentration of ERK around the nucleus supports the conclusion from the Western data, that defects in ERK activation (phosphorylation) are not the cause for reduced nuclear dpERK after knockdown of DIM-7.
Integrins Are Required for Nuclear dpERK
Adhesion to the ECM has been shown to promote nuclear localization of activated ERK in NIH-3T3 cells (Aplin et al., 2001). Adhesion dependence of nuclear translocation is often addressed by comparing cells attached to a substratum with those in suspension. Using characterized mutant proteins, we sought to determine specifically whether there is an integrin requirement for ERK import in Drosophila S2 cells. All of the cells we examined were attached to and spread on the substratum, and to define specific integrin requirements we used S2 cells containing transgenes that express either wild-type αPS2βPS integrins or αPS2 combined with one of two βPS mutant subunits, corresponding to mutations (mysG1 and mysG4) that have been isolated and studied in developing flies. βPS-G4 has a mutation in the second serine of the MIDAS domain (DXSXS), which would be expected to inhibit extracellular ligand binding (Xiong et al., 2001; Jannuzi et al., 2002). Phenotypically, this allele behaves similarly to loss of function alleles in vivo, and it shows little ability to mediate cell spreading on ECM ligands in culture (Jannuzi et al., 2002). βPS-G1 contains a frameshift mutation in the cytoplasmic domain that eliminates the two NPXY motifs that are critical for interaction with a number of cytoplasmic proteins, including talin (Calderwood et al., 2002; Garcia-Alvarez et al., 2003). This allele has dominant-negative properties in developing animals, and experiments with S2 cells suggest that integrins with this mutation may mediate cell spreading, but the integrins are not regulated properly (Jannuzi et al., 2002). All of these experiments were performed using cells treated with dsRNA specific for the 3′-untranslated region of the endogenous βPS transcript; previous work has demonstrated that this eliminates effects due to expression of the endogenous wild-type genes (Jannuzi et al., 2002). (In the case of βPS-G4, we take advantage of the fact that integrins are not required for S2 cells to spread in the absence of serum.)
Cells expressing either βPS-G4 or βPS-G1 mutant integrins show greatly reduced nuclear dpERK in both the “cell spreading” and “insulin-stimulated” paradigms (Figure 4). This difference was not a consequence of simple adhesion or overall shape, because only cells that were spread to similar degrees were selected for the comparisons.
As noted for the DIM-7 dsRNA-treated cells, we do not see increases in cytoplasmic dpERK staining that match the nuclear decreases. Although we sometimes see slight reductions in dpERK on Western blots from integrin mutant cells (Figure 5), these are never similar to the magnitude of the decrease in nuclear dpERK seen cytologically (Figure 4). We therefore examined total ERK distribution after insulin stimulation for the βPS-G1 mutant, which spreads via integrin binding to RBB-Tiggrin. As seen for spreading cells (Figure 3), the cytoplasmic ERK in insulin-stimulated cells is found mostly in aggregates, which outline the relatively dark nuclei after treatment with DIM-7 dsRNA (Figure 6). These aggregates are not seen with the dpERK antibody, indicating that the dpERK epitope is probably inaccessible in these structures. Compared with DIM-7 dsRNA-treated cells, the distribution of the aggregates in the integrin mutant cells is more variable, and cells with ERK concentrated around the nucleus are mixed with cells that display a more diffuse distribution of the cytoplasmic aggregates, similar to control cells. Together, these data indicate that expression of βPS-G1 has its greatest effect on ERK import, as opposed to ERK phosphorylation.
Integrins Affect DIM-7 Phosphorylation
The above-mentioned data indicate that integrin function affects the DIM-7–mediated nuclear import of activated ERK, and mutations in the moleskin (DIM-7) gene suppress an integrin gain of function phenotype in developing animals (Baker et al., 2002). These data suggested the possibility that integrin function may facilitate ERK nuclear import by affecting the activity of DIM-7. DIM-7 is tyrosine phosphorylated in response to insulin (Lorenzen et al., 2001; Supplemental Figure 7), and we examined the integrin dependence of this phosphorylation, by using cells stably transformed with various integrin genes and treated with dsRNA to reduce expression of the endogenous β subunit. Cells were stimulated with insulin, and DIM-7 was immunopurified from postnuclear fractions. Equal amounts of total DIM-7 protein are found in cells expressing no integrins, wild-type integrins, or either the βPS-G4 or βPS-G1 mutant proteins. However, tyrosine phosphorylation of DIM-7 is greatest in the cells expressing wild-type βPS (Figure 7); hormone stimulation is not sufficient to maximize this modification of the importin in cells expressing mutant integrins.
Figure 7.
DIM-7 tyrosine phosphorylation is affected by integrins. Proteins were immunoprecipitated by anti-DIM-7 from cells 10 min after stimulation by insulin (or after no insulin, lane 1). The cell lines used were transformed to express no integrin or αPS2 in combination with the βPS subunit indicated. All cells (including controls) were grown in dsRNA to inhibit expression of endogenous βPS. Expression of the mutant βPS subunits does not significantly alter the amount of DIM-7 that is brought down, but it does reduce its tyrosine phosphorylation.
DIM-7 Colocalizes with Integrins at the Cell Periphery
Importins are generally thought of as functioning in and around the nuclear envelope. However, the findings that integrins can affect both DIM-7 functions (ERK import), and, more directly, the biochemical modification of DIM-7, suggest the possibility that DIM-7 may be regulated by events at or near the plasma membrane. This hypothesis predicts that there should be significant concentrations of DIM-7 at the cell periphery, away from the nucleus. Using an antibody against the DIM-7 protein, we examined the subcellular distribution of DIM-7 by immunolocalization in αPS2βPS-expressing S2 cells spread on RBB-Tiggrin–coated slides. As expected, the majority of DIM-7 seems to be perinuclear. However, distinct concentrations of DIM-7 are seen at the periphery of spreading cells. Peripheral DIM-7 typically is seen in regions characterized by brightly stained integrin dots near the spreading cell edges (Figure 8, A and B). This and all of the DIM-7 immunostaining patterns described below are greatly reduced in cells treated with DIM-7 dsRNA (which reduces DIM-7 protein levels by >90%), indicating that the staining is specific for DIM-7 (e.g., Figure 1). Indeed, as DIM-7 protein levels are reduced by increasing levels of dsRNA, the peripheral staining is the first to disappear (data not shown). (In these experiments, integrins are visualized with the mouse monoclonal CF.6G11, which is highly specific for βPS, e.g., Bunch et al., 1992.) The βPS-G1 and βPS-G4 mutants affect ERK nuclear import, but DIM-7 is still found at the periphery in cells expressing either mutant (data not shown).
Figure 8.
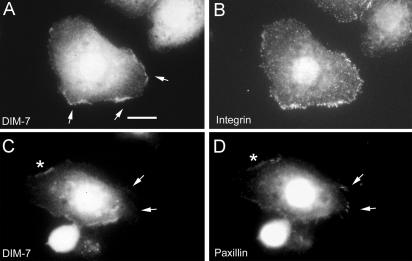
DIM-7 and integrin colocalization. Drosophila S2 cells expressing αPS2βPS integrins were spread on a fragment of the ECM ligand Tiggrin, and fixed and stained to show the distributions of various components. (A and B) DIM-7 is found at high levels around the cell nucleus, and it also is localized at the cell periphery where concentrations of integrins (stained with anti-βPS) are mediating cell spreading (arrows). (C and D) DIM-7 is specifically absent from focal adhesions (visualized by high-density accumulations of paxillin, arrows) and the surrounding areas of the cell periphery. More diffuse paxillin and DIM-7 are seen along spreading edges of the cell (e.g., asterisks). Bar, 10 μm.
DIM-7 Is Not at Focal Adhesions
In many cell types, integrins also are concentrated in focal adhesions, the largest of which are typically found at the ends of actin stress fibers. Obvious actin stress fibers are relatively rare in our S2 cells, but when found they also terminate in integrin-dense focal adhesions (Zavortink et al., 1993). In such cells, we do not see accumulations of DIM-7 along the fibers or at the focal adhesions. To examine this further, we double-stained cells for DIM-7 and paxillin, a cytoplasmic protein that is a prominent component of focal adhesions. Diffuse paxillin staining is often found along the spreading edges of the cells, where DIM-7 and integrins are also common. However, DIM-7 is never found at the discrete paxillin-stained focal adhesions (Figure 8, C and D). Moreover, although both diffuse paxillin and DIM-7 may be present in nearby regions, DIM-7 is conspicuously absent from the cortex immediately around the focal adhesions, regardless of focal plane.
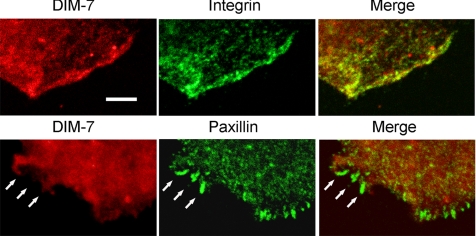
This absence of DIM-7 from regions around stress fiber-associated focal adhesions is quite stunning, and we decided to examine DIM-7 localization at higher resolution in more dynamic adhesive complexes in spreading cells. Again, DIM-7 is often seen colocalized with integrins at the edges of spreading lamellae (Figure 9). Spreading S2 cells sometimes display relatively short paxillin-positive adhesions along their extended edges. Unlike the larger stress fiber-associated focal adhesions, these shorter structures may occasionally overlap with diffuse DIM-7 patches. However, these sites also do not concentrate DIM-7 into the paxillin-defined adhesions (Figure 9). Thus, the integrin-DIM-7 colocalization clearly depends on the nature of the integrin-associated cytoplasmic complexes.
Figure 9.
DIM-7 colocalizes with integrins but not with organized adhesive sites. Confocal images of peripheral areas of cells plated on RBB-Tiggrin, as in Figure 8, but at higher resolution. As shown in the top row, DIM-7 and integrins are largely concentrated in the same regions near the edge of the cell. However, where more organized adhesive sites are formed (shown by paxillin staining, lower row), there is no concomitant concentration of DIM-7. Bar, 5 μm.
Peripheral DIM-7 Requires Corkscrew
DIM-7 has been shown to interact physically with Corkscrew, the Drosophila SHP-2 homologue, in both the two-hybrid system and via coimmunoprecipitation (Lorenzen et al., 2001), and SHP-2/Corkscrew has been shown to function in both RTK and integrin-induced ERK signaling (Oh et al., 1999). These findings led us to ask whether Corkscrew is necessary for the peripheral localization of DIM-7 seen in S2 cells. Cells treated with corkscrew dsRNA show a significant decrease in Corkscrew protein levels (Supplemental Figure 1), and peripheral localization of DIM-7 in these cells is typically undetectable (Figure 10). Thus, the biochemical interaction between Corkscrew (SHP-2) and DIM-7 is necessary for recruitment of the importin to sites of integrin function.
Figure 10.
Peripheral localization of DIM-7 is Corkscrew dependent. (A) Integrin-expressing cells were spread on RBB-Tiggrin and stained for DIM-7. Note the DIM-7 staining around virtually the entire periphery in this cell (arrows). (B) DIM-7 peripheral localization is absent in cells that have been treated with dsRNA against corkscrew, although DIM-7 staining seems greater overall than in A; Western blots confirm that DIM-7 levels increase in cells with reduced Corkscrew (data not shown). Bar, 10 μm.
DISCUSSION
Importin-7 Is a General ERK Nuclear Import Factor
We show that a vertebrate homologue of DIM-7 can rescue the ERK localization phenotype of DIM-7 dsRNA treated cells. Thus, ERK nuclear translocation is a property of members of the Importin-7 family of proteins generally. This function cannot necessarily be extended to other MAP kinases; for example, we see no change in nuclear localization of the p38 MAP kinase in S2 cells grown in DIM-7 dsRNA, although c-Jun NH2-terminal kinase transport does seem to involve DIM-7 (unpublished data).
Role for PS2 Integrins in ERK Localization
The ability of growth factors to activate ERK signaling is often linked to integrins (Aplin et al., 2001; Howe et al., 2002); however, specific integrin functions typically have not been examined (but see Hirsch et al., 2002; discussed below). The reduced levels of nuclear dpERK in cells expressing βPS-G1 or βPS-G4 show that ERK signaling seen in Drosophila S2 cells is dependent on functional integrins and that it is not due solely to changes in cell adhesion or shape. Specifically, both the extracellular and cytoplasmic integrin domains must be able to interact properly with ECM or intracellular components for the integrins to support high levels of nuclear dpERK.
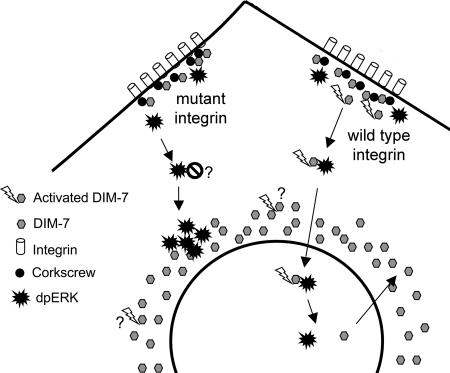
A simple model in which soluble dpERK (activated by integrins or growth factors) finds DIM-7 (“activated” by integrins) for import cannot explain all of the data. The experiments that examine total ERK distribution in the integrin mutants suggest that most of the activated ERK cannot enter the nucleus even after translocation into the DIM-7–rich perinuclear region. One would expect that the importins here are actively working with various other cargos and that a significant fraction of the DIM-7 is generally capable of import. This result suggests a model in which ERK phosphorylation must be spatially coupled to DIM-7 activation to make a complex that can be imported efficiently (Figure 11). Interestingly, a specific membrane localization of DIM-7 has been suggested as a regulatory mechanism in developing Drosophila eyes; however, in this case the targeting to apical epithelia has been seen as an inhibitory mechanism (Vrailas et al., 2006).
Figure 11.
Model for the role of integrins in ERK nuclear import in S2 cells. DIM-7 is localized near functioning integrins, at least in part by its association with the SHP-2 phosphatase Corkscrew. dpERK (activated via either hormone or cell spreading) must associate with activated (perhaps by tyrosine phosphorylation) DIM-7 at the periphery, after which the ERK/DIM-7 complex is imported into the nucleus. In cells expressing mutant integrins, ERK can be phosphorylated and transported centrally, but this ERK cannot efficiently cross the nuclear envelope, even though there is a high perinuclear concentration of DIM-7. It is not known whether this is because the ERK associates with an inhibitor in the absence of activated DIM-7 at the periphery or whether the perinuclear DIM-7 is not activated in the same way as the cortical importin. Note that DIM-7 is not required for nuclear directed transport, only nuclear import, because the perinuclear ERK aggregates are formed in DIM-7–depleted cells as well as those expressing mutant integrins.
moleskin (DIM-7) function is required for normal cell proliferation in animals, where patches of mutant cells disappear in developing epithelia (Baker et al., 2002; Vrailas et al., 2006; Pepple et al., 2007). Examples in which a 50% reduction in DIM-7 function has produced phenotypes in developing flies have involved circumstances in which a signaling pathway has been stimulated to high levels by gene overexpression (Baker et al., 2002; Pepple et al., 2007). In our study DIM-7 levels are reduced significantly, but they are not eliminated, and the cells show no obvious phenotype during normal growth. However, clear effects are seen after acute stimulation of the ERK pathway. The integrin-mediated activation of DIM-7 may be especially important as a regulatory component in such cases of acute, high-level signaling.
Similarities to Mammalian Integrin Studies
Barberis et al. (2000) found that mouse embryo fibroblasts expressing a β1 mutant similar to βPS-G1 transiently display elevated phospho-ERK after stimulation with growth factor, but subsequently the same groups (Hirsch et al., 2002) reported that ERK does not necessarily enter the nucleus after plating on fibronectin. Interestingly, the import defect seen in the integrin mutants can be rescued by adding constitutively active Rac. Although multiple pathways may couple integrins to ERK activation and transport in different cell types, Importin-7 family members are likely to be a common feature of dpERK nuclear translocation, and it will be interesting to see whether various pathways leading from integrins to import converge at this protein.
Perhaps most intriguing with respect to our studies is the work on a natural human β1 variant. β1C is an alternatively spliced form that, like βPS-G1, replaces the cytoplasmic NPXY motifs with other sequence. Cells that express β1C show reduced proliferation and reduced activation of the Ras–ERK pathway, relative to cells expressing the more common β1A (Meredith et al., 1995; Fornaro et al., 2000). β1A-containing integrins seem to form a complex that includes insulin-like growth factor-I receptor and the insulin receptor substrate-1 (IRS-1), and the addition of insulin leads to cell proliferation and inhibition of adhesion to laminin (Goel et al., 2004). In contrast, β1C expression leads to decreased proliferation and increased adhesion, and these effects seem to be mediated by a complex that includes Gab1 and SHP-2, but not IRS-1. There is no β1C variant naturally in Drosophila, but the βPS-G1 mutant does show some dominant-negative effects in flies (Jannuzi et al., 2002), and the work reported here suggests that this might result at least in part from disruptive effects on intracellular signaling.
Components of the Integrin–DIM-7 Connection
We find significant amounts of cortical DIM-7 where integrins are located at the spreading edges of cells. Consistent with a role of integrins in peripheral DIM-7 localization, cells that are protease treated, heat shocked to induce integrin expression, and spread in serum-free media (where spreading is integrin independent), DIM-7 is not found at the periphery in fully spread cells at early times, but it appears when integrin expression is detected after a few hours. One striking feature of the peripheral DIM-7 is that it does not colocalize with integrins in more organized cell–substratum adhesion sites. Thus, peripheral integrin-DIM-7 associations seem to depend on the functional state of the integrins.
Vertebrate SHP-2 is necessary in many contexts for growth factor activation of ERK, and SHP-2 has also been shown to be involved in integrin-dependent signaling (Neel et al., 2003). However, the data from different cell types fail to paint a simple, cohesive picture of SHP-2 molecular function, especially with respect to signaling downstream of integrins (Yu et al., 1998; Manes et al., 1999; Oh et al., 1999; Inagaki et al., 2000). Previous biochemical data indicate that DIM-7 binds Drosophila Corkscrew (SHP-2) (Lorenzen et al., 2001). Corkscrew is generally an essential component in signaling via receptor tyrosine kinases (Perkins et al., 1992, 1996; Allard et al., 1996; Johnson Hamlet and Perkins, 2001); however, Corkscrew lacking tyrosine phosphatase activity can rescue some phenotypes when reintroduced into corkscrew mutants, suggesting that in some contexts Corkscrew functions primarily as a scaffolding protein (Allard et al., 1998). The role of Corkscrew in DIM-7 activation may be largely as a scaffold, because DIM-7 disappears from the periphery when Corkscrew is depleted. This does not seem to be an indirect result of a defect in DIM-7 activation, because cortical DIM-7 remains in other situations that affect its ability to import dpERK, for example, in both of the integrin mutants tested.
Interestingly, the screen that identified moleskin (DIM-7) as a suppressor of Blistermaker assayed only ∼40% of the Drosophila genome (the third chromosome) (Baker et al., 2002). Further elucidation of the molecular mechanisms underlying the DIM-7/integrin connection is likely to be facilitated by the identification of additional Blistermaker suppressors on other chromosomes. Screens for such loci are in progress.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dirk Görlich for the Xenopus DNA, Alison Jannuzi and Carl Boswell for help with the confocal microscopy, Emily Smith for introduction to NIH ImageJ, Garth Powis for use of facilities, and Candida Morris for assistance in preparation of the manuscript. This study was supported by national Institutes of Health grants R01 GM-61707 and P01 HD-39942, Project2 (to L.A.P.), R01 GM-42474 (to D.L.B.), and K12 GM-00708 (to B.P.J.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-07-0659) on August 15, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Adachi M., Fukuda M., Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–5358. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard J. D., Chang H. C., Herbst R., McNeill H., Simon M. A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- Allard J. D., Herbst R., Carroll P. M., Simon M. A. Mutational analysis of the SRC homology 2 domain protein-tyrosine phosphatase Corkscrew. J. Biol. Chem. 1998;273:13129–13135. doi: 10.1074/jbc.273.21.13129. [DOI] [PubMed] [Google Scholar]
- Aplin A. E., Stewart S. A., Assoian R. K., Juliano R. L. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 2001;153:273–282. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Schwartz M. A. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Baker S. E., Lorenzen J. A., Miller S. W., Bunch T. A., Jannuzi A. L., Ginsberg M. H., Perkins L. A., Brower D. L. Genetic interaction between integrins and moleskin, a gene encoding a Drosophila homolog of importin-7. Genetics. 2002;162:285–296. doi: 10.1093/genetics/162.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis L., Wary K. K., Fiucci G., Liu F., Hirsch E., Brancaccio M., Altruda F., Tarone G., Giancotti F. G. Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem. 2000;275:36532–36540. doi: 10.1074/jbc.M002487200. [DOI] [PubMed] [Google Scholar]
- Bianchi E., Denti S., Granata A., Bossi G., Geginat J., Villa A., Rogge L., Pardi R. Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate AP-1 activity. Nature. 2000;404:617–621. doi: 10.1038/35007098. [DOI] [PubMed] [Google Scholar]
- Bokel C., Brown N. H. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Brabant M. C., Fristrom D., Bunch T. A., Brower D. L. Distinct spatial and temporal functions for PS integrins during Drosophila wing morphogenesis. Development. 1996;122:3307–3317. doi: 10.1242/dev.122.10.3307. [DOI] [PubMed] [Google Scholar]
- Brower D. L. Platelets with wings: the maturation of Drosophila integrin biology. Curr. Opin. Cell Biol. 2003;15:607–613. doi: 10.1016/s0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Brower D. L., Wilcox M., Piovant M., Smith R. J., Reger L. A. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. USA. 1984;81:7485–7489. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch T. A., Salatino R., Engelsgjerd M. C., Mukai L., West R. L., Brower D. L. Characterization of mutant alleles of myospheroid, the gene encoding the β subunit of the Drosophila PS integrins. Genetics. 1992;132:519–528. doi: 10.1093/genetics/132.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D. A., Yan B., de Pereda J. M., Alvarez B. G., Fujioka Y., Liddington R. C., Ginsberg M. H. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 2002;277:21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- Clemens J. C., Worby C. A., Simonson-Leff N., Muda M., Maehama T., Hemmings B. A., Dixon J. E. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio P. M., Boccaccio C., Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Fassati A., Görlich D., Harrison I., Zaytseva L., Mingot J. M. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22:3675–3685. doi: 10.1093/emboj/cdg357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty F. J., Fessler L. I., Bunch T. A., Yaron Y., Parker C. G., Nelson R. E., Brower D. L., Gullberg D., Fessler J. H. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila αPS2βPS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Fornaro M., Steger C. A., Bennett A. M., Wu J. J., Languino L. R. Differential role of β1C and β1A integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/Mitogen-activated protein kinase pathways. Mol. Biol. Cell. 2000;11:2235–2249. doi: 10.1091/mbc.11.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman N. D., Yamamoto K. R. Importin 7 and Importin α/Importin β are nuclear import receptors for the glucocorticoid receptor. Mol. Biol. Cell. 2004;15:2276–2286. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Gotoh Y., Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarez B., de Pereda J. M., Calderwood D. A., Ulmer T. S., Critchley D., Campbell I. D., Ginsberg M. H., Liddington R. C. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11:49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Goel H. L., Fornaro M., Moro L., Teider N., Rhim J. S., King M., Languino L. R. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J. Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D. Transport into and out of the cell nucleus. EMBO J. 1998;17:2721–2727. doi: 10.1093/emboj/17.10.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Dabrowski M., Bischoff F. R., Kutay U., Bork P., Hartmann E., Prehn S., Izaurralde E. A novel class of RanGTP binding proteins. J. Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E., Barberis L., Brancaccio M., Azzolino O., Xu D., Kyriakis J. M., Silengo L., Giancotti F. G., Tarone G., Fassler R., Altruda F. Defective Rac-mediated proliferation and survival after targeted mutation of the β1 integrin cytodomain. J. Cell Biol. 2002;157:481–492. doi: 10.1083/jcb.200111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A. K., Aplin A. E., Juliano R. L. Anchorage-dependent ERK signaling–mechanisms and consequences. Curr. Opin. Genet. Dev. 2002;12:30–35. doi: 10.1016/s0959-437x(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Noguchi T., Matozaki T., Horikawa T., Fukunaga K., Tsuda M., Ichihashi M., Kasuga M. Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene. 2000;19:75–84. doi: 10.1038/sj.onc.1203204. [DOI] [PubMed] [Google Scholar]
- Jäkel S., Albig W., Kutay U., Bischoff F. R., Schwamborn K., Doenecke D., Görlich D. The importin β/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–2423. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannuzi A. L., Bunch T. A., Brabant M. C., Miller S. W., Mukai L., Zavortink M., Brower D. L. Disruption of C-terminal cytoplasmic domain of βPS integrin subunit has dominant negative properties in developing Drosophila. Mol. Biol. Cell. 2002;13:1352–1365. doi: 10.1091/mbc.01-08-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Hamlet M. R., Perkins L. A. Analysis of corkscrew signaling in the Drosophila epidermal growth factor receptor pathway during myogenesis. Genetics. 2001;159:1073–1087. doi: 10.1093/genetics/159.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Reddig P., Alahari S., Edin M., Howe A., Aplin A. Integrin regulation of cell signalling and motility. Biochem. Soc. Trans. 2004;32:443–446. doi: 10.1042/BST0320443. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev A. V., Canagarajah B., Wilsbacher J., Robinson M., Atkinson M., Goldsmith E., Cobb M. E. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kumar J. P., Hsiung F., Powers M. A., Moses K. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development. 2003;130:3703–3714. doi: 10.1242/dev.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. M., Baskaran R., Taagepera S., Schwartz M. A., Wang J. Y. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc. Natl. Acad. Sci. USA. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen J. A., Baker S. E., Denhez F., Melnick M. B., Brower D. L., Perkins L. A. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development. 2001;128:1403–1414. doi: 10.1242/dev.128.8.1403. [DOI] [PubMed] [Google Scholar]
- Manes S., Mira E., Gomez-Mouton C., Zhao Z. J., Lacalle R. A., Martinez A. C. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenda D. R., Vrailas A. D., Rodrigues A. B., Cook S., Powers M. A., Lorenzen J. A., Perkins L. A., Moses K. MAP kinase subcellular localization controls both growth and proliferation in the developing Drosophila wing. Development. 2005;133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith J., Jr, Takada Y., Fornaro M., Languino L. R., Schwartz M. A. Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science. 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- Muda M., Worby C. A., Simonson-Leff N., Clemens J. C., Dixon J. E. Use of double-stranded RNA-mediated interference to determine the substrates of protein tyrosine kinases and phosphatases. Biochem. J. 2002;366:73–77. doi: 10.1042/BJ20020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Gu H., Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos S. N., Blaikie P., Yoshioka T., Guo W., Puri C., Tacchetti C., Giancotti F. G. Targeted deletion of the integrin β4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-κB, causing defects in epidermal growth and migration. Mol. Cell. Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. S., Gu H., Saxton T. M., Timms J. F., Hausdorff S., Frevert E. U., Kahn B. B., Pawson T., Neel B. G., Thomas S. M. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 1999;19:3205–3215. doi: 10.1128/mcb.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepple K. L., Anderson A. E., Frankfort B. J., Mardon G. A genetic screen in Drosophila for genes interacting with senseless during neural development identifies the importin moleskin. Genetics. 2007;175:125–141. doi: 10.1534/genetics.106.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Johnson M. R., Melnick M. B., Perrimon N. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev. Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Larsen I., Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Volmat V., Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- Quelo I., Gauthier C., Hannigan G. E., Dedhar S., St-Arnaud R. Integrin-linked kinase (ILK) regulates the nuclear entry of the C-JUN coactivator α NAC and its coactivation potency. J. Biol. Chem. 2004;279:43893–43899. doi: 10.1074/jbc.M406310200. [DOI] [PubMed] [Google Scholar]
- Roux P. P., Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. R., Smedberg J. L., Rula M. E., Xu X. X. Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells. J. Cell Biol. 2004;164:689–699. doi: 10.1083/jcb.200312028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taagepera S., McDonald D., Loeb J. E., Whitaker L. L., McElroy A. K., Wang J. Y., Hope T. J. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc. Natl. Acad. Sci. USA. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrailas A. D., Marenda D. R., Cook S. E., Powers M. A., Lorenzen J. A., Perkins L. A., Moses K. smoothened and thickveins regulate Moleskin/Importin 7-mediated MAP kinase signaling in the developing Drosophila eye. Development. 2006;133:1485–1494. doi: 10.1242/dev.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehurst A. W., Wilsbacher J. L., You Y., Luby-Phelps K., Moore M. S., Cobb M. H. ERK2 enters the nucleus by a carrier-independent mechanism. Proc. Natl. Acad. Sci. USA. 2002;99:7496–7501. doi: 10.1073/pnas.112495999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J. P., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott D. L., Joachimiak A., Goodman S. L., Arnaout M. A. Crystal structure of the extracellular segment of integrin αVβ3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. H., Qu C. K., Henegariu O., Lu X., Feng G. S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- Yu Z., Ahmad S., Schwartz J. L., Banville D., Shen S. H. Protein-tyrosine phosphatase SHP2 is positively linked to proteinase-activated receptor 2-mediated mitogenic pathway. J. Biol. Chem. 1997;272:7519–7524. doi: 10.1074/jbc.272.11.7519. [DOI] [PubMed] [Google Scholar]
- Zavortink M., Bunch T. A., Brower D. L. Functional properties of alternatively spliced forms of the Drosophila PS2 integrin a subunit. Cell adhesion and communication. 1993;1:251–264. doi: 10.3109/15419069309097258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.