Abstract
Three distinct dendritic cell (DC) subsets capable of stimulating allogeneic naive T cells were isolated from human thymus. The most abundant subset was represented by plasmacytoid DCs (pDCs), which secreted high amounts of IFN-α upon stimulation with inactivated influenza virus and thus likely correspond to the recently identified peripheral blood natural IFN-α/β–producing cells (IPCs). Like those latter cells, thymic pDCs had distinctive phenotypic features (i.e., Lin–, HLA-DRint, IL-3Rαhi, CD45RAhi, CD11c–, CD13–, and CD33lo) and developed into mature DCs upon culture in IL-3 and CD40L. Of the two other DC subsets, one displayed a phenotype of immature myeloid DCs (imDCs) (HLA-DRint, CD11c+, CD13+, CD33+), and the other represented HLA-DRhi CD11c+ mature DCs (mDCs). Since they also expressed DC-LAMP, these mDCs appear to correspond to interdigitating dendritic cells (IDCs). Thymic pDCs, but not myeloid imDCs, strongly expressed lymphoid-specific transcripts such as pre-Tα, λ-like, and Spi-B, thereby suggesting a possible lymphoid origin. The detection of Spi-B mRNA, not only upon in vitro maturation of pDCs, but also in freshly purified IDCs, suggests that in vivo pDCs may differentiate into IDCs.
Introduction
Dendritic cells (DCs) are unique leukocyte subpopulations within antigen-presenting cells (APCs). DCs capture antigen at the periphery of the organism and present processed antigen to CD4+ and CD8+ naive T cells, thereby initiating primary cellular immune responses (1). Depending on their anatomical localization, DCs exist in different maturational states, show distinct pathways of differentiation, and exhibit functional and phenotypic differences. In humans, two subsets of DCs were originally identified in the peripheral blood (PB) based on the differential expression of CD11c (2). They have in common the expression of CD4, MHC class II molecules, and absence of lineage-specific markers (2–4). The CD11c+ population constitutes immature PB DCs (imDCs) that express CD45R0 and myeloid antigens (CD13 and CD33); within secondary lymphoid organs imDCs are localized to B-cell areas and are designated germinal center dendritic cells (GCDCs) (5). The CD11c– subset is negative for myeloid markers, expresses high level of CD45RA and IL-3Rα, and displays a characteristic plasmacytoid morphology (6–9); hence, these DCs are referred to as plasmacytoid DCs (pDCs).
The pDCs, for which a lymphoid origin has been proposed (8), are identical to blood natural IFN-α/β–producing cells (IPCs), which secrete high amounts of IFN-α/β in response to viruses (9, 10). pDCs have the potential to differentiate into mature DCs when cultured with IL-3 and CD40L (8) or with viruses (11, 12). Within secondary lymphoid organs, pDCs are localized around the high endothelial venules and in T-cell areas (8, 9). The T zones of lymphoid tissues are also populated by mature DCs (mDCs) referred to as interdigitating DCs (IDCs) (13–15). These cells are characterized by a high expression of MHC class II molecules and of the costimulatory molecules CD80 and CD86 (13), as well as by the expression of the specific DC maturation marker DC-LAMP (14). Whether IDCs correspond to the mature stage of the imDCs and/or of the pDCs remains to be determined.
Studies in the mouse showed that DCs could be of distinct ontogenic origins. In contrast to the myeloid CD8α– DC subset, CD8α+ thymic DCs have been proposed to be of lymphoid origin because they derive from an early intrathymic CD4lo precursor common to T and B cells (16, 17). In humans, in vitro studies have shown that DCs together with T, B, and NK cells may derive from a common precursor identified in thymus (18, 19), bone marrow (20), and fetal liver (21). Because DCs are capable of inducing T-cell immunity (1) or central tolerance in the thymus (22), it is important to determine which DCs populate human thymus and for which function. Initial studies have characterized human thymic DCs as a homogeneous MHC class II+ population that differs from its mouse counterpart in being mainly CD4+8– (23–26). However, recent studies reported that thymic medulla contains both mDCs and IL-3Rα+ pDCs (15, 27, 28). Interestingly, Res et al. (15) showed that thymic pDCs expressed pre–TCR-α chain mRNA, a finding that further emphasizes their possible lymphoid origin.
We report here the first identification, to our knowledge, of three distinct DC populations in the human thymus: (a) plasmacytoid HLA-DRint CD11c– DCs (pDCs) representing the most abundant subset; (b) HLA-DRint CD11c+ imDCs expressing myeloid-related markers; and (c) IDCs expressing high levels of HLA-DR and DC-LAMP, a marker unique to mDCs. We demonstrate that, like pDCs isolated from tonsils (8), thymic pDCs differentiate into mDCs that express DC-LAMP upon culture with IL-3 and CD40L. Unlike imDCs, HLA-DRint CD11c– pDCs expressed lymphoid-specific transcripts such as pre-Tα, λ-like, and Spi-B. The observation that Spi-B was also detected in mDCs as well as in pDCs upon in vitro maturation suggests that a subset of IDCs may derive from pDCs. Besides their capacity to activate allogeneic T cells, thymic pDCs also produce significant levels of IFN-α in response to influenza virus and, thus, are functionally related to the blood IPCs.
Methods
Isolation procedure for human thymic DCs.
Normal human thymuses from 15-day- to 7-year-old children undergoing corrective cardiovascular surgery (Hôpital Marie Lannelongue, Le Plessis Robinson, France) were cut into small pieces and digested for 25 minutes at 37°C with collagenase IV (1 mg/ml; Sigma Chemical Co., St. Louis, Missouri, USA) and deoxyribonuclease I (50 KU/ml; Sigma Chemical Co.) in RPMI 1640 supplemented with EDTA 0.5 M for the final 5 minutes of incubation. Lineage marker–negative (Lin–) mononuclear cells were obtained by the Ficoll-Rosetting method followed by immunomagnetic depletion of contaminating cells reactive to a mixture of CD3, CD7 (T lineage), CD14 (monocytes), CD19 (B cells), and CD56 (NK cells) antibodies, as described previously (8). This DC-enriched cell population was further stained with anti–HLA-DR FITC, anti-CD11c PE (both from Becton Dickinson Immunocytometry Systems, Mountain View, California, USA) and a cocktail of Lin PE-Cy5 anti-CD3, -CD14, -CD19 (IOTEST; Immunotech, Marseille, France), and anti-CD7 (Caltag Laboratories Inc., Burlingame, California, USA) antibodies. Then Lin– DCs were sorted into HLA-DRhi CD11c+ (mDC), HLA-DRint CD11c+ (imDC), and HLA-DRint CD11c– (pDC) subpopulations using a FACStarplus flow cytometer (Becton Dickinson Immunocytometry Systems), and their percentages of purity were 94.2 ± 3.6%, 93.7 ± 2%, and 95.8 ± 1.3%, respectively (n = 7 thymuses).
Cell cultures.
Thymic pDCs were cultured with IL-3 (20 ng/ml; Schering-Plough Research Institute [SPRI], Kenilworth, New Jersey, USA) in the presence or absence of CD40L-transfected fibroblasts (8) or with IL-3 and GM-CSF (200 ng/ml; SPRI) and TNF-α (2.5 ng/ml; Genzyme Pharmaceuticals, Boston, Massachusetts, USA). Monocytes were cultured for 6 days in GM-CSF and IL-4 (50 U/ml; SPRI).
Phenotypic analysis of isolated thymic DC subsets.
PE-conjugated antibodies specific for CD2, CD4, CD5, CD11c, CD13, CD14, CD19, CD34, CD45RO, and CD62L were purchased from Becton Dickinson Immunocytometry Systems; those for CD1a, CD3, CD7, CD33, CD40, CD45RA, CD56, CD83, CD116, and CD127 were purchased from Immunotech; those for CD86 and CD123 were purchased from PharMingen (San Diego, California, USA); and those for CD8α were from BioSource International (Camarillo, California, USA). Anti-CD13 PE-Cy5 was obtained from DAKO A/S (Glostrup, Denmark). Anti–DC-LAMP mAb was produced in our laboratory (14).
Transmission electron microscopy.
Freshly purified thymic cell suspensions were analyzed as described elsewhere (29).
Immunohistochemical analysis of sorted thymic DCs.
Cytospin preparations of thymic DC subsets were fixed in acetone for 20 minutes at 4°C. Double staining with mouse IgG1 anti–DC-LAMP and mouse IgG2a anti–HLA-DR (Becton Dickinson Immunocytometry Systems) was revealed by sheep anti-mouse IgG1 (The Binding Site, Birmingham, United Kingdom) followed by mouse anti-alkaline phosphatase-alkaline phosphatase complexes (APAAP technique; DAKO A/S), and biotinylated sheep anti-mouse IgG2a (The Binding Site) followed by ExtraVidin-peroxidase (Sigma Chemical Co.). Alkaline phosphatase and peroxidase activities were revealed using Fast Blue substrate (Sigma Chemical Co.) and 3-amino-9-ethylcarbazole (Vector Laboratories, Burlingame, California, USA), respectively.
Immunohistological localization/characterization of thymic DCs.
Double stainings on human thymus sections were performed by immunohistochemistry using mouse IgG2b anti-CD86 and IgG2a anti–IL-3Rα (PharMingen) together with mouse IgG1 anti–DC-LAMP. Human thymus sections were also stained using mouse IgG1 anti-CD40 (mAb 89, produced in our laboratory; ref. 30), anti-CD68 (DAKO A/S), and anti-CD45RA (Immunotech), saturated for 10 minutes with 10% mouse serum and stained again using anti–DC-LAMP biotin. Indirect and biotinylated mAb’s were revealed with appropriate reagents as already described here. Double stainings using immunofluorescence were also performed using mouse IgG1 anti–DC-LAMP biotin or IgG2a anti–IL-3Rα (PharMingen) revealed by Red Texas–conjugated streptavidin (Pharmacia Biotech Europe, Saclay, France) and by Red Texas-labeled goat anti-mouse mAb (Pharmacia Biotech Europe), respectively. FITC-labeled mouse IgG1 anti-CD11c (DAKO A/S), IgG1 anti-CD45RA (Becton Dickinson Immunocytometry Systems), or IgG1 anti–DC-LAMP were next incubated. Control sections were incubated with the fluorescent conjugates only. Fluorescence microscopy was performed using a Leica confocal system (Leica Lasertechnik GmbH, Heidelberg, Germany).
RT-PCR analysis of isolated thymic DCs.
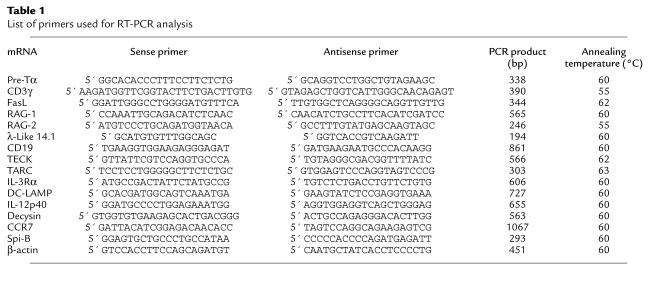
Total RNA was extracted from the FACS-sorted or cultured thymic DCs following established procedures (31). After DNase I treatment (in the presence of RNase inhibitor), first-strand cDNAs were prepared using oligo(dT) primers (Pharmacia Biotech A/B, Uppsala, Sweden) and Superscript RNase-H RT (Life Technologies Inc., Gaithersburg, Maryland, USA). PCR was performed in a DNA thermal cycler (PE Applied Biosystems, Foster City, California, USA) for 35 or 40 cycles (1-minute denaturation at 94°C, 1-minute annealing at 55°C or 60°C, and 2-minute elongation at 72°C) with AmpliTaq enzyme and buffer (Perkin-Elmer), dNTPS at 10 mM (Perkin-Elmer), and DMSO at 5% final concentration. β-Actin mRNA amplification was performed for 28 cycles on the cDNA as positive control of reaction efficiency. Oligonucleotide primers and the expected sizes of PCR products are listed in Table 1.
Table 1.
List of primers used for RT-PCR analysis
Quantitative RT-PCR analysis of Spi-B expression.
To compare the amount of Spi-B mRNAs expressed in the three thymic DC populations, expression was related to the level of expression of the housekeeping gene β-actin. Standard curve for Spi-B was established by amplification of serial dilutions of Spi-B cDNA cloned into pCRII vector (TA cloning kit; Invitrogen, San Diego, California, USA) containing 105 to 1 copy of plasmid. Standard curve for β-actin was established by amplification of serially diluted cDNA prepared from the B-cell line JY (dilution from 1:2 to 1:128). PCR was performed in a LightCycler instrument (Roche Diagnostic, Meylan, France) in a total volume of 15 μl using the LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostic). Samples were heated to 95°C for 10 minutes followed by amplification for at least 30 cycles of 15 seconds at 95°C, 5 seconds at 60°C (β-actin and Spi-B), and 10 seconds at 72°C. After the last cycle, a melting curve analysis was performed to assess the specificity of the amplified PCR products. Oligonucleotide primers used for Spi-B and β-actin are listed in Table 1.
T-cell proliferation assay.
Naive CD4+ CD45RA+ T cells were isolated from PBMCs by negative selection (32). A total of 5 × 104 allogeneic naive CD4+ T cells were cocultured with fresh thymic DC subsets, IL-3–matured pDCs, and monocyte-derived DCs in round-bottomed 96-well culture plates in RPMI 1640 supplemented with 10% of FCS, 2 mM L-glutamine, antibiotics, and 10% human AB+ serum. After 5 days, cells were pulsed with 1 μCi [3H]thymidine (Amersham Pharmacia Biotech, Saclay, France) for 8 hours before harvesting.
IFN-α production.
pDCs sorted from PB, tonsils (8), and thymus, and thymic cell suspensions at different steps of enrichment were used. Cells were incubated with 1 HAU/ml formaldehyde-inactivated influenza virus strain Beijing/262/95 (kindly provided by N. Kuehn, Aventis, Pasteur Mérieux, Val de Reuil, France) in duplicate wells (2 × 105 cells in 200 μl of culture medium per well in 96-well culture plates). IFN-α levels in supernatants from 24-hour cultures were measured by ELISA that specifically recognizes IFN-α2 (Immunotech). The sensitivity of this assay was 0.6 IU/ml.
Results
Isolation of three distinct thymic DC populations.
A multistep isolation method was developed including (a) depletion of sheep erythrocyte rosette-forming cells on a Ficoll-density gradient; (b) magnetic bead depletion of B cells (CD19+), T-lineage cells (CD3+, CD7+), monocytes (CD14+), and NK cells (CD56+); and (c) fluorescence-activated sorting of cells negative for CD3, CD7, CD14, and CD19 (27 ± 15%; range 13.7–54%; n = 8) on the basis of CD11c expression and either intermediate or high levels of HLA-DR. As illustrated in Figure 1a, three Lin– HLA-DR+ populations were isolated: HLA-DRint CD11c– (R2, 25.5 ± 12% of Lin– cells; range 13.6–50.8%; n = 8), HLA-DRint CD11c+ (R3, 10.2 ± 4%; range 5.4–16.7%; n = 8), and a population expressing high levels of both HLA-DR and CD11c (R4, 4.5 ± 2%; range 0–6.7%; n = 8). Freshly isolated HLA-DRint CD11c– cells displayed a characteristic plasma cell–like morphology (Figure 1, b and e) resembling the plasmacytoid T cells (8), whereas both CD11c+ populations possessed an irregular outline and a polylobulated nucleus (Figure 1, c, d, f, and g). HLA-DRint CD11c+ cells had a heterogeneous morphology, and 50% of them had the features of the one presented in Figure 1f. Numerous fine dendrites characterized the HLA-DRhi subset (Figure 1d).
Figure 1.
Purification of thymic DC subsets. (a) DC-enriched Lin– cells (R1) contain HLA-DRint CD11c– pDC (R2), HLA-DRint CD11c+ imDC (R3), and HLA-DRhi CD11c+ mDC (R4) subpopulations. FSC, forward scatter; PE-Cy5, phycoerythrin–cyanin 5.1. (b–d) Giemsa staining. (e–g) Transmission electron microscopy. Bar = 1 μm. (h–j) HLA-DR (red) and DC-LAMP (blue) stainings. Original magnification (b–d, h–j): ×1,000.
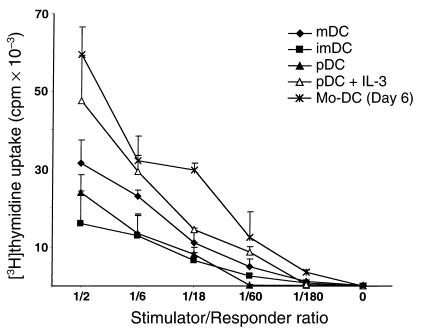
We next tested the capacities of the three HLA-DR+ populations to stimulate CD4+ CD45RA+ allogeneic T cells. All three subsets induced naive T-cell proliferation, thereby demonstrating that they all exhibited dendritic cell function (Figure 2). We accordingly refer to immature HLA-DRint CD11c–, HLA-DRint CD11c+, and mature HLA-DRhi CD11c+ subpopulations as plasmacytoid DCs (pDCs), immature DCs (imDCs), and mDCs, respectively.
Figure 2.
Thymic DCs induce proliferation of CD4+ CD45RA+ T cells. A total of 5 × 104 PB naive CD4+ T cells were cocultured with graded doses of fresh mDCs, imDCs, pDCs, or IL-3–treated pDCs, and monocyte-derived DCs (Mo-DC) as positive control, for 5 days. Results are expressed as mean ± SD of triplicate cultures and are representative of three experiments.
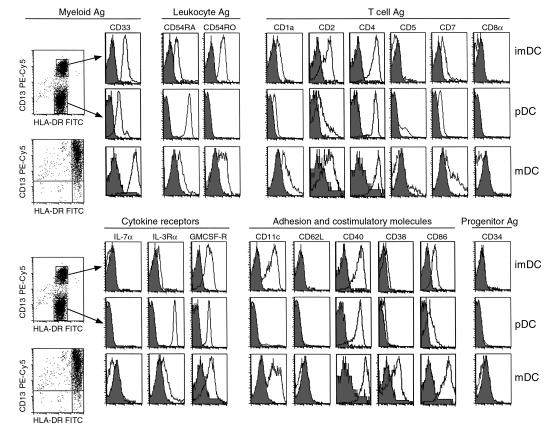
Phenotypic analysis of thymic DC populations.
To characterize the thymic DC subsets with a panel of PE-conjugated mAb’s (Figure 3), we modified the isolation procedure described here by omitting the use of anti-CD11c PE. The modification exploited the similar expression of CD11c and CD13 on thymic DC subsets. Thus, Lin– cells (identified using PC5-conjugated anti-CD3, -CD7, -CD14, and -CD19 mAb’s) were sorted into either HLA-DRint or HLA-DRhi (using a FITC-conjugated mAb). The sorted cells were devoid of CD3-, CD14-, CD19-, and CD56-contaminating cells (data not shown) and also did not express IL-7Rα and CD34, excluding thymocyte or progenitor contamination. All the sorted cells expressed high levels of CD40 and CD4, but not CD8α. HLA-DRint cells contained (a) pDCs, a lymphoid-related population; CD13–, CD33lo, CD11c–, CD45RO–, CD45RA+, IL-3Rα+, and CD86lo, CD83–; and (b) imDCs, a myeloid-related population; CD13+, CD33+, CD11c+, CD45RO+, CD45RA–, IL-3Rα–, and CD86lo, CD83–. The HLA-DRhi mDC/IDCs had a broad range of staining intensity with CD13 and CD11c mAb’s and expressed a phenotype of mDCs indicated by high levels of HLA-DR and expression of CD86 and CD83. Only mDC/IDCs were labeled intracellularly by an mAb specific for DC-LAMP, a newly described mDC-specific lysosomal marker (14) (Figure 1, h–j). Unlike blood DC subsets, thymic DCs were CD62L–, suggesting that, as previously observed in tonsils, emigration of circulating cells from blood into peripheral lymphoid organs results in CD62L cleavage (9). Finally, all three subsets expressed low levels of CD1a, intermediate levels of GMCSF-Rα, and heterogeneous levels of the T cell–related markers CD2, CD5, and CD7.
Figure 3.
Immunophenotype of isolated thymic DC subsets analyzed by flow cytometry. Thymic DCs were sorted into Lin– (PE-Cy5) HLA-DRint (FITC) and Lin– HLA-DRhi subsets. Anti–CD13-PE-Cy5 labeling of HLA-DRint cells clearly resolved two distinct populations. CD13+ HLA-DRint, CD13– HLA-DRint, and CD13+ HLA-DRhi DCs were analyzed using PE-conjugated mAb’s for the expression of a number of lymphoid, myeloid, costimulatory, and adhesion markers. Data shown are representative of three experiments. Ag, antigen.
Thymic mDCs have the features of IDCs.
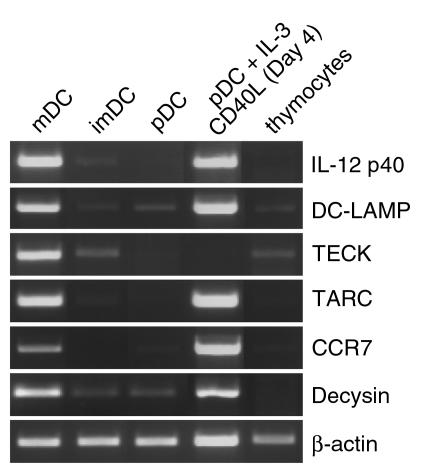
Purification of the mature thymic DC subset enabled their extensive characterization. In addition to CD83, CD86, and DC-LAMP proteins, mDCs were shown to express mRNA characteristic of mature IDCs. Depending on their maturation or activation stage, DCs produce a series of soluble molecules implicated in the initiation of the immune response. IL-12p40 mRNA was strongly detected in mDC, which also expressed DC-LAMP mRNA, and a faint band of IL-12p40 mRNA was also observed in imDCs but not in pDCs (Figure 4). Two chemokines, TECK and TARC, have been shown to be expressed by murine thymic DCs (33, 34). Both TECK and TARC mRNA were restricted to the mDC subset, although they were barely detectable in the total thymocyte population. The expression of the chemokine receptor is also strictly regulated during the maturation process of DCs in vivo. For example, CCR7 has been described only in mDCs (35). In line with the mature phenotype of the HLA-DRhi CD11c+ DC, CCR7 mRNA was only found in mDCs. A disintegrin metalloproteinase designated decysin (36) was demonstrated to be highly induced upon spontaneous or CD40-dependent maturation of CD11c+ imDC subset. The mDC subset displayed decysin mRNA expression, in contrast to the imDCs and pDCs. Taken together, these observations showed that HLA-DRhi population expressed all the properties of mDCs and corresponded to the thymic IDCs.
Figure 4.
Thymic mDCs express genes specific to IDCs. Fresh thymic DC subsets, in vitro–matured pDCs, and total thymocytes were analyzed for expression of mRNA for IL-12p40, DC-LAMP, TECK, TARC, CCR7, decysin, and β-actin.
Thymic DCs are localized in the medulla.
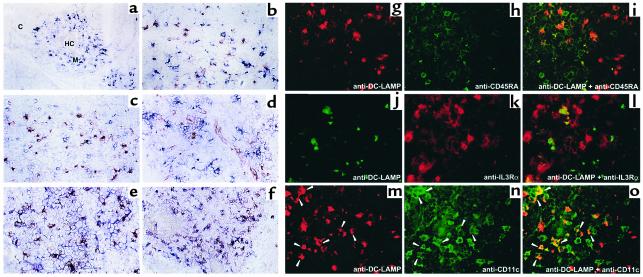
The anatomic localization of the three DC subsets was analyzed by in situ stainings using either immunohistochemistry or immunofluorescence. Figure 5, a–f, shows that DC-LAMP+ cells and IL-3Rα+ CD45RA+ cells are both located in the medulla. As with the phenotype observed for freshly isolated mDCs, all DC-LAMP+ cells coexpressed the mDC markers CD40 and CD86 (Figure 5, e and f). Two-color immunofluorescence analyses of thymus sections further clearly demonstrated that DC-LAMP+ cells were CD45RA– and IL-3Rα– (Figure 5, i and l). Different levels of CD11c intensity on DC-LAMP+ cells could suggest heterogeneity among this population (Figure 5o). The CD11chi DC-LAMP– cells could be either imDCs, macrophages, or granulocytes, cell types that in humans also express CD11c. Using anti–DC-LAMP and anti-CD68 mAb’s, we showed that CD68+ macrophages did not express DC-LAMP (Figure 5b), confirming that DC-LAMP was uniquely expressed in mature thymic IDCs. It appears that unlike T cells, both thymic IL-3Rα+ CD45RA+ pDCs and DC-LAMP+ mDC/IDCs were located in the medulla, regardless of their stage of differentiation.
Figure 5.
The thymic medulla contains both pDCs and mDCs. All DC-LAMP+ cells were located in the medulla (M), identified by the presence of Hassall’s corpuscles (HC), either using anti–DC-LAMP antibody revealed in blue (a, d, and f) or in red (b, c, and e). Double stainings identified DC-LAMP+ cells coexpressing CD40 (blue, e) and CD86 (red, f), characteristic of mDCs that are clearly distinct from CD68+ macrophages (blue, b). CD45RA+ (blue, c) IL-3Rα+ (red, d) pDCs are also located in the medulla and are distinct from mature CD45RA– IL-3Rα– CD11c+ DC-LAMP+ DCs (i, l, and o). Patterns of the individual colors are depicted separately for anti–DC-LAMP (red, g and m; or green, j), -CD45RA (h), -IL3Rα (k), and -CD11c (n). M, medulla; C, cortex; HC, Hassall’s corpuscles. Original magnification: (a, f) ×200; (b–e, g–o) ×400.
Thymic pDCs express lymphoid-associated transcripts.
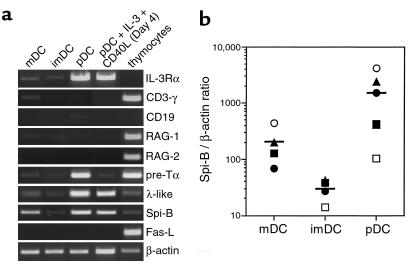
IL-3Rα mRNA was selectively present in pDC (Figure 6a), whereas the abundance of GM-CSFR transcripts was comparable in the three DCs subsets (data not shown). Because the thymus contains precursor cells capable of giving rise to DCs and to T, B, and NK cells (16, 18, 19), we investigated in purified thymic DCs the expression of lymphoid-related transcripts. Figure 6a shows that although the three DC subsets lacked CD3γ, CD19, RAG-1, and RAG-2 mRNA, pDCs expressed pre-Tα and λ-like 14.1 mRNA, that are T and B progenitor genes (37, 38). Moreover, while searching for pDC specific markers (M.-C. Rissoan, unpublished data), we found a tag sequence corresponding to the Ets family transcription factor Spi-B (39) that has been described as exclusively expressed in lymphoid cells (40). As shown in Figure 6a, thymic pDCs expressed high levels of Spi-B transcripts that are, unlike pre-Tα and λ-like transcripts, also found in mDCs. The levels of Spi-B mRNA expression in the different thymic DC subsets, was analyzed by quantitative PCR. Approximately tenfold higher levels of Spi-B mRNA were observed in mDCs than in imDCs, which increased a further tenfold in pDCs (Figure 6b). It has been hypothesized that murine CD8α+ FasL-expressing DCs negatively regulate T-cell activation through Fas-FasL–induced apoptosis (41). None of the human thymic DC subsets expressed FasL mRNA (Figure 6a). Overall, expression of lymphoid specific genes in thymic pDCs further argues in favor of their lymphoid origin. In addition, the presence of both myeloid and lymphoid markers within the thymic IDC population suggest that IDCs are heterogeneous.
Figure 6.
Thymic pDCs express lymphoid-associated transcripts. (a) Fresh thymic DC subsets, IL-3/CD40L–treated pDCs, and total thymocytes were analyzed for expression of mRNA for IL-3Rα, CD3γ, CD19, RAG-1, RAG-2, pre-Tα, λ-like 14.1, Spi-B, FasL, and β-actin. (b) Expression levels of Spi-B mRNA in subsets of thymic DCs. Spi-B/β-actin ratios were determined by quantitative RT-PCR. Standard curves were calculated in which the first point of the curve containing the highest amount of cDNA was set arbitrarily to 100 U for β-actin and to 560,000 U for Spi-B. Each symbol represents an independent experiment.
Thymic pDCs develop into mature DCs upon stimulation with IL-3 and CD40L.
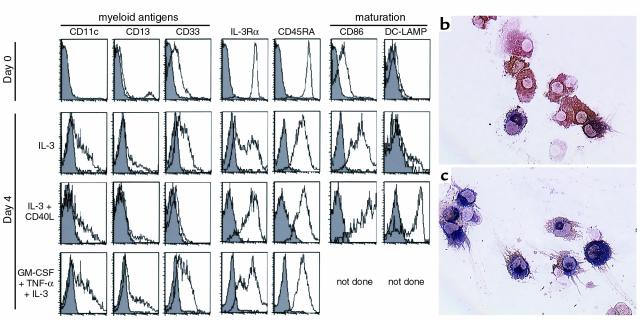
As described in tonsils (8), IL-3 rescued thymic pDCs from apoptosis and allowed their maturation with a rapid upregulation of CD86 (Figure 7a) correlating with a higher T-cell stimulatory activity (Figure 2). However, expression of certain surface molecules varied upon culture conditions. Thymic pDCs cultured in IL-3 or, most strikingly, in IL-3/GM-CSF/TNF-α expressed heterogeneous levels of IL-3Rα, CD11c, CD13, and CD33 (Figure 7a). Stimulation by CD40L together with IL-3 resulted in the differentiation of pDCs into mDCs principally characterized by the induction of DC-LAMP, both at the protein and mRNA levels (Figure 4; and Figure 7, a–c), and maintained expression of IL-3Rα and CD45RA (Figure 7a). The in vitro–matured DCs also accumulated mRNA for IL-12p40, TARC, CCR7, and decysin (Figure 4), markers that are specific for fully mDCs, whereas TECK was not induced. Regarding the lymphoid transcripts, in vitro–matured DCs no longer expressed pre-Tα mRNA, suggesting that pre-Tα is downregulated concomitantly with the acquisition of a mature phenotype. Unlike pre-Tα mRNA, λ-like and Spi-B mRNA were still observed in thymic pDCs after 4 days of culture with IL-3 and CD40L. Taken together, these results show that pDCs mature in vitro into cells exhibiting all the characteristics of mDCs and sharing most markers of thymic IDC. Moreover, the maintained expression of Spi-B in in vitro–matured DCs combined with the presence of Spi-B transcripts in pDCs and mDCs suggests that thymic IDCs may represent a mature stage arising from both the lymphoid-related pDC subset and the myeloid imDC subset.
Figure 7.
Phenotype and morphology of thymic pDCs after in vitro–induced maturation. (a) In vitro–cultured pDCs display a phenotype specific for immature (IL-3 or GM-CSF/TNF-α/IL-3) or mature DCs (IL-3/CD40L). Filled histograms represent isotype-matched controls; open histograms represent staining with specific antibodies. (b) IL-3–treated pDCs acquire a dendritic morphology showing strong staining for HLA-DR, and (c) develop into mature DC-LAMP+ DCs upon further CD40 activation. Original magnification (b and c): ×200.
Thymic CD11c– pDCs produce IFN-α in response to influenza virus.
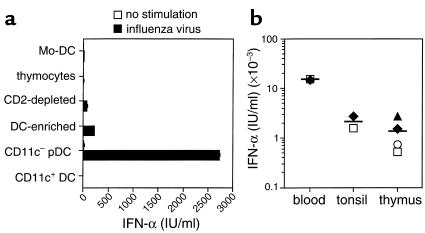
It was recently reported that PB pDCs are the natural IFN-α–producing cells (9, 10). To investigate the capacity of thymic pDCs to produce IFN-α, we exposed them to formaldehyde-inactivated influenza virus for 24 hours after cell sorting, and then IFN-α was measured by ELISA. As shown in Figure 8a, production of IFN-α correlated with the enrichment of the pDC fraction. Indeed, total thymic suspensions (thymocytes) did not produce IFN-α, although some IFN-α became detectable after elimination by rosetting of CD2-positive cells, and even more after CD3, CD7, CD14, CD19, and CD56 depletion (DC enriched). Highly purified sorted pDCs produced high levels of IFN-α (2,721 IU/ml) in response to influenza virus, unlike CD11c+ DCs (including imDCs and mDCs). In a separate experiment, we also confirmed that only pDCs and neither imDCs nor mDCs were capable of producing IFN-α (data not shown). The IFN-α–producing capacities of pDCs isolated from thymus, blood, or tonsils were compared. Thymic pDCs produced IFN-α at levels comparable to those obtained with tonsillar pDCs (respectively, 1,371 ± 500 IU/ml [n = 4] and 2,158 ± 590 IU/ml [n = 2]), but lower than those produced by circulating blood pDCs (15,063 ± 257 U/ml, n = 2) (Figure 8b). Monocyte-derived DCs were unable to produce IFN-α under such stimulation (Figure 8b).
Figure 8.
Thymic pDCs produce large amounts of IFN-α after being stimulated with influenza virus. Cells (2 × 105) were cultured for 24 hours with inactivated influenza virus. Without influenza virus, IFN activity from all cell types used was less than 5 IU/ml. (a) Mo-DC: monocyte-derived DCs after 6 days of culture with GM-CSF and IL-4. Thymocytes: total thymic suspension. CD2-depleted: thymic mononuclear cells devoid of CD2+ cells by Ficoll Rosetting. DC-enriched: thymic CD2– mononuclear cells that were depleted of Lin+ cells. CD11c– pDC: FACS-sorted Lin– HLA-DRint CD11c– cells. CD11c+ DC: FACS-sorted Lin– HLA-DRint and HLA-DRhi CD11c+ cells. (b) IFN-α production by thymic HLA-DRint CD11c– pDCs and CD4+ CD11c– pDCs purified from blood and tonsils. Each symbol represents an independent experiment.
Discussion
Only a few reports have dealt with the purification of thymic DCs in humans, and it has always been assumed that thymic DCs were a homogeneous population (23–25). In this study, we identified three distinct CD4+Lin– DC populations in human thymus: (a) HLA-DRint CD11c– pDC, representing the most abundant population of thymic DCs; (b) HLA-DRint CD11c+ imDCs; and (c) HLA-DRhi CD11c+ mDC/IDCs.
Thymic pDCs clearly share common features with pDCs present in PB (2, 4, 9) and in secondary lymphoid organs (8) and were most recently designated as pre-DC2 (32) or IPCs (9, 10) based on their functional properties. Like blood and tonsillar pDC, thymic pDCs (a) have a plasmacytoid morphology, (b) express CD45RA, IL-3Rα, and low levels of myeloid-related antigens such as CD13 and CD33, (c) are rescued from apoptosis by IL-3, (d) produce high levels of IFN-α in response to virus, and (e) following culture in IL-3 and CD40L, acquire all the properties of mDCs, including expression of DC-LAMP, CCR7, decysin, and IL-12p40 and a high capacity to stimulate allogeneic naive T cells.
The second subset is the myeloid-related HLA-DRint CD11c+ imDC population, which is analogous to the CD11c+ DCs isolated from PB (2) or from tonsil germinal centers (5). GCDCs may present antigen to both T and B cells (42) and may directly contribute to the germinal center reaction both in vitro in human (43) and in vivo in mouse (44) and in rat (42). Germinal center formations have been observed in normal thymuses, and their numbers are increased in pathological situations such as myasthenia gravis (45). As CD11c+ DCs were shown to be involved in the induction of B-cell responses (1, 46), the possibility exists that in autoimmune diseases, thymic HLA-DRint CD11c+ imDCs could play a role in the induction of the differentiation of B cells–producing autoantibodies.
Third, we purified a minor population of mDCs, HLA-DRhi CD11c+, comprising 0–7% of thymic DCs, that represent the thymic IDCs. They exhibit all the properties of mDCs, including expression of DC-LAMP, decysin, CCR7, and IL-12. Like pDCs, these thymic IDCs are located in the medullary area (14, 15). Thymic mDCs express high levels of chemokines such as TARC and TECK that have been reported as important factors in the regulation of thymocytes homing/trafficking within the thymus.
A key feature of thymic DCs is their role in the negative, but not positive, selection of thymocytes (22). Studies in mice have shown that lymphoid DCs expressed FasL or a FasL-related molecule that could be responsible for this property (41). However, we found that none of the human thymic DC populations studied expressed FasL. Thus, it remains to be demonstrated whether a given thymic DC subset in humans as well as in mice can induce T-cell apoptosis and by which mechanism. Although they represent only a minor component of thymic cells, mDCs may play critical roles (a) in the deletion of autoreactive thymocytes owing to their efficient antigen-presenting capacity reflected on high levels of MHC class II and (b) in the production of high quantities of key factors such as cytokines (IL-12) or chemokines (TECK, TARC).
In the mouse, a thymic precursor to both T cells and DCs has been demonstrated (16). Several experiments in humans have also supported the existence of a common multipotent progenitor population for DCs and for T, B, and NK cells (18–20). Although some evidence suggests that pDCs could be of lymphoid origin, this remains to be formally demonstrated. Recently, it has been shown that human committed DC precursors that are present in adult PB (47) and in thymus (15), and which have a phenotype similar to the pDCs we have isolated from thymus, share with T-cell precursors the expression of pre-Tα mRNA (37). We extended these observations by showing that, within human thymic DC subsets, only the HLA-DRint CD11c– pDC subset expressed pre-Tα transcripts. Importantly, we showed that thymic pDCs expressed also the lymphoid-associated B cell–specific transcripts Spi-B (39) and λ-like (38). The finding of transcripts associated with the T- or B-cell differentiation pathways adds support to the putative lymphoid origin of pDCs.
Having identified different DC populations in the human thymus, we attempted to determine whether mDCs originate from the differentiation of CD11c– pDC, CD11c+ imDC, or both. The expression of CD11c on all mDC/IDCs does not exclude their differentiation from pDCs, as pDCs in vitro can express at least moderate levels of CD11c upon culture in the presence of IL-3 (this study and ref. 3). Of the lymphoid-specific genes expressed in pDC, pre-Tα mRNA is rapidly lost upon in vitro maturation, whereas the expression of λ-like and Spi-B mRNAs is maintained. Of these two latter genes that could represent markers of mature pDCs in vivo, Spi-B but not λ-like is expressed in thymic mDC/IDCs. Thus, the expression of Spi-B in mDC/IDCs argues in favor of differentiation in vivo of pDCs into mDC/IDCs. Heterogeneous expression of markers such as Spi-B, CD13, and CD11c in mDC/IDCs might suggest that both pDCs and imDCs could give rise to mDC/IDCs in vivo. The presence of mDCs of two distinct lineages is also supported by a recent study showing that the CD11c+ CD11b– mDC subset, but not the CD11chi CD11b+ mDCs, produced IL-12 (28).This may suggest different functions for thymic IDCs. The myeloid pathway may restrict its function to immunogenicity, trapping antigen in the periphery and eliciting T-cell response. The plasmacytoid (lymphoid-related) pathway may provide a resident population of cells that express self-antigens and actually represent the IDC part in the steady state. Lymphoid-related DCs may function in the thymic medulla to induce central tolerance and in the T-cell areas to maintain peripheral tolerance to self-antigens (48).
In conclusion, we characterized three CD4+ DC populations that were purified from human thymus. A lymphoid-related pDC and a myeloid-related imDC that likely correspond to the populations previously described as pre-DC2/IPCs and GCDCs, respectively, as well as DC-LAMP+ mDC/IDCs representing a heterogeneous subset including the mature forms of both lymphoid and myeloid DCs. The characterization of new markers for human DC subsets will be helpful for the eventual clarification of the intriguing differences between human and mouse DC heterogeneity.
Acknowledgments
We thank F. Jambou and P. Goettelfinger for providing us with thymus; S. Poea for the quantitative PCR analysis; C. Alexandre and M. Vatan for editorial assistance; M. Billaud for excellent graphical work; O. Clear and B. Michat for maintenance support; and M.-C. Rissoan, J.-M. Bridon, and T. Duhen for helpful discussions. N. Bendriss-Vermare is a recipient of a grant from Fondation Marcel Mérieux, Lyon, France.
Footnotes
Preliminary results were presented at the 6th International Symposium on Dendritic Cells in Port Douglas, Australia, in May 2000.
References
- 1.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.O’Doherty U, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 3.Kohrgruber N, et al. Survival, maturation, and function of CD11c- and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol. 1999;163:3250–3259. [PubMed] [Google Scholar]
- 4.Robinson SP, et al. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ. Dendritic cells capable of stimulating T cells in germinal centers. Nature. 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 6.Lennert K, Kaiserling E, Müller-Hermelink HK. T-associated plasma cells. Lancet. 1975;1:1031–1032. doi: 10.1016/s0140-6736(75)91974-1. [DOI] [PubMed] [Google Scholar]
- 7.Facchetti F, de Wolf-Peeters C. The plasmacytoid monocyte (the so called plasmacytoid T-cell). An enigmatic cell of the human lymphoid tissue. Biotest Bulletin. 1991;4:225–240. [Google Scholar]
- 8.Grouard G, et al. The enigmatic plasmacytoid T cells develop into dendritic cells with IL-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 10.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 13.Björck P, Florès-Romo L, Liu YJ. Human interdigitating dendritic cells directly stimulate CD40-activated naive B cells. Eur J Immunol. 1997;27:1266–1274. doi: 10.1002/eji.1830270531. [DOI] [PubMed] [Google Scholar]
- 14.de Saint-Vis B, et al. A novel lysosome associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 15.Res PC, Couwenberg F, Vyth-Dreese FA, Spits H. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–2657. [PubMed] [Google Scholar]
- 16.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Antica M, Johnson GR, Scollay R, Shortman K. Development potential of the earliest precursor cells from the adult mouse thymus. J Exp Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquez C, et al. Identification of a common developmental pathway for thymic natural killer cells and dendritic cells. Blood. 1998;91:2760–2771. [PubMed] [Google Scholar]
- 19.Res P, et al. CD34+ CD38dim cells in the human thymus can differentiate into T, natural killer, and dendritic cells but are distinct from pluripotent stem cells. Blood. 1996;87:5196–5206. [PubMed] [Google Scholar]
- 20.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 21.Plum J, et al. In vitro intrathymic differentiation kinetics of human fetal liver CD34+CD38- progenitors reveals a phenotypically defined dendritic/T-NK precursor split. J Immunol. 1999;162:60–68. [PubMed] [Google Scholar]
- 22.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier M, et al. Characterization of human thymic dendritic cells in culture. Immunology. 1986;58:263–270. [PMC free article] [PubMed] [Google Scholar]
- 24.Lafontaine M, Landry D, Montplaisir S. The human thymic dendritic cell phenotype and its modification in culture. Cell Immunol. 1992;142:238–251. doi: 10.1016/0008-8749(92)90286-x. [DOI] [PubMed] [Google Scholar]
- 25.Sotzik F, et al. Assessment of CD4 expression by early T precursor cells and by dendritic cells in the human thymus. J Immunol. 1994;152:3370–3377. [PubMed] [Google Scholar]
- 26.Beaulieu S, Landry D, Bergeron D, Cohen EA, Montplaisir S. An improved method for purifying human thymic dendritic cells. J Immunol Methods. 1995;180:225–236. doi: 10.1016/0022-1759(94)00318-q. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt C, et al. Identification of mature and immature human thymic dendritic cells that differentially express HLA-DR and interleukin-3 receptor in vivo. J Leukoc Biol. 2000;68:836–844. [PubMed] [Google Scholar]
- 28.Vandenabeele, S., Hochrein, H., Mavaddat, N., Winkel, K., and Shortman, K. 2000. The dendritic cell network in the human thymus. 6th International Symposium on Dendritic Cells. Port Douglas, Australia. p. 65. (Abstr. no. 1.44.)
- 29.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF-α. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallé A, et al. Activation of human B lymphocytes through CD40 and interleukin 4. Eur J Immunol. 1989;19:1463–1467. doi: 10.1002/eji.1830190818. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Rissoan M-C, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 33.Vicari AP, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 34.Lieberam I, Forster I. The murine beta-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol. 1999;29:2684–2694. doi: 10.1002/(SICI)1521-4141(199909)29:09<2684::AID-IMMU2684>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Dieu MC, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:1–14. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller CGF, et al. PCR selects a novel disintegrin-proteinase from CD40-activated germinal center dendritic cells. J Exp Med. 1997;186:655–663. doi: 10.1084/jem.186.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Porto P, Bruno L, Mattei MG, von Boehmer H, Saint-Ruf C. Cloning and comparative analysis of the human pre-T-cell receptor alpha-chain gene. Proc Natl Acad Sci USA. 1995;92:12105–12109. doi: 10.1073/pnas.92.26.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiff C, et al. The immunoglobulin λ-like gene cluster (14.1; 16.1 and Fλ1) contains gene(s) selectively expressed in pre-B cells and is the human counterpart of the mouse λ5 gene. Int Immunol. 1990;2:201–207. doi: 10.1093/intimm/2.3.201. [DOI] [PubMed] [Google Scholar]
- 39.Ray D, et al. Characterization of Spi-B, a transcription factor related to the putative oncoprotein Spi-1/PU.1. Mol Cell Biol. 1992;12:4297–4304. doi: 10.1128/mcb.12.10.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su GH, et al. The Ets protein Spi-B is expressed exclusively in B cells and T cells during development. J Exp Med. 1996;184:203–214. doi: 10.1084/jem.184.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Süss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-Ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 43.Dubois B, et al. Dendritic cells directly modulate B cell growth and differentiation. J Leukoc Biol. 1999;66:224–230. doi: 10.1002/jlb.66.2.224. [DOI] [PubMed] [Google Scholar]
- 44.Berney C, et al. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190:851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes BF, Hale LP. The human thymus. A chimeric organ comprised of central and peripheral lymphoid components. Immunol Res. 1998;18:175–192. doi: 10.1007/BF02788778. [DOI] [PubMed] [Google Scholar]
- 46.Dubois B, et al. Toward a role of dendritic cells in the germinal center reaction: triggering of B cell proliferation and isotype switching. J Immunol. 1999;162:3428–3436. [PubMed] [Google Scholar]
- 47.Bruno L, Res P, Dessing M, Cella M, Spits H. Identification of a committed T cell precursor population in adult human peripheral blood. J Exp Med. 1997;185:875–884. doi: 10.1084/jem.185.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]