Summary
Local protein synthesis regulates the turning of growth cones to guidance cues yet little is known about which proteins are synthesized or how they contribute to directional steering. Here we show that β-actin mRNA resides in Xenopus retinal growth cones where it binds to the RNA-binding protein, Vg1RBP. Netrin-1 induces the movement of Vg1RBP granules into filopodia suggesting that it may direct the localization and translation of mRNAs in growth cones. Indeed, a gradient of netrin-1 activates the translation initiation regulator, eIF4E-BP, asymmetrically and triggers a polarized increase in β-actin translation on the near side of the growth cone prior to growth cone turning. Inhibition of β-actin translation abolishes both the asymmetric rise in β-actin and attractive, but not repulsive, turning. Our data suggest that newly synthesized β-actin, concentrated near sites of signal reception, provides the directional bias for polymerizing actin in the direction of an attractive stimulus.
Keywords: netrin-1, β-actin, retinal ganglion cell, translation, Vg1RBP
Introduction
Axonal growth cones navigate through the developing nervous system in response to guidance cues. To turn appropriately in response to guidance cue gradients, they transduce extracellular gradients into intracellular asymmetry. Previous studies have shown that guidance cue gradients induce asymmetries of signalling components such as Ca2+1 and receptor association with lipid rafts2. Similarly, turning can be induced by artificial asymmetries of Ca2+3,4, lipid rafts2, PLCγ activation5, or cAMP6. Recent findings show that chemotropic responses to netrin-1, Semaphorin 3A (Sema3A), Slit2 and brain derived neurotrophic factor (BDNF) require local translation7-10 raising the question of whether asymmetrical protein synthesis influences growth cone turning in guidance cue gradients.
β-actin is a candidate for asymmetrical cue-induced synthesis. Of the three isoforms of actin, α, β, and γ, β-actin is the isoform implicated in directional movement11. In situ hybridization shows that β-actin mRNA is present in axonal growth cones9,12 and stimulation with neurotrophins, such as NT-3, BDNF or NGF, increases the transport of β-actin mRNA in both embryonic and adult axons13-15. Asymmetric actin polymerization is thought to play a major role in growth cone turning, since asymmetrical application of cytochalasin, an actin depolymerizing drug, causes repulsive turning16. These findings suggest that asymmetrical β-actin synthesis may be involved in growth cone turning.
Protein synthesis is regulated by translation initiation factors that globally regulate the recruitment of ribosomes to mRNAs17. Guidance cues such as netrin-1 and Sema3A activate the rate-limiting translation initiation factor eIF4E10. mRNA-specific regulation can be achieved by RNA-binding proteins which bind to target mRNAs, transport them to a specific cytoplasmic compartment and, in some cases, help regulate or silence their translation18. The localization of β-actin mRNA requires a cis-acting element located in the 3’ untranslated region (UTR) of the mRNA, called the “zipcode” 19. In chick, zipcode binding protein 1 (ZBP1) associates with the zipcode to regulate the localization of β-actin mRNA to the leading edge of fibroblasts and growth cones14,19, and recent evidence shows that β-actin translation can be regulated by the phosphorylation of ZBP1 through Src kinase20. The Xenopus homolog of ZBP1, Vg1RBP (also known as Vera), is required for the motility of neural crest cells, binds several mRNAs such as Vg1, VegT and cofilin, and controls mRNA localization in Xenopus oocytes8,21. However, it is not known whether it binds to β-actin mRNA or whether it is present in developing retinal axons where it might help to regulate translation-dependent steering.
Here, we investigate the mechanisms that underlie protein synthesis-dependent steering of growth cones to netrin-1. We show that netrin-1 stimulates a rapid translocation of Vg1RBP granules into filopodia and increases β-actin synthesis. A directional gradient of netrin-1 induces a translation-dependent increase in β-actin on the side of the growth cone closest to the gradient source (near-side) and a similarly asymmetric activation of a global translational regulator (4EBP). These asymmetric changes precede growth cone turning and inhibition of β-actin synthesis by antisense morpholinos blocks the netrin-1-induced attractive turning, suggesting that spatially asymmetric translation of β-actin, biased to the near-side, directs turning towards the stimulus.
Results
Vg1RBP interacts with β-actin mRNA in retinal growth cones
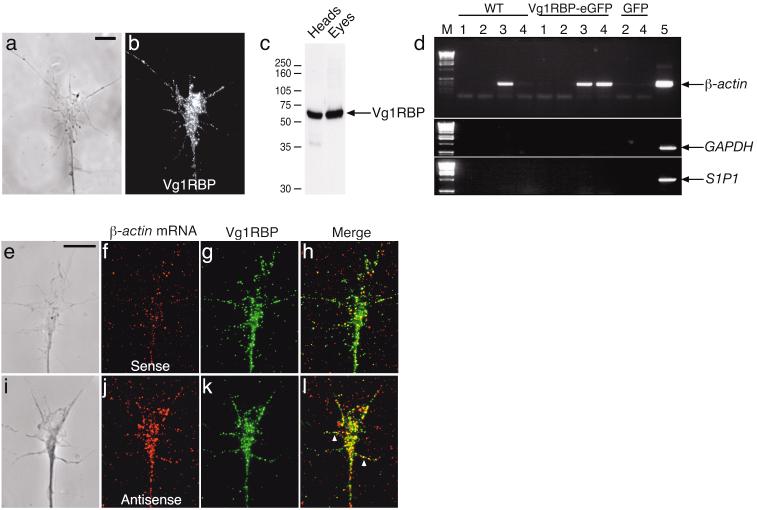
Immunostaining with an antibody raised against Xenopus Vg1RBP showed abundant expression of Vg1RBP in retinal growth cones in culture between stages 24 and 40 (Fig. 1a,b). Consistent with RNA-binding protein assembly in granules22, Vg1RBP protein has a punctate distribution along the axon shaft and in the growth cone central domain and filopodia (Fig. 1b). On Western blots, the Vg1RBP antibody detected a predominant band of the expected size (65 kDa) in both head and eye lysates, verifying the specificity of the antibody (Fig. 1c).
Figure 1. Vg1RBP is expressed in retinal growth cones and interacts with β-actin mRNA.
Cultured stage 33/34 retinal explants were stained with Vg1RBP antibody (a: phase, b: anti-Vg1RBP). Vg1RBP is detected in the axon shaft, central domain and filopodia. Western blot analysis using Vg1RBP antibody shows a predominant 65 kDa band in stage 33/34 head or eye lysates (c). Vg1RBP was immunoprecipitated from head lysates of stage 33/34 wildtype (WT) embryos, injected with Vg1RBP-eGFP mRNA or GFP mRNA (d). RNA extracted from the immunoprecipitates was subjected to RT-PCR using primers to the 3’UTR of β-actin mRNA (top panel), GAPDH mRNA (middle panel) or S1P1 mRNA (bottom panel). 1: protein A beads only. 2: purified rabbit IgG. 3: Vg1RBP antiserum. 4: GFP antiserum. 5: Positive RT-PCR control. M: molecular DNA marker. β-actin mRNA is immunoprecipitated with both Vg1RBP and Vg1RBP-eGFP. GAPDH and S1P1 mRNAs do not interact with Vg1RBP. FISH using riboprobes directed against 3’UTR of β-actin mRNA (j, antisense) or control RNA (f, sense) together with immunostaining of Vg1RBP (g,k) reveals partial colocalization of Vg1RBP and β-actin mRNA in the growth cone (e,i: phase, f: control sense RNA, j: β-actin antisense RNA, g,k: Vg1RBP, h,l: merge). Arrowheads refer to complexes of Vg1RBP and β-actin mRNA in a filopodium. Scale bars 5 μm.
Vg1RBP associates with β-actin mRNA as shown by immunoprecipitation followed by RT-PCR (Fig.1d). β-actin mRNA was detected by RT-PCR in samples immunoprecipitated from embryo head lysates using antibodies against endogenous Vg1RBP or expressed Vg1RBP-eGFP (Fig. 1d; lanes 3 and 4), but not in control immunoprecipitates (Fig. 1d; lanes 1 and 2). In contrast, the mRNAs of GAPDH and Sphingosine-1-phosphate receptor 1 (S1P1), an mRNA present in retinal growth cones (Strochlic and Holt, unpublished observation), did not associate with Vg1RBP (Fig. 1d; middle and lower panel).
Association of β-actin mRNA and Vg1RBP in vitro does not prove that Vg1RBP binds β-actin mRNA in vivo. To investigate whether Vg1RBP and β-actin mRNA interact in the growth cone, we performed fluorescent in situ hybridization (FISH) with digoxigenin-labelled antisense probes against the β-actin 3’UTR followed by immunostaining for endogenous Vg1RBP (Fig. 1e-l). Consistent with previous studies12,14, we found a punctate pattern of the β-actin mRNA signal in growth cones (Fig. 1i-l). In contrast, FISH using sense probes detected only background signal (Fig. 1e-h). Vg1RBP granules overlapped with β-actin mRNA puncta compared to sense riboprobe puncta (antisense versus sense: 56.7% ± 1.2% versus 38.5% ± 1.0%, mean ± s.e.m., P < 0.0001 Mann Whitney test), indicating significant co-localization of Vg1RBP and β-actin mRNA. Vg1RBP/β-actin mRNA complexes could also be detected in the growth cone filopodia (Fig. 1h, arrowheads). These data indicate that Vg1RBP interacts with β-actin mRNA and suggest that Vg1RBP may, like ZBP1, transport β-actin mRNA to and within retinal growth cones.
Dynamic movements of Vg1RBP-eGFP granules in growth cones
mRNA transport in neuronal cells involves both anterograde and retrograde movements of messenger ribonucleoprotein (mRNP) complexes14. mRNP complexes are actively transported along microtubules and actin filaments23. We therefore investigated whether Vg1RBP granules show characteristics of active transport.
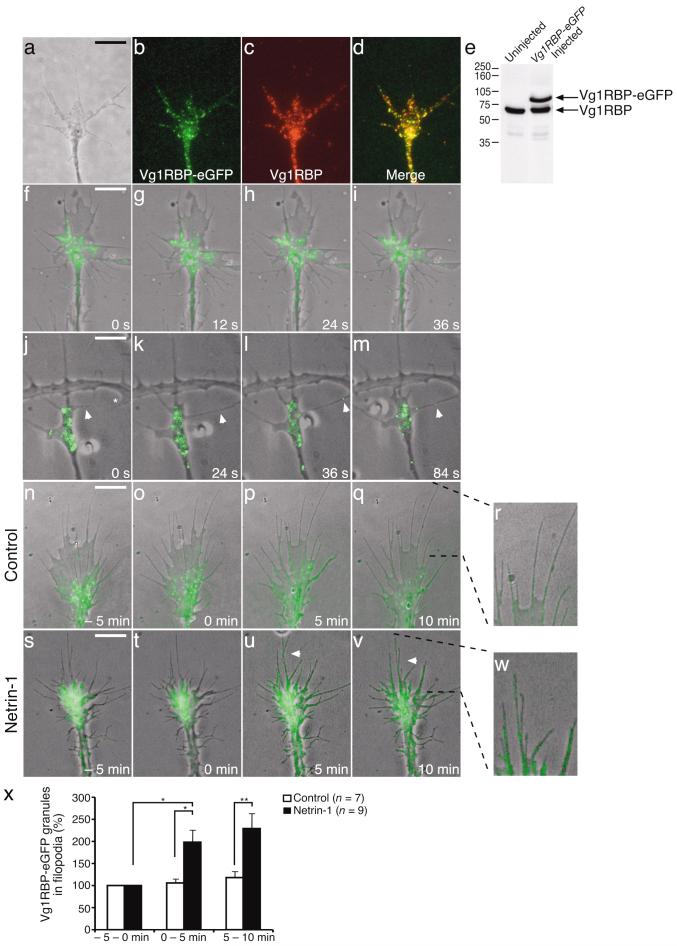
To this end, we visualized Vg1RBP dynamics using live cell imaging of single growth cones expressing eGFP-tagged Vg1RBP. Vg1RBP-eGFP mRNA was introduced by blastomere injection at the 8-cell stage and retinal cultures were made from eGFP-expressing embryos at stage 33/34. Both endogenous and eGFP-tagged proteins are recognized by the Vg1RBP antibody (Fig. 2e) using immunostaining or Western blot analysis and exhibit similar granular localization patterns and levels of expression (Fig. 2d,e). Furthermore, both endogenous Vg1RBP and Vg1RBP-eGFP can interact with β-actin mRNA in vitro (Fig. 1d).
Figure 2. Netrin-1 or cell-contact induce translocation of Vg1RBP-eGFP into filopodia.
Retinal explants taken from stage 33/34 embryos expressingVg1RBP-eGFP were stained with Vg1RBP antibody. Both endogenous and overexpressed proteins are recognized by the Vg1RBP antibody and show similar patterns of localization (a: phase, b: Vg1RBP-eGFP, c: Vg1RBP, d: merge). Vg1RBP-eGFP is expressed at similar levels as the endogenous Vg1RBP as shown by Western blot analysis of head lysates from injected and uninjected embryos (e). Time-lapse analysis of Vg1RBP-eGFP shows bidirectional movement of Vg1RBP-eGFP granules in the axon shaft, growth cone central domain and filopodia. Images were taken every 12 seconds (f-i and Supplementary movie 1). Vg1RBP-eGFP granules are also detected moving along filopodial contact-contact sites (j-m, arrowheads and Supplementary movie 2). The asterisk indicates the branch/filopodium, which is going to contact the Vg1RBP-eGFP expressing filopodium. Live imaging of Vg1RBP-eGFP expressing retinal growth cones stimulated with control medium (n-r) or netrin-1 (s-w and Supplementary movie 3) at T = 0 min. The percentage of Vg1RBP-eGFP granules in the filopodia was calculated in each frame and averaged per 5 minutes (x). Application of netrin-1 results in an increase of Vg1RBP-eGFP granules in the filopodia compared to control. * P < 0.05, ** P < 0.01, Kruskal-Wallis test. Error bars s.e.m. Scale bars 5 μm.
Live cell imaging revealed bidirectional movements of Vg1RBP-eGFP granules in retinal growth cones (Fig. 2f-i and Supplementary movie 1). In unstimulated cultures, Vg1RBP-eGFP granules were enriched in the growth cone central domain and were also occasionally observed in filopodia. Granule movement patterns ranged from oscillations with no net movement, to uni- and bi-directional movements over small and longer (> 10μm) distances. The average speed ranged from 0 to 0.7 μm s-1. Interference with the actin cytoskeleton using cytochalasin D resulted in a complete retraction of Vg1RBP granules from the growth cone (Supplementary movie 2). In contrast, depolymerizing microtubules with nocodazole had no obvious effects on Vg1RBP movement in the growth cone (data not shown).
Filopodia can be considered as the long-range (5-10 μm) sensors of the growth cone, enabling it to detect and respond to new cues along the pathway. It is interesting, therefore, that we occasionally observed that Vg1RBP granules move rapidly to the site where a filopodium forms a new contact with another filopodium or axon shaft (Fig. 2j-m). An example is shown (Fig.2j-m and Supplementary movie 3) which illustrates anterograde transport of a Vg1RBP-eGFP granule moving to, and subsequently away from, a transient contact site between two filopodia (different cellular origins). This suggests that the trafficking of Vg1RBP granules in filopodia might be regulated by external cues.
Netrin-1 induces transport of Vg1RBP into filopodia
The observation that Vg1RBP granules move to contact sites prompted us to investigate whether an axon guidance cue could alter granule movement. Netrin-1 plays an important role in retinal axon guidance24 and can trigger local protein synthesis in growth cones10; therefore, we hypothesized that it might also elicit the transport of Vg1RBP granules into filopodia to bring mRNA close to sites of signal reception. To test this, live imaging was used to determine the percentage of Vg1RBP-eGFP granules in the central domain versus filopodia before and during global stimulation with netrin-1 (Fig. 2n-x). Bath application of netrin-1, but not control medium, increased the percentage of Vg1RBP granules present in filopodia after 5 and 10 minutes (Fig. 2n-x and Supplementary movie 4). The percentage of granules in the central domain of the growth cone did not change significantly (data not shown) and no obvious changes in growth cone morphology were observed. Netrin-1 also increased diffuse Vg1RBP signal in filopodia, which was not included in the quantification (Fig. 2x). To test whether these Vg1RBP granules contain β-actin mRNA, we used quantitative immunofluorescence (QIF)/FISH. Netrin-1 induced an increase in both Vg1RBP and β-actin mRNA signal in filopodia after 10 minutes. Moreover, similar levels of colocalization between Vg1RBP and β-actin mRNA in filopodia were detected before and after netrin-1 stimulation (Supplementary Fig. 1). Together, these results show that netrin-1 induces the transport of Vg1RBP granules into the filopodia and suggest that a significant fraction of these carry β-actin mRNA cargo.
Netrin-1 triggers a translation-dependent rise in β-actin
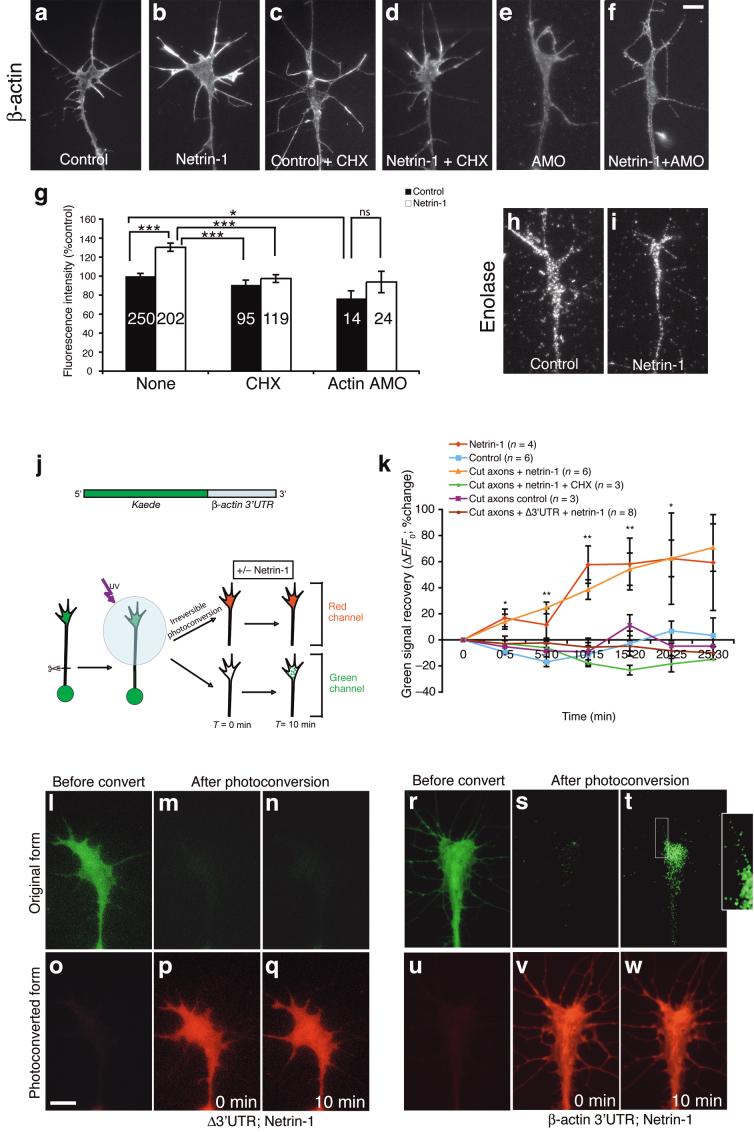
Since netrin-1 regulates the movement of an RNA-binding protein that can interact with β-actin mRNA, we asked whether netrin-1 might trigger β-actin mRNA translation in growth cones. Quantitative IF using an antibody that specifically detects β-actin revealed a 30% increase in the average pixel intensity per unit area after 5-minute stimulation with netrin-1 (Fig. 3a,b,g) compared to unstimulated growth cones8,10,25,26. Cycloheximide (CHX), a protein synthesis inhibitor, blocked this increase (Fig. 3c,d,g) but did not significantly affect growth cone β-actin mRNA levels (control versus CHX: 100% ± 6.2% versus 93% ± 6.8%, mean ± s.e.m., P = 0.106 Mann Whitney test), suggesting that the netrin-1-induced increase in β-actin protein is mediated via protein synthesis. A blocking antibody against Deleted in Colorectal Cancer (DCC), the receptor that mediates growth cone chemotropic responses to netrin-124, also prevented this increase in β-actin QIF signal, indicating that netrin-1’s effects on translation are mediated by DCC (Supplementary Fig. 2).
Figure 3. Netrin-1 induces β-actin translation driven by its 3’UTR.
Stage 24 retinal growth cones were stimulated with netrin-1 for 5 minutes, stained for β-actin and fluorescence intensities were measured. Netrin-1induced an increase in β-actin QIF signal, which was blocked by CHX and β-actin AMO (a-g). *** P < 0.0001, Kruskal-Wallis test. Growth cones from β-actin AMO injected retina showed reduced β-actin QIF signal (e,g), which was not affected by netrin-1 stimulation (f,g). * P = 0.02, ns = non-significant, Mann-Whitney test. Enolase QIF signal was not affected by netrin-1 stimulation (h,i). Schematic diagram of Kaede construct and experimental design (j). Time-lapse imaging showed that only Kaede-green was detected before photoconversion (l,r). At T = 0 min, Kaede was photoconverted and only Kaede-red was detected (p,v). Netrin-1 had no effect on Kaede-green (l-n) or Kaede-red signals (o-q) in growth cones expressing Kaede-Δ3’UTR (k). However, netrin-1 induced a recovery of Kaede-green in both intact and severed growth cones expressing Kaede-β actin 3’UTR (k,s,t), which was blocked by CHX (k). The recovery was also seen in some filopodia (t, boxed area and inset). The exposure gain for inset picture is increased for visualization of filopodia. Kaede-red remained unchanged (v,w). The change in Kaede-green signal (ΔF) was compared to the image taken at T = 0 min (F0) and presented as ΔF/F0. * P < 0.05, ** P < 0.01, Mann-Whitney test. Scale bars 5 μm. Error bars s.e.m. Numbers inside bars indicate the number of growth cones analyzed.
To specifically inhibit β-actin translation, we used an antisense morpholino oligonucleotide (AMO) directed against the AUG start site of β-actin mRNA. Lissamine-tagged AMOs were injected into blastomeres fated to give rise to eyes. At the concentrations used, the AMO-injected embryos developed normally and retinal cultures were made from lissamine-positive eyes at stage 24. Fluorescent labelling of AMOs enabled us to restrict our analyses to growth cones known to contain AMOs (see Supplementary Fig. 4). The β-actin QIF signal was significantly lower in AMO-containing growth cones than in control growth cones (23%; Fig. 3a,e,g), verifying that the β-actin AMO inhibits β-actin translation. Moreover, netrin-1 did not significantly alter β-actin QIF signal in AMO-containing growth cones, indicating that the netrin-1-induced rise in β-actin levels depends on translation of β-actin mRNA (Fig. 3f,g).
In contrast, the QIF signal of a non-cytoskeletal protein, enolase, whose mRNA is abundant in retinal growth cones (Strochlic and Holt, unpublished observation) remained unchanged following netrin-1 stimulation (Fig. 3h,i; control versus netrin-1: 100% ± 5% versus 97% ± 4%, mean ± s.e.m., P = 0.648 unpaired t-test), indicating that netrin-1 does not elicit the translation of all mRNAs present in growth cones. Consistent with this, a previous study in Xenopus retinal growth cones showed that netrin-1 does not induce translation of cofilin-1 mRNA, while Slit2, a repellent guidance factor, induces the translation of cofilin but not β-actin mRNA8. To test whether β-actin translation is a common response to attractants, we used a second chemoattractant, BDNF. BDNF has been shown to increase protein synthesis in neurons27 and elicits protein synthesis-dependent turning responses in Xenopus spinal neurons7. We found that exposure of retinal growth cones to BDNF increased the β-actin QIF signal by 61% in 10 minutes and this rise was blocked by CHX (Supplementary Fig. 2). These results suggest that β-actin mRNA is translated locally in growth cones in response to chemoattractants.
β-actin 3’UTR mediates netrin-1-induced reporter synthesis
The translation of mRNAs is commonly regulated by the untranslated regions28. To test directly whether the β-actin 3’UTR drives the synthesis of new protein in response to netrin-1, we constructed a photoconvertible fluorescent protein 29, linked to the β-actin 3’UTR (Kaede-β-actin 3’UTR; Fig. 3j). Kaede protein is originally green but can be irreversibly, proteolytically converted to the red form by UV illumination, thereby permitting the detection of newly synthesized protein by visualizing the return of green fluorescence. Plasmid cDNA encoding Kaede-β-actin 3’UTR was introduced by blastomere injection and stage 24 eyes were cultured. Kaede-positive growth cones were UV illuminated for 6 seconds, which efficiently converted the green into red fluorescence (Fig. 3j,l-q). Netrin-1 stimulation significantly increased the green, but not red, signal within 5 minutes (Fig. 3k,r-w). This increase occurred mainly in the body of the growth cone but, with time, could also be detected in filopodia (Fig. 3t; inset). The elevation in Kaede-green occurred even when axons were severed from their cell bodies, demonstrating that the increase was not from new protein transported from the soma (Fig. 3k). Green fluorescence did not increase if the β-actin 3’UTR was replaced by a ‘control’ UTR mutated to escape regulation by Vg1RBP (Δ3’UTR; Fig. 3k-q), or if growth cones received a control vehicle instead of netrin-1 (Fig. 3k). In fact, in these control conditions, there was a slight decrease in green fluorescence due to photobleaching, suggesting that the netrin-1-induced increase in green fluorescence is underestimated. Importantly, the netrin-1-induced return of green fluorescence was blocked by CHX added before photoconversion (Fig.3k), indicating that it represented the synthesis of new protein, not just folding of Kaede protein synthesized immediately before photoconversion. These data indicate that the β-actin 3’UTR drives the translation of new protein in response to netrin-1.
Netrin-1 gradient induces asymmetric Vg1RBP transport
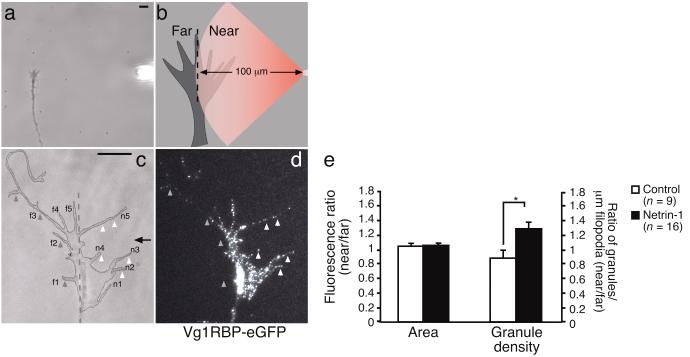
Our results with globally added netrin-1 suggest that guidance cues activate mechanisms that transport mRNA granules closer to sites of signal reception, potentially helping the growth cone respond to a directional stimulus. Thus, we reasoned that a directional stimulus might produce polarized transport of granules. To test this, we applied a netrin-1 gradient to growth cones and asked whether it elicited asymmetric trafficking of Vg1RBP. The gradient was produced by pulsatile ejection from a micropipette6 positioned at a 90° angle to the growth cone (Fig. 4a,b) to achieve a steep difference between the ‘near’ and ‘far’ sides of the growth cone. The line of symmetry was determined by tracing the perpendicular from the pipette to the axon shaft. Retinal growth cones expressing Vg1RBP-eGFP were subjected to a gradient of netrin-1 or control medium for 5 minutes, fixed and imaged. The number of granules per micrometer filopodia as well as the average pixel intensity per unit area were compared between the ‘near’ and ‘far’ sides of the growth cone, yielding a near/far ratio (Fig. 4b). Netrin-1, but not control medium, increased the number of granules per micrometer in filopodia on the near side by approximately 30% (Fig. 4c-e). Prolonged netrin-1 stimulation has been shown to increase filopodial length in retinal growth cones30. However, a 5-minute directional stimulation with netrin-1 did not significantly change the average length of the filopodia at the far versus the near side (6.48 μm versus 6.22 μm; P = 0.6, paired t-test). Furthermore, the overall fluorescence intensity of each side did not change, indicating that the asymmetric distribution is due to local transport of granules (Fig. 4e). Thus, a netrin-1 gradient elicits the polarized transport of Vg1RBP into filopodia.
Figure 4. Vg1RBP granules move into filopodia closest to a netrin-1 source.
Experimental design showing the pipette tip positioned 100 μm from the growth cone at an angle of 90°; a dashed line divides the growth cone into ‘near’ and ‘far’ sides with respect to the pipette. (a,b). Vg1RBP-eGFP expressing retinal growth cones were stimulated with a gradient of culture medium (control) or netrin-1 for 5 minutes and subsequently fixed (c-e). Mean fluorescence intensities as well as the number of granules per μm filopodia were calculated for both the near and far sides (c-e). A gradient of netrin-1 induces an increase in the number of Vg1RBP-eGFP granules per μm filopodia at the near side. No difference in Vg1RBP fluorescence intensity is observed in the far versus the near side (e). Near-side and far-side filopodia are numbered n1-n5 and f1-f5 respectively. Arrowheads indicate filopodia containing low-density (grey arrowheads) and high-density (white arrowheads) areas of Vg1RBP-eGFP granules. * P < 0.03 Mann Whitney test. Arrow indicates direction of pipette. Error bars s.e.m. Scale bars 5 μm.
Netrin-1 gradient induces asymmetric 4EBP phosphorylation
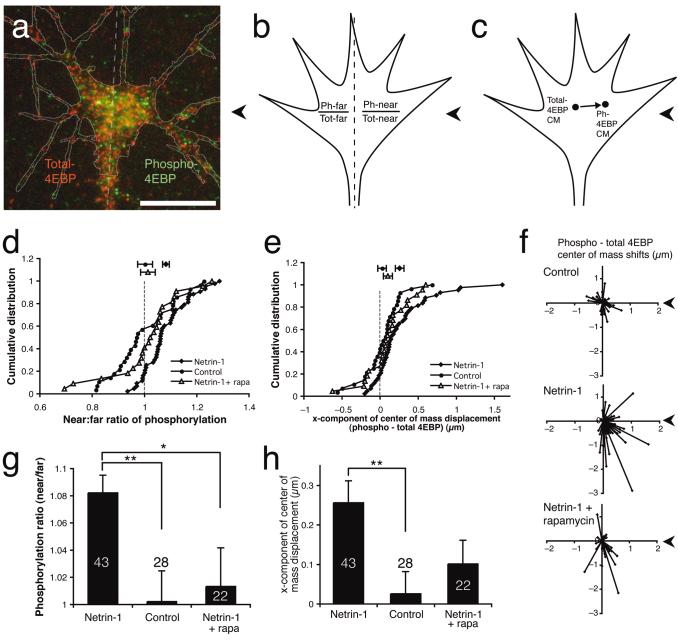
Bath application of netrin-1 has been shown to cause the rapid phosphorylation of the translation initiation factor eIF-4E-binding protein 1 (4EBP)10. The phosphorylation of 4EBP is a marker for cap-dependent translation initiation, because hypophosphorylated 4EBP blocks translation initiation by sequestering the rate-limiting translation initiation factor eIF-4E17.We therefore asked whether a gradient of netrin-1 could similarly induce asymmetric 4EBP phosphorylation.
Growth cones were stimulated with a netrin-1 gradient for 5 minutes, fixed and double-stained for Ser65-phosphorylated-4EBP and total-4EBP (Fig. 5a). 4EBP is phosphorylated hierarchically, with Ser65 being the final residue phosphorylated31. Two methods were used to assess asymmetric phosphorylation. First, the degree of phosphorylation on the ‘near’ and ‘far’ sides was taken as the ratio of phospho-4EBP to total-4EBP intensity per unit area (Fig. 5b). Phosphorylation asymmetry was calculated as the near/far ratio of 4EBP phosphorylation. Second, phosphorylation asymmetry was calculated as the difference between the ‘centers of mass’ of phospho- and total-4EBP intensity (Experimental Procedures and Fig. 5c). By both methods, the netrin-1 gradient produced a significant asymmetry in 4EBP phosphorylation, while a control gradient did not (Fig. 5d-h).
Figure 5. Netrin-1 gradient causes asymmetric activation of translation regulator.
Growth cone double-stained for total-4EBP (red) and phospho-4EBP (green) with a line dividing near/far sides (a). Lack of overlap may be due to stochastic mutual exclusion by large Zenon Fc-antibody complexes (see Methods). Asymmetric phosphorylation of 4EBP was assessed by the near/far ratio method (b) or by the ‘center of mass’ method (c). Phosphorylation is higher on the near side than on the far side in netrin-1-stimulated growth cones. (P < 0.000001, paired t-test), but not control-stimulated growth cones (P = 0.9) or netrin-1-stimulated growth cones treated with rapamycin (d,g; P > 0.95). The center of mass of phospho-4EBP staining is significantly closer to the pipette than that of total-4EBP in netrin-1-stimulated growth cones (P < 0.0001, paired t-test), but not in control-stimulated growth cones (P > 0.65) or netrin-1-stimulated growth cones treated with rapamycin (e,h; P > 0.1). The difference in center of mass shifts between the netrin-1 and netrin-1 + rapamycin conditions is almost significant (P = 0.06, Welch-corrected unpaired t-test). Vector plots of center of mass shifts show that only the netrin-1 condition exhibits consistent center of mass shifts toward the pipette (f). Each vector represents the center of mass shift of phospho-4EBP relative to total-4EBP in one growth cone. The axon shaft is down. Numbers in (g) and (h) indicate number of growth cones per condition. ** P < 0.005, * P < 0.05, Welch-corrected unpaired t-test. Arrowheads indicate direction of pipette. Scale bar 10 μm. Error bars s.e.m.
4EBP is phosphorylated by the kinase target of rapamycin (TOR)17. To test whether asymmetrical phosphorylation of 4EBP is mediated by TOR, we used the inhibitor of TOR, rapamycin. Rapamycin-treated growth cones exhibited no significant asymmetry in 4EBP phosphorylation (Fig. 5d-h). Although we cannot formally rule out the possibility that phospho-4EBP is selectively transported across the growth cone in a rapamycin-sensitive manner, the more parsimonious explanation is that an external gradient of netrin-1 is translated into an internal gradient of translation initiation through asymmetrical activation of signal transduction pathways.
Netrin-1 gradient elicits spatially asymmetric β-actin synthesis
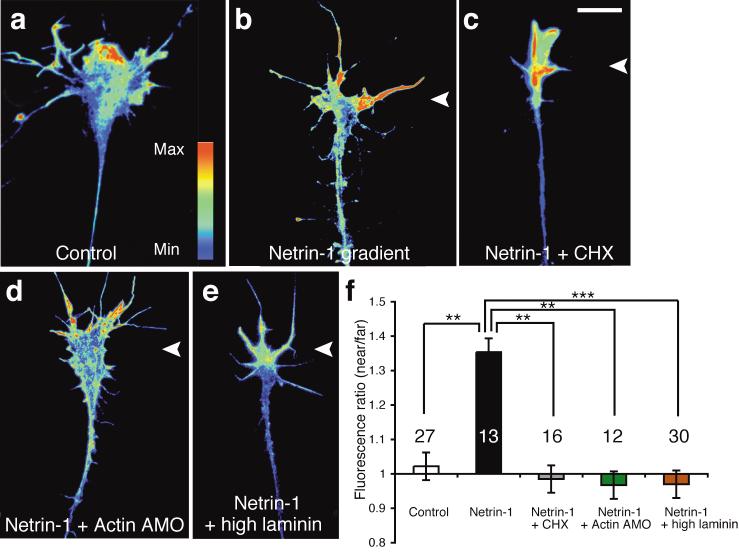
Since a netrin-1 gradient stimulates asymmetric activation of 4EBP and movement of Vg1RBP, we next asked whether it elicits asymmetric protein synthesis-dependent changes in β-actin. Growth cones were stimulated with a netrin-1 gradient for 5 minutes, immunostained for β-actin, and the near/far ratio of average pixel intensity per unit area was determined. Growth cones stimulated with a netrin-1 gradient, but not control growth cones, commonly exhibited an asymmetric distribution of β-actin QIF signal with a near/far ratio of 1.35:1 (Fig. 6a,b,f). The netrin-1-induced near/far bias in β-actin QIF signal was abolished in growth cones treated with CHX or β-actin AMO (Fig. 6c,d,f). These observations show that a netrin-1 gradient induces an asymmetric rise in β-actin in growth cones that is abolished by inhibition of translation and specifically inhibition of β-actin mRNA translation, thereby implicating newly synthesized β-actin in establishing the asymmetry.
Figure 6. Netrin-1 gradient elicits asymmetric increase of β-actin across the growth cone.
Growth cones from stage 24 retinal explants were exposed to a gradient of netrin-1 for 5 minutes and then stained for β-actin. Fluorescence intensities of both sides of the growth cone were measured and the near/far ratios of the different groups were compared (f). In control condition, β-actin was expressed at similar levels on both sides of the growth cone (a). Netrin-1 gradient induced an asymmetric rise in β-actin QIF signal on the near side (b), which was blocked by CHX (c) and β-actin AMO (d). Netrin-1 did not cause β-actin asymmetry in growth cones grown on high laminin substrate (e). Images were pseudo-coloured (see colour bar in a). ** P = 0.003, *** P < 0.001, Kruskal-Wallis test. Scale bar 10 μm. Error bars s.e.m. Numbers inside bars indicate the number of growth cones analyzed.
β-actin AMOs block netrin-1-induced attraction
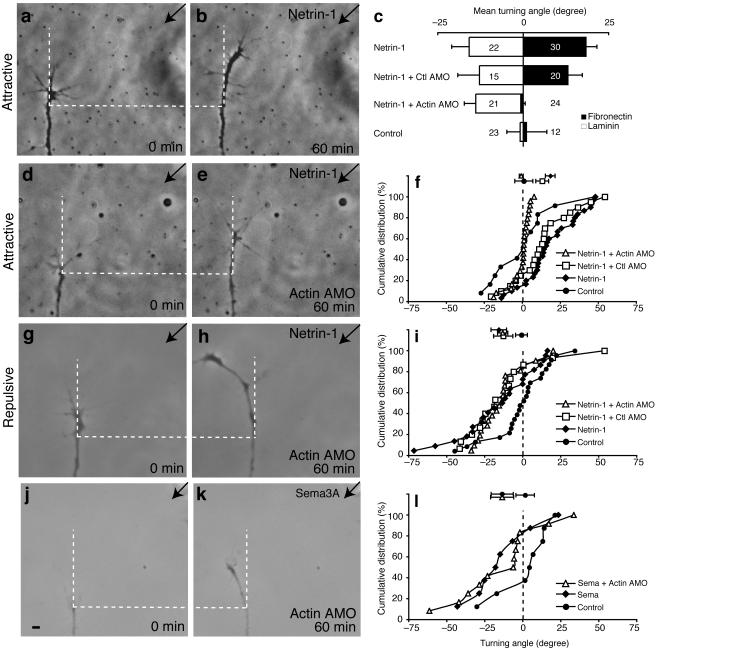
Finally, we hypothesized that the spatially asymmetric translation of β-actin mRNA was critical to the turning response to netrin-1. To test this, β-actin synthesis was inhibited with β-actin AMOs during a standard turning assay with netrin-130. Consistent with previous studies30, growth cones were attracted by a netrin-1 gradient (Fig. 7a-c,f; mean turning angle 18.4°) but not a control gradient (Fig. 7c,f; 0.9°). Growth cones containing control AMOs also turned toward the netrin-1 gradient (Fig. 7c,f; 13.1°) but growth cones containing β-actin AMOs were not attracted to a netrin-1 gradient (Fig. 7c-f; −0.7°). The lack of turning by the β-actin AMO-growth cones was not due to compromised mobility, because the average extension rate was not significantly different between β-actin-AMO and control-AMO growth cones (Fig. 7e; 0.28μm min-1 versus 0.30μm min-1; P = 0.7, unpaired t-test), consistent with previous findings that protein synthesis is not required for axon extension10,32. These results suggest that the attractive turning to netrin-1 requires rapid local β-actin synthesis.
Figure 7. β-actin morpholinos block netrin-1-induced attractive turning.
Eye primordia from stage 24 embryos injected with β-actin or control AMO were cultured for 24 hours. Under attractive conditions, netrin-1 caused strong positive turning (a-c,f; 18.4° ± 3.2°) whereas control medium did not (0.9° ± 6.0°). This attractive response was blocked in β-actin AMO-containing growth cones (c-f; −0.7° ± 1.3°) whereas growth cones containing control AMO retained attractive turning in response to netrin-1 (c and f; 13.1° ± 4.2°). *** P < 0.001. Under repulsive conditions, growth cones turned away from the source of netrin-1 (c and i, −15.8° ± 5.1°) but not control medium (−0.9° ± 3.9°). Introduction of β-actin AMO, as well as control AMO, into growth cones did not affect the repulsive turning response (c,g-i; −13.2° ± 3.3° and −12.8° ± 6.3° respectively). *** P < 0.001. The repulsive turning triggered by Sema3A in control growth cones (−13.6° ±7.3°) is similar to that in β-actin AMO-containing growth cones (j-l; −13.6° ±7.5°). P > 0.05. The control supernatant did not cause repulsive turning (1.5° ± 6.1°). Kolmogorov-Smirnov test. Mean turning angle ± s.e.m.. Error bars s.e.m.
To test whether AMOs inhibit the general responsiveness of growth cones to chemotropic cues we examined whether β-actin-AMO-containing growth cones were sensitive to repulsive cues. First, we used Sema3A and found that both the collapse and repulsive turning responses of β-actin-AMO growth cones were indistinguishable from control growth cones, demonstrating that the sensitivity to repellents was not compromised (Fig.7j-l and Supplementary Fig. 2). Second, we used netrin-1 on growth cones grown on a high concentration of laminin (20 μg ml-1), which converts attraction to repulsion by lowering cAMP levels in retinal growth cones26,33. Under these repulsive conditions, growth cones containing β-actin-AMOs were also repelled by a netrin-1 gradient (Fig. 7c,g-i; −13.2°). β-actin AMO and control AMO growth cones grew on laminin at the same rate (0.5 μm min-1). Consistent with this, growth cones stimulated with a netrin-1 gradient for 5 minutes under these repulsive conditions did not show an asymmetric increase in β-actin QIF signal on the near side (Fig. 6e,f). A slight bias towards the far side was sometimes observed but this was not, in our hands, statistically significant. Our results show that inhibition of β-actin translation differentially affects attractive and repulsive responses and indicate that β-actin translation is of particular importance for attractive turning.
Discussion
This study addressed the question of whether asymmetrical protein synthesis has a role in growth cone turning. Our results indicate that new β-actin protein is synthesized in response to netrin-1 and that an external gradient of netrin-1 causes a polarized increase of β-actin on the side of the growth cone nearest to the source. The increase occurs just 5 minutes after addition of netrin-1 and is abolished by inhibitors of translation. Significantly, β-actin AMOs block attractive but not repulsive turning suggesting that β-actin synthesis is particularly important for directional guidance towards a positive cue.
The finding that the β-actin AMOs abolished the netrin-1-induced increases in β-actin in growth cones indicates that the AMOs effectively inhibited β-actin translation over the time period examined (5-60 minutes). Overall, the AMOs caused a 20-25% drop in β-actin QIF signal. This relatively small knockdown of β-actin probably reflects the large maternal pool of actin that is recycled for the first 24-36 hours of embryonic development and likely predominates in the pioneering population of retinal axons, which develop just 28 hours post-fertilization. Indeed, the knockdown in β-actin QIF signal was higher in older growth cones than in younger ones (approximately 40% in stage 35/36 versus 20% in stage 24, data not shown). Importantly, AMO-containing axons grew at a normal rate and exhibited appropriate chemotropic responses to Sema3A and netrin-1 under repulsive conditions. It could be argued that attractive turning is more sensitive to β-actin levels (and hence actin polymerization rates) than axon extension or repulsive turning, and the β-actin AMO knocks down baseline β-actin translation levels just enough to block attractive turning but not extension or repulsion. Such an argument, however, would predict that the AMO would at least reduce axon extension and repulsive turning, while in fact we observed that extension and repulsion remained normal. Together, these lines of evidence indicate that β-actin translation is specifically required in attractive turning, not undirected growth, repulsive turning, or general responsiveness to netrin-1.
What role does β-actin translation play in attractive turning? At first glance it would seem that β-actin synthesis is not necessary for actin polymerization given that the pool of unpolymerized actin in growth cones is thought to be large34. Indeed, it has been estimated that β-actin synthesis provides only 7% of the total actin needed for polymerization in migrating fibroblasts19, making it unlikely that asymmetrical β-actin synthesis makes the growth cone turn by sheer mass alone. It has been proposed that newly synthesized β-actin can polymerize or nucleate polymerization more efficiently than “older” actin due to chaperone binding to the nascent β-actin chain11,35 and protecting it from glutathionylation, which restricts the rate of polymerization36. Thus, given that the rate-limiting step of actin polymerization is nucleation, an appealing model is that local β-actin synthesis provides spatially localized nucleation sites for actin polymerization11,19,37. The restricted size of the growth cone compartment would concentrate newly synthesized β-actin monomers in a small volume, thereby contributing to rapid formation of nucleation sites. We therefore suggest that a gradient of netrin-1 elicits spatially biased actin polymerization by inducing the asymmetric actin nucleation sites on the side of the growth cone closest to the pipette (see Supplementary Fig. 3). The netrin-1-stimulated increase in the β-actin signal is particularly evident in filopodia, suggesting that the new actin contributes to actin-filament bundles in these structures that radiate from the less polarized actin mesh network in the body of the growth cone38. As β-actin synthesis occurs at least 10 minutes before overt turning, we hypothesize that asymmetrical β-actin translation prefigures the turn itself.
Asymmetrical synthesis requires spatial regulation of β-actin translation. One possible mechanism is transport of β-actin mRNA to the side closest to the source of the gradient. Previous studies have shown that the neurotrophin NT-3 elicits the transport of the β-actin mRNA-ZBP1 complex into growth cones14 and serum stimulation induces the transport of β-actin mRNA to the leading edge of fibroblasts39. An independent study shows that both ZBP1 and β-actin mRNA become asymmetrically distributed in the growth cones of Xenopus spinal neurons upon BDNF stimulation (J. Zheng, personal communication). In agreement, our experiments show that Vg1RBP binds β-actin mRNA and that netrin-1 stimulation induces transport of Vg1RBP granules into filopodia, asymmetrically if netrin-1 is presented in a gradient. We also find that sites of filopodial contact can induce the transport of Vg1RBP-eGFP granules into filopodia, suggesting that external cues recruit RNA-binding proteins and their mRNA cargo to local sites of stimulation. Although our dynamic imaging studies could not demonstrate that the Vg1RBP-eGFP granules moving into filopodia were specifically transporting β-actin mRNA, fixed samples showed that Vg1RBP colocalizes with β-actin mRNA and the netrin-1-induced increase in filopodial Vg1RBP is accompanied by an increase in filopodial β-actin mRNA.
Another mechanism for spatial regulation of β-actin synthesis is the asymmetrical activation of translation. We found that a gradient of netrin-1 induced a significant asymmetry in the phosphorylation of the translation initiation factor 4EBP, suggesting a corresponding asymmetry in the global rate of translation. The translation of β-actin mRNA, like that of most eukaryotic mRNAs, is cap-dependent40, making it subject to asymmetrical activation of translation initiation factors. Both attractants and repellents stimulate activation of translation initiation factors10,41, though they presumably induce synthesis of different proteins. Indeed, in contrast to our result that netrin-1 stimulates synthesis of β-actin, the repellents Sema3A and Slit2 stimulate synthesis of RhoA and cofilin respectively, proteins that promote actin depolymerization8,9. An external gradient can activate signalling cascades asymmetrically to generate an internal gradient of translation activation oriented toward both attractants and repellents, while the identity of the asymmetrically synthesized proteins and, hence, the polarity of the turning response, is determined by mRNA-specific regulation. For ‘Class 1’ (Ca2+-dependent) guidance cues like netrin-1, Ca2+ is a candidate switch mechanism3, especially since laminin seems to switch netrin-induced attraction to repulsion by lowering cAMP and thereby reducing Ca2+-induced Ca2+ release26,33. Global translation initiation regulation combined with mRNA-specific regulation is consistent with our finding that enolase protein levels do not change with netrin-1 stimulation, despite both the presence of enolase mRNA and upregulation of global translation initiation10. Further studies will be needed to test this idea, especially the identity of proteins synthesized in attractive versus repulsive conditions.
mRNA-specific regulation may not entail separate RNA binding proteins. Proteomic and colocalization studies have revealed that RNP complexes consist of multiple RNA binding proteins and can transport several different mRNAs42. Consistent with this, we have shown that Vg1RBP also interacts with cofilin mRNA, which is translated in response to the chemorepellent Slit-28. It may be that in attractive conditions, Vg1RBP activates β-actin translation after being phosphorylated by Src, as occurs for the chick homology of Vg1RBP, ZBP120, while an alternative signaling pathway causes Vg1RBP to activate cofilin-1 translation but suppress β-actin translation in repulsive conditions. The exact sequence of signalling between DCC and Src remains largely unknown, but Src might in turn be activated by DCC through direct binding or via focal adhesion kinase (FAK), which interacts with DCC43.
The differential sensitivity to β-actin translation inhibition of attraction versus repulsion and collapse suggests that repulsion and attraction are not simply mirror-symmetrical processes but operate through distinct pathways. This may not be surprising because attraction involves near-side filopodial extension, while repulsion involves near-side filopodial withdrawal16. Indeed, it is thought that repulsive turning is essentially localized collapse, because guidance cues or drugs that cause collapse when added globally generally elicit repulsive turning when presented asymmetrically44. Consistent with this, the repellent Slit2 does not induce β-actin synthesis, and indeed causes a significant decrease in β-actin levels8, possibly caused by depolymerization due to Slit2-induced cofilin synthesis, combined with suppression of β-actin translation. Similarly, we observed that a gradient of netrin-1 in repulsive conditions did not induce near side β-actin synthesis, but rather caused a slight, though non-significant, decrease of β-actin on the near side. This model predicts that although inhibition of β-actin synthesis does not block repulsive turning, de-regulation of β-actin synthesis might. In agreement with this model, an independent study detects a decrease of β-actin on the near side in response to a repulsive gradient and shows that an antisense oligonucleotide directed against the ‘zipcode’ in β-actin mRNA’s 3’ UTR blocks both the asymmetrical decrease of β-actin and repulsive turning (J. Zheng, personal communication).
In summary, we provide evidence that netrin-1 induces a rapid local asymmetric increase in β-actin mRNA translation in retinal growth cones, which is necessary for the attractive turning response and might be achieved via directed transport of Vg1RBP and/or asymmetrical translation initiation. We speculate that newly synthesized β-actin could provide spatially targeted de novo nucleation sites for actin polymerization and hence direct the migration of the growth cone towards the cue (see Supplementary Fig. 3). Our data agree with those previously obtained in fibroblasts19 and suggest that similar mechanisms occur to direct migration in the two cell types. Together with the fact that numerous cytoskeletal mRNAs and cytoskeletal-regulatory protein mRNAs have been identified in axons, this suggests that the ability of growth cones to transduce external gradients into matching internal asymmetries of cytoskeletal protein synthesis might be a conserved mechanism.
Experimental procedures
Reagents
Purified netrin-145 was used at 300 ng ml-1 for bath-application, 10 μg ml-1 in turning assays, and 5 μg ml-1 in eIF4E-BP1 assays. Cycloheximide (25 μM, Sigma) and rapamycin (10 nM, Calbiochem) were bath-applied to retinal cultures immediately prior to the addition of netrin-1. Antibodies: β-actin (1:400, AC15, Abcam), enolase (1:100, H300, Santa Cruz Biotechnologies), Vg1RBP antiserum46, Ser65-phosphorylated eIF-4EBP1 and total eIF-4EBP1 (1:50 and 1:67, Cell Signaling Technology). Vg1RBP antiserum was affinity purified using recombinant Vg1RBP protein. The Zenon Rabbit IgG Labeling Kit (Molecular Probes) was used for 4EBP double staining (5:1 Zenon reagent:primary antibody ratio for phospho-4EBP, 3:1 for total-4EBP).
Morpholinos and plasmids
Antisense morpholinos (AMOs) conjugated to lissamine were designed and supplied by GeneTools: Xenopus β-actin AMO: 5’-CAATATCGTCTTCCATTGTGATCTG-3’; control: 5’-CCTCTTACCTCAGTTACAATTTATA-3’. pCS2 Kaede-3’UTR was generated by fusing CoralHue™ Kaede (MBL; Accession No. AB085641) to the 3’UTR of Xenopus β-actin (Accession No. BC041203) into pCS2+. Kaede-Δ3’UTR construct lacks the first 419 bp of the 3’UTR. We used pET21d-Vg1RBP-GFP (Accession No. AF064634)47and pSP64T-Vg1RBP-eGFP as described48. See Supplementary procedures for cloning details.
Immunoprecipitation and β-actin mRNA detection
Analysis of Vg1RBP and β-actin mRNA interaction was performed as described8. PCR was performed with the following primers: β-actin mRNA: 5’CCTGTGCAGGAAGATCACAT3’ and 5’TGTTAAAGAGAATGAGCCCC3’, S1P1 mRNA: 5’ATCGGCAACTTGGCTCTTTCGG3’ and 5’ATTCCCCCTCATCTTTCTGCGG3, GAPDH mRNA: 5’GACTCCACCCACGGCCGC3’ and 5’CCATTGAAGTCAGTGGAG3’.
In situ hybridization
Probes were generated of a 446 bp fragment (1172-1618bp, Accession No. BC041203) comprising the 3’UTR of β-actin mRNA, which was amplified by PCR using primers 5’AAAGGATCC AAGGACAGACCCTTTCAACATG3’ and 5’AAAGAATTCGTGAAACAA CATAAGTTTTATTTTTTC3’ and cloned into pBluescript. For fluorescence in situ hybridization, retinal cultures from stage 33/34 embryos were fixed in 4% PFA, 7.5% sucrose in PBS for 1 hour at RT. Hybridization was performed using 0.6 ng/μl riboprobes and subsequent detection of β-actin mRNA and Vg1RBP (1:300)12,49.
Quantitation of fluorescence intensity
Immunostaining, image-capture and quantitation of fluorescence intensity were performed as described previously8. Statistical analyses were performed using Graphpad InStat3.
Growth cone turning and gradient assay
Gradients of diffusible netrin-1 protein and Sema3A supernatant (or control) were established as described previously10,30. In 5-minute gradient stimulation experiments, a source of netrin-1 was place at 90° to the direction of the axon shaft. Images were taken every 30 seconds for 5 minutes. Samples were rapidly fixed and analyzed immediately (in case of Vg1RBP-eGFP), stained using β-actin antibodies to detect β-actin protein levels, or double-stained using phospho-4EBP and total-4EBP antibodies to detect 4EBP phosphorylation levels. For the 4EBP experiments, the pipette was placed 67 μm from the growth cone. For asymmetry experiments, the growth cone was bissected into two equal areas by a line drawn through the axon shaft (90° to the pipette) by an experimenter ‘blind’ to the direction of the gradient. 4EBP phosphorylation on each side of the growth cone was calculated as phospho-4EBP intensity divided by total-4EBP intensity. The center of mass was analyzed using ImageJ software (NIH). The background fluorescence level was subtracted from all pixels of the growth cone. The centers of mass of phospho-4EBP and total-4EBP fluorescence within the growth cone were calculated as the average of all pixel locations weighted by intensity. Filopodia length was measured using Openlab software (Improvision). Growth cones exhibiting less than 2 filopodia on each side were excluded from the analysis, as were filopodia shorter than 1 μm.
Live cell imaging
Fluorescence recovery
Stage 24 Kaede-β-actin 3’UTR injected retinal primordia were cultured for 24 hours and imaged for 30 minutes. Kaede-positive growth cones were selected and the whole neurite including the axon shaft was photoconverted by exposure to 340-380 nm irradiation. Images were taken every 2.5 minutes and the intensities of the signals were quantified in each frame and averaged per 5 minutes. In some cases, axons were severed from their cell bodies prior to the photoconversion. Fluorescent intensity was normalized to the first frame after photoconversion and presented against relative time (min).
Vg1RBP-eGFP. Stage 33/34 Vg1RBP-eGFP injected retinal primordial were cultured for 24 hours. Images of Vg1RBP-eGFP positive growth cones viewed at 100X with a Nikon inverted microscope equipped with a cooled CCD camera were captured every 12 seconds under reduced fluorescence intensities to avoid bleaching. To measure granule speed, images were captured every second. Openlab and Volocity software (Improvision) was used to calculate the number and speed of granules.
Supplementary Material
Movement of Vg1RBP-eGFP granules in retinal growth cones. Retinal explants taken from stage 33/34 embryos injected with Vg1RBP-eGFP mRNA were cultured for 24 hours and analyzed in real time. Images were taken every 12 seconds. Vg1RBP-eGFP granules show bidirectional movement in the axon shaft, growth cone central domain and filopodia.
The effect of cytochalasin D on Vg1RBP-eGFP granules movement. Live imaging of Vg1RBP-eGFP positive growth cones. Bath application of cytochalasin D (0.1 μM) results in retraction of Vg1RBP-eGFP granules from the growth cone. Images were taken every 12 seconds.
Movement of Vg1RBP-eGFP granules into filopodial contact sites. Live imaging of Vg1RBP-eGFP positive growth cones. The arrows indicate anterograde transport of a Vg1RBP-eGFP granule moving to the contact site, followed by a pause and retrograde movement to the base of the filopodium. Images were taken every 12 seconds.
Netrin-1 induces transport of Vg1RBP-eGFP granules into filopodia. Vg1RBP-eGFP expressing retinal growth cones were stimulated with netrin-1 after 5 minutes and followed in real time. Images were taken every 12 seconds for 15 minutes. The arrows indicate filopodia in which the increased transport of Vg1RBP-eGFP granules is very prominent.
Acknowledgements
We thank M. Spira for suggesting use of Kaede-β-actin 3’UTR, R. Adams for suggesting use of the Zenon labelling kit and J. Ireland and B. Kvinlaug for preliminary data on β-actin translation. We also thank J. Zheng, D. Campbell and L. Strochlic for sharing unpublished data, A. Dwivedy, I. Pradel and K. Zivraj for technical assistance and L. Poggi and J. Falk for help with image analysis. We thank W. Harris, M. Piper and D. Campbell for comments on the manuscript. This work was supported by a Croucher Scholarship (KL), an EMBO Long Term Fellowship (FvH), an NSF Graduate Research Fellowship (ACL), a BBSRC Studentship (RA) and a Wellcome Trust Programme Grant (CEH). Author contributions: K-M.L did the experiments on β-actin synthesis and function in Fig. 3, 6, 7 and Supplementary Fig. 2. F.v.H did the experiments on Vg1RBP in Fig. 1, 2, 4, Supplementary Fig. 1, 2k-o and movies 1-4. A.L did the gradient assays on 4EBP and β-actin in Fig. 5, 6e. R.A and N.S provided Vg1RBP reagents and constructs, and discussed experiments. K-M.L, F.v.H, A.L and C.H wrote the manuscript and discussed experiments.
References
- 1.Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–30. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 3.Wen Z, Guirland C, Ming GL, Zheng JQ. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron. 2004;43:835–46. doi: 10.1016/j.neuron.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–8. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 6.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–61. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming GL, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–8. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 8.Piper M, et al. Signaling mechanisms underlying slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–28. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu KY, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–4. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–26. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 11.Shestakova EA, Singer RH, Condeelis J. The physiological significance of beta-actin mRNA localization in determining cell polarity and directional motility. Proc Natl Acad Sci U S A. 2001;98:7045–50. doi: 10.1073/pnas.121146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassell GJ, et al. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–65. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis D, et al. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–91. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HL, et al. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–75. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HL, Singer RH, Bassell GJ. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan XB, et al. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- 17.Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–35. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloc M, Zearfoss NR, Etkin LD. Mechanisms of subcellular mRNA localization. Cell. 2002;108:533–44. doi: 10.1016/s0092-8674(02)00651-7. [DOI] [PubMed] [Google Scholar]
- 19.Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell. 2005;97:97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- 20.Huttelmaier S, et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–5. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 21.Yisraeli JK. VICKZ proteins: a multi-talented family of regulatory RNA-binding proteins. Biol Cell. 2005;97:87–96. doi: 10.1042/BC20040151. [DOI] [PubMed] [Google Scholar]
- 22.Sundell CL, Singer RH. Requirement of microfilaments in sorting of actin messenger RNA. Science. 1991;253:1275–7. doi: 10.1126/science.1891715. [DOI] [PubMed] [Google Scholar]
- 23.Lopez de Heredia M, Jansen RP. mRNA localization and the cytoskeleton. Curr Opin Cell Biol. 2004;16:80–5. doi: 10.1016/j.ceb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Deiner MS, et al. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–89. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DS, et al. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21:8538–47. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 27.Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276:42818–25. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- 28.Wilkie GS, Dickson KS, Gray NK. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem Sci. 2003;28:182–8. doi: 10.1016/S0968-0004(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 29.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–6. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Torre JR, et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–24. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 31.Gingras AC, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eng H, Lund K, Campenot RB. Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J Neurosci. 1999;19:1–9. doi: 10.1523/JNEUROSCI.19-01-00001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooashi N, Futatsugi A, Yoshihara F, Mikoshiba K, Kamiguchi H. Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. The Journal of Cell Biology. 2005;170:1159. doi: 10.1083/jcb.200503157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J Neurosci. 1999;19:1965–75. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen WJ, Cowan NJ, Welch WJ. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol. 1999;145:265–77. doi: 10.1083/jcb.145.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, et al. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–6. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 37.Kislauskis EH, Zhu X, Singer RH. beta-Actin messenger RNA localization and protein synthesis augment cell motility. J Cell Biol. 1997;136:1263–70. doi: 10.1083/jcb.136.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis AK, Bridgman PC. Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. The Journal of Cell Biology. 1992;119:1219. doi: 10.1083/jcb.119.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oleynikov Y, Singer RH. Real-time visualization of ZBP1 association with beta-actin mRNA during transcription and localization. Curr Biol. 2003;13:199–207. doi: 10.1016/s0960-9822(03)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci U S A. 1996;93:1065–70. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunet I, et al. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–8. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–25. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–32. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan J, Raper JA. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 45.Shirasaki R, Mirzayan C, Tessier-Lavigne M, Murakami F. Guidance of circumferentially growing axons by netrin-dependent and -independent floor plate chemotropism in the vertebrate brain. Neuron. 1996;17:1079–88. doi: 10.1016/s0896-6273(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, et al. Vg1 RBP intracellular distribution and evolutionarily conserved expression at multiple stages during development. Mech Dev. 1999;88:101–6. doi: 10.1016/s0925-4773(99)00162-8. [DOI] [PubMed] [Google Scholar]
- 47.Yaniv K, Fainsod A, Kalcheim C, Yisraeli JK. The RNA-binding protein Vg1 RBP is required for cell migration during early neural development. Development. 2003;130:5649–61. doi: 10.1242/dev.00810. [DOI] [PubMed] [Google Scholar]
- 48.Chang P, et al. Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum. Mol Biol Cell. 2004;15:4669–81. doi: 10.1091/mbc.E04-03-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blichenberg A, et al. Identification of a cis-Acting Dendritic Targeting Element in MAP2 mRNAs. Journal of Neuroscience. 1999;19:8818. doi: 10.1523/JNEUROSCI.19-20-08818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vignali R, Poggi L, Madeddu F, Barsacchi G. HNF1(beta) is required for mesoderm induction in the Xenopus embryo. Development. 2000;127:1455–65. doi: 10.1242/dev.127.7.1455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movement of Vg1RBP-eGFP granules in retinal growth cones. Retinal explants taken from stage 33/34 embryos injected with Vg1RBP-eGFP mRNA were cultured for 24 hours and analyzed in real time. Images were taken every 12 seconds. Vg1RBP-eGFP granules show bidirectional movement in the axon shaft, growth cone central domain and filopodia.
The effect of cytochalasin D on Vg1RBP-eGFP granules movement. Live imaging of Vg1RBP-eGFP positive growth cones. Bath application of cytochalasin D (0.1 μM) results in retraction of Vg1RBP-eGFP granules from the growth cone. Images were taken every 12 seconds.
Movement of Vg1RBP-eGFP granules into filopodial contact sites. Live imaging of Vg1RBP-eGFP positive growth cones. The arrows indicate anterograde transport of a Vg1RBP-eGFP granule moving to the contact site, followed by a pause and retrograde movement to the base of the filopodium. Images were taken every 12 seconds.
Netrin-1 induces transport of Vg1RBP-eGFP granules into filopodia. Vg1RBP-eGFP expressing retinal growth cones were stimulated with netrin-1 after 5 minutes and followed in real time. Images were taken every 12 seconds for 15 minutes. The arrows indicate filopodia in which the increased transport of Vg1RBP-eGFP granules is very prominent.