Abstract
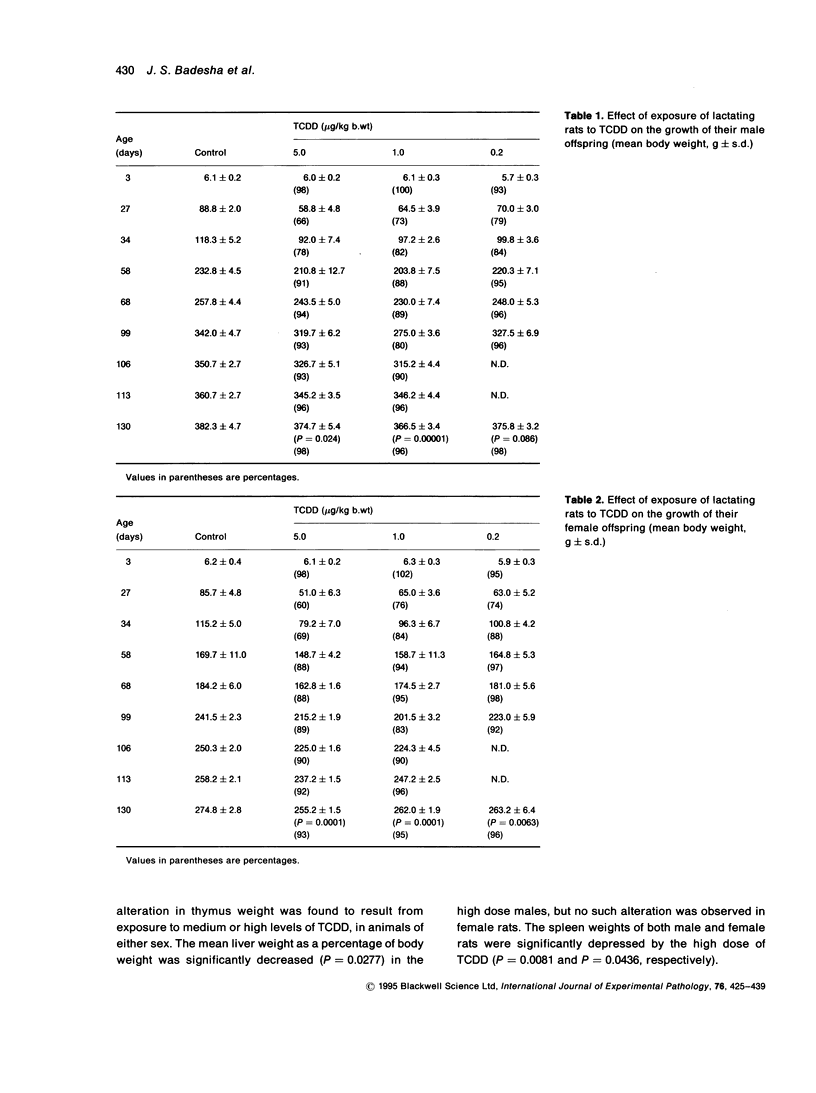
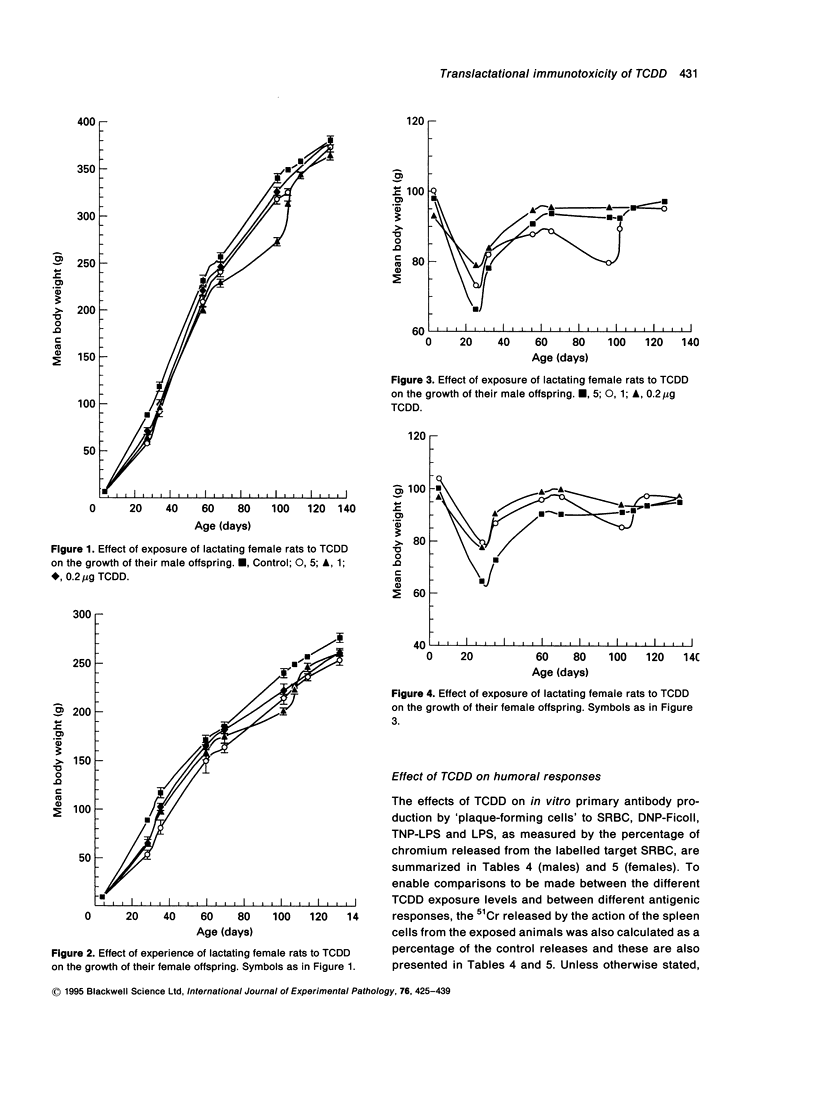
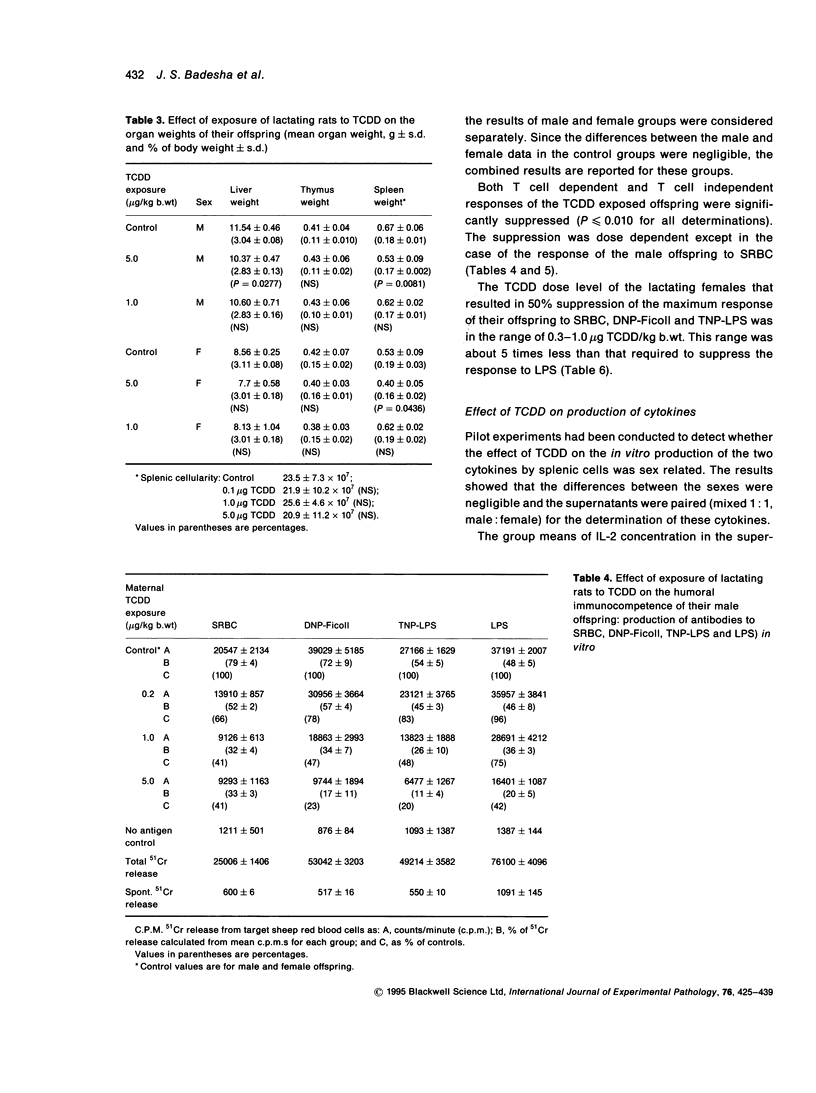
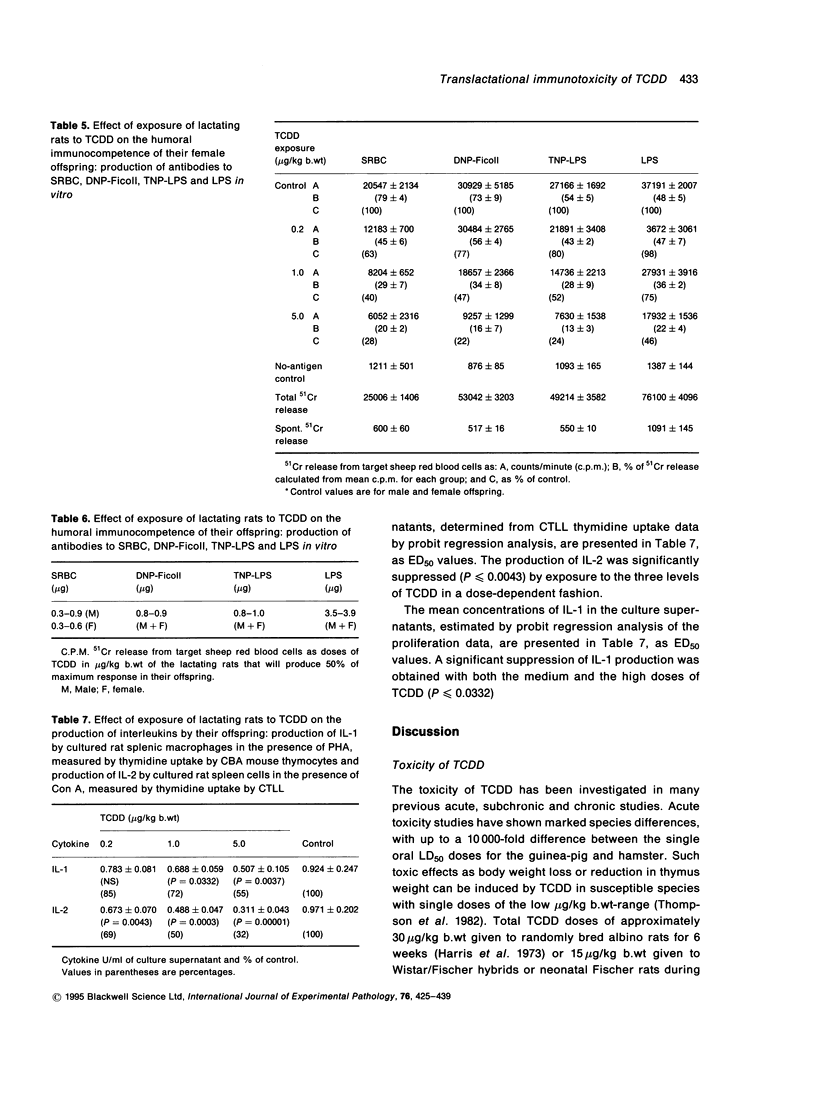
Exposure of lactating female Leeds rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) resulted in a reduction in the body, spleen and liver weights of their male offspring at 130 days of age. None of the total administered doses (0.2, 1.0 or 5.0 micrograms/kg b.wt over 18 days) induced thymic atrophy in the offspring of either sex as adults. Most of the growth inhibition occurred during the suckling period and the effect was near maximal following maternal exposure to the lowest dose of TCDD. After this dose, at post-natal day 130 the body weights of the female offspring remained depressed, while those of the males had recovered to untreated control values. Maternal exposure to TCDD affected the immunocompetence of the adult offspring: in vitro T cell dependent and T cell independent responses and mitogen induced in vitro production of interleukin 1 (IL-1) and interleukin 2 (IL-2) were suppressed at post-natal day 130. The total dose of TCDD that has to be administered to dams over an 18-day nursing period in order to reduce the humoral responses of their offspring as adults by 50% of the maximum was estimated to be in the range 0.3-1.0 micrograms/kg b.wt to the antigens SRBC, DNP-Ficoll or TNP-LPS and 3.5-3.9 micrograms/kg b.wt. to the antigen LPS.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsharif N. Z., Schlueter W. J., Stohs S. J. Stimulation of NADPH-dependent reactive oxygen species formation and DNA damage by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat peritoneal lavage cells. Arch Environ Contam Toxicol. 1994 Apr;26(3):392–397. doi: 10.1007/BF00203568. [DOI] [PubMed] [Google Scholar]
- Bagchi M., Stohs S. J. In vitro induction of reactive oxygen species by 2,3,7,8-tetrachlorodibenzo-p-dioxin, endrin, and lindane in rat peritoneal macrophages, and hepatic mitochondria and microsomes. Free Radic Biol Med. 1993 Jan;14(1):11–18. doi: 10.1016/0891-5849(93)90504-n. [DOI] [PubMed] [Google Scholar]
- Bailey M., Williams N. A., Wilson A. D., Stokes C. R. PROBIT: weighted probit regression analysis for estimation of biological activity. J Immunol Methods. 1992 Aug 30;153(1-2):261–262. doi: 10.1016/0022-1759(92)90329-r. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï N. P., Chanh P. H., Sesque G., Azum-Gelade M. C., Saint-Ruf G. Organs as targets of "dioxin" (2,3,7,8-tetrachlorodibenzo-p-dioxin) intoxication. Naturwissenschaften. 1972 Apr;59(4):174–175. doi: 10.1007/BF00637374. [DOI] [PubMed] [Google Scholar]
- Chastain J. E., Jr, Pazdernik T. L. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced immunotoxicity. Int J Immunopharmacol. 1985;7(6):849–856. doi: 10.1016/0192-0561(85)90047-5. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dooley R. K., Holsapple M. P. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988 Nov-Dec;16(3):167–180. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Dooley R. K., Morris D. L., Holsapple M. P. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: II. The role of the T-lymphocyte. Immunopharmacology. 1990 Jan-Feb;19(1):47–58. doi: 10.1016/0162-3109(90)90026-b. [DOI] [PubMed] [Google Scholar]
- Faith R. E., Luster M. I. Investigations on the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on parameters of various immune functions. Ann N Y Acad Sci. 1979 May 31;320:564–571. doi: 10.1111/j.1749-6632.1979.tb56634.x. [DOI] [PubMed] [Google Scholar]
- Faith R. E., Moore J. A. Impairment of thymus-dependent immune functions by exposure of the developing immune system to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J Toxicol Environ Health. 1977 Oct;3(3):451–464. doi: 10.1080/15287397709529578. [DOI] [PubMed] [Google Scholar]
- Fruman D. A., Mather P. E., Burakoff S. J., Bierer B. E. Correlation of calcineurin phosphatase activity and programmed cell death in murine T cell hybridomas. Eur J Immunol. 1992 Oct;22(10):2513–2517. doi: 10.1002/eji.1830221008. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Maness S. C., Leonard L. S., Greenlee W. F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-dependent regulation of transforming growth factors-alpha and -beta 2 expression in a human keratinocyte cell line involves both transcriptional and post-transcriptional control. J Biol Chem. 1992 Dec 5;267(34):24591–24595. [PubMed] [Google Scholar]
- Gillis C. N., Huxtable R. J., Roth R. A. Effects of monocrotaline pretreatment of rats on removal of 5-hydroxytryptamine and noradrenaline by perfused lung. Br J Pharmacol. 1978 Jul;63(3):435–443. doi: 10.1111/j.1476-5381.1978.tb07795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B. N., Vos J. G., Moore J. A., Zinkl J. G., Bullock B. C. Pathologic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals. Environ Health Perspect. 1973 Sep;5:125–140. doi: 10.1289/ehp.7305125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. W., Moore J. A., Vos J. G., Gupta B. N. General biological effects of TCDD in laboratory animals. Environ Health Perspect. 1973 Sep;5:101–109. doi: 10.1289/ehp.7305101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsapple M. P., Dooley R. K., McNerney P. J., McCay J. A. Direct suppression of antibody responses by chlorinated dibenzodioxins in cultured spleen cells from (C57BL/6 x C3H)F1 and DBA/2 mice. Immunopharmacology. 1986 Dec;12(3):175–186. doi: 10.1016/0162-3109(86)90001-9. [DOI] [PubMed] [Google Scholar]
- Hornshaw T. C., Aulerich R. J., Johnson H. E. Feeding Great Lakes fish to mink: effects on mink and accumulation and elimination of PCBS by mink. J Toxicol Environ Health. 1983 Apr-Jun;11(4-6):933–946. doi: 10.1080/15287398309530396. [DOI] [PubMed] [Google Scholar]
- Håkansson H., Ahlborg U. G. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the uptake, distribution and excretion of a single oral dose of [11,12-3H]retinyl acetate and on the vitamin A status in the rat. J Nutr. 1985 Jun;115(6):759–771. doi: 10.1093/jn/115.6.759. [DOI] [PubMed] [Google Scholar]
- Håkansson H., Waern F., Ahlborg U. G. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the lactating rat on maternal and neonatal vitamin A status. J Nutr. 1987 Mar;117(3):580–586. doi: 10.1093/jn/117.3.580. [DOI] [PubMed] [Google Scholar]
- Ismadi S. D., Olson J. A. Dynamics of the fetal distribution and transfer of Vitamin A between rat fetuses and their mother. Int J Vitam Nutr Res. 1982;52(2):112–119. [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Jansen H. T., Cooke P. S., Porcelli J., Liu T. C., Hansen L. G. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993 May-Jun;7(3):237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Jenkins J. K., Arend W. P. Interleukin 1 receptor antagonist production in human monocytes is induced by IL-1 alpha, IL-3, IL-4 and GM-CSF. Cytokine. 1993 Sep;5(5):407–415. doi: 10.1016/1043-4666(93)90030-9. [DOI] [PubMed] [Google Scholar]
- Jenkins J. K., Malyak M., Arend W. P. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994 Feb;13(1):47–54. [PubMed] [Google Scholar]
- Jensen D. J., Hummel R. A. Secretion of TCDD in milk and cream following the feeding of TCDD to lactating dairy cows. Bull Environ Contam Toxicol. 1982 Oct;29(4):440–446. doi: 10.1007/BF01605609. [DOI] [PubMed] [Google Scholar]
- Karras J. G., Holsapple M. P. Inhibition of calcium-dependent B cell activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1994 Apr;125(2):264–270. doi: 10.1006/taap.1994.1072. [DOI] [PubMed] [Google Scholar]
- Kerkvliet N. I., Brauner J. A. Mechanisms of 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD)-induced humoral immune suppression: evidence of primary defect in T-cell regulation. Toxicol Appl Pharmacol. 1987 Jan;87(1):18–31. doi: 10.1016/0041-008x(87)90080-9. [DOI] [PubMed] [Google Scholar]
- Kerkvliet N. I., Oughton J. A. Acute inflammatory response to sheep red blood cell challenge in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): phenotypic and functional analysis of peritoneal exudate cells. Toxicol Appl Pharmacol. 1993 Apr;119(2):248–257. doi: 10.1006/taap.1993.1066. [DOI] [PubMed] [Google Scholar]
- Kimura S., Eldridge J. H., Michalek S. M., Morisaki I., Hamada S., McGhee J. R. Immunoregulation in the rat: ontogeny of B cell responses to types 1, 2, and T-dependent antigens. J Immunol. 1985 May;134(5):2839–2846. [PubMed] [Google Scholar]
- Kramer C. M., Sando J. J., Holsapple M. P. Lack of direct effect of 2,3,7,8-tetrachlorodibenzo-P-dioxin (TCDD) on protein kinase C activity in EL4 cells. Biochem Biophys Res Commun. 1986 Oct 15;140(1):267–272. doi: 10.1016/0006-291x(86)91085-5. [DOI] [PubMed] [Google Scholar]
- Kremer J., Gleichmann E., Esser C. Thymic stroma exposed to arylhydrocarbon receptor-binding xenobiotics fails to support proliferation of early thymocytes but induces differentiation. J Immunol. 1994 Sep 15;153(6):2778–2786. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lundberg K., Dencker L., Grönvik K. O. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits the activation of antigen-specific T-cells in mice. Int J Immunopharmacol. 1992 May;14(4):699–705. doi: 10.1016/0192-0561(92)90133-6. [DOI] [PubMed] [Google Scholar]
- Lundberg K., Dencker L., Grönvik K. O. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) treatment in vivo on thymocyte functions in mice after activation in vitro. Int J Immunopharmacol. 1990;12(4):459–466. doi: 10.1016/0192-0561(90)90029-m. [DOI] [PubMed] [Google Scholar]
- Lundberg K., Grönvik K. O., Goldschmidt T. J., Klareskog L., Dencker L. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters intrathymic T-cell development in mice. Chem Biol Interact. 1990;74(1-2):179–193. doi: 10.1016/0009-2797(90)90066-v. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Faith R. E., Clark G. Laboratory studies on the immune effects of halogenated aromatics. Ann N Y Acad Sci. 1979 May 31;320:473–486. doi: 10.1111/j.1749-6632.1979.tb56628.x. [DOI] [PubMed] [Google Scholar]
- McConnell E. E., Lucier G. W., Rumbaugh R. C., Albro P. W., Harvan D. J., Hass J. R., Harris M. W. Dioxin in soil: bioavailability after ingestion by rats and guinea pigs. Science. 1984 Mar 9;223(4640):1077–1079. doi: 10.1126/science.6695194. [DOI] [PubMed] [Google Scholar]
- McConnell E. E., Moore J. A., Dalgard D. W. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rhesus monkeys (Macaca mulatta) following a single oral dose. Toxicol Appl Pharmacol. 1978 Jan;43(1):175–187. doi: 10.1016/s0041-008x(78)80042-8. [DOI] [PubMed] [Google Scholar]
- McConnell E. E., Moore J. A., Haseman J. K., Harris M. W. The comparative toxicity of chlorinated dibenzo-p-dioxins in mice and guinea pigs. Toxicol Appl Pharmacol. 1978 May;44(2):335–356. doi: 10.1016/0041-008x(78)90195-3. [DOI] [PubMed] [Google Scholar]
- Moos A. B., Baecher-Steppan L., Kerkvliet N. I. Acute inflammatory response to sheep red blood cells in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: the role of proinflammatory cytokines, IL-1 and TNF. Toxicol Appl Pharmacol. 1994 Aug;127(2):331–335. doi: 10.1006/taap.1994.1169. [DOI] [PubMed] [Google Scholar]
- Nagayama J., Tokudome S., Kuratsune M., Masuda Y. Transfer of polychlorinated dibenzofurans to the foetuses and offspring of mice. Food Cosmet Toxicol. 1980 Apr;18(2):153–157. doi: 10.1016/0015-6264(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Nau H., Bass R., Neubert D. Transfer of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) via placenta and milk, and postnatal toxicity in the mouse. Arch Toxicol. 1986 May;59(1):36–40. doi: 10.1007/BF00263955. [DOI] [PubMed] [Google Scholar]
- Neal G. E., Cabral J. R. Effect of partial hepatectomy on the response of rat liver to aflatoxin B1. Cancer Res. 1980 Dec;40(12):4739–4743. [PubMed] [Google Scholar]
- Nebert D. W., Puga A., Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci. 1993 Jun 23;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Neubert R., Jacob-Müller U., Helge H., Stahlmann R., Neubert D. Polyhalogenated dibenzo-p-dioxins and dibenzofurans and the immune system. 2. In vitro effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on lymphocytes of venous blood from man and a non-human primate (Callithrix jacchus). Arch Toxicol. 1991;65(3):213–219. doi: 10.1007/BF02307311. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Bliven M. L., Eskra J. D., Reinke M., Hanson D. C. The pharmacologic regulation of interleukin-1 production: the role of prostaglandins. Cell Immunol. 1988 Jul;114(2):385–397. doi: 10.1016/0008-8749(88)90330-9. [DOI] [PubMed] [Google Scholar]
- Poland A., Teitelbaum P., Glover E., Kende A. Stimulation of in vivo hepatic uptake and in vitro hepatic binding of [125I]2-lodo-3,7,8-trichlorodibenzo-p-dioxin by the administration of agonist for the Ah receptor. Mol Pharmacol. 1989 Jul;36(1):121–127. [PubMed] [Google Scholar]
- Rappe C., Nygren M., Marklund S., Keller L. O., Bergqvist P. A., Hansson M. Assessment of human exposure to polychlorinated dibenzofurans and dioxins. Environ Health Perspect. 1985 May;60:303–304. doi: 10.1289/ehp.8560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schecter A., Startin J., Wright C., Kelly M., Päpke O., Lis A., Ball M., Olson J. Dioxins in U.S. food and estimated daily intake. Chemosphere. 1994 Nov-Dec;29(9-11):2261–2265. doi: 10.1016/0045-6535(94)90393-x. [DOI] [PubMed] [Google Scholar]
- Skelly R. R., Munkenbeck P., Morrison D. C. Stimulation of T-independent antibody responses by hapten-lipopolysaccharides without repeating polymeric structure. Infect Immun. 1979 Feb;23(2):287–293. doi: 10.1128/iai.23.2.287-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter T. R., Guzman K., Dold K. M., Greenlee W. F. Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science. 1991 Oct 18;254(5030):415–418. doi: 10.1126/science.1925598. [DOI] [PubMed] [Google Scholar]
- Thompson H. J., Meeker L. D., Becci P. J., Kokoska S. Effect of short-term feeding of sodium selenite on 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in the rat. Cancer Res. 1982 Dec;42(12):4954–4958. [PubMed] [Google Scholar]
- Thunberg T., Ahlborg U. G. Effects of dietary vitamin A (retinol) on the reduction of hepatic retinol storage caused by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Dev Toxicol Environ Sci. 1980;8:521–524. [PubMed] [Google Scholar]
- Thunberg T., Ahlborg U. G., Johnsson H. Vitamin A (retinol) status in the rat after a single oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Toxicol. 1979 Sep;42(4):265–274. doi: 10.1007/BF00334840. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., Vore S. J., Luster M. I. Suppression of B cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1986 Apr;29(4):372–377. [PubMed] [Google Scholar]
- Vanden Heuvel J. P., Clark G. C., Tritscher A. m., Lucier G. W. Accumulation of polychlorinated dibenzo-p-dioxins and dibenzofurans in liver of control laboratory rats. Fundam Appl Toxicol. 1994 Oct;23(3):465–469. doi: 10.1006/faat.1994.1128. [DOI] [PubMed] [Google Scholar]
- Vecchi A., Mantovani A., Sironi M., Luini W., Spreafico F., Garattini S. The effect of acute administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on humoral antibody production and cell-mediated activities in mice. Arch Toxicol Suppl. 1980;4:163–165. doi: 10.1007/978-3-642-67729-8_35. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Moore J. A. Suppression of cellular immunity in rats and mice by maternal treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Int Arch Allergy Appl Immunol. 1974;47(5):777–794. doi: 10.1159/000231268. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Moore J. A., Zinkl J. G. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the immune system of laboratory animals. Environ Health Perspect. 1973 Sep;5:149–162. doi: 10.1289/ehp.7305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., de Klerk A., Krajnc E. I., Kruizinga W., van Ommen B., Rozing J. Toxicity of bis(tri-n-butyltin)oxide in the rat. II. Suppression of thymus-dependent immune responses and of parameters of nonspecific resistance after short-term exposure. Toxicol Appl Pharmacol. 1984 Sep 30;75(3):387–408. doi: 10.1016/0041-008x(84)90177-7. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu Rev Pharmacol Toxicol. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Young E. T., Nicholls D. M. Liver enzyme induction by 1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane (DDT) is accompanied by an increase in the specific activity of elongation factor 1. Biochem J. 1978 Jun 15;172(3):479–486. doi: 10.1042/bj1720479. [DOI] [PMC free article] [PubMed] [Google Scholar]


