Abstract
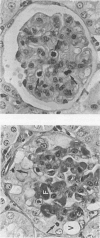
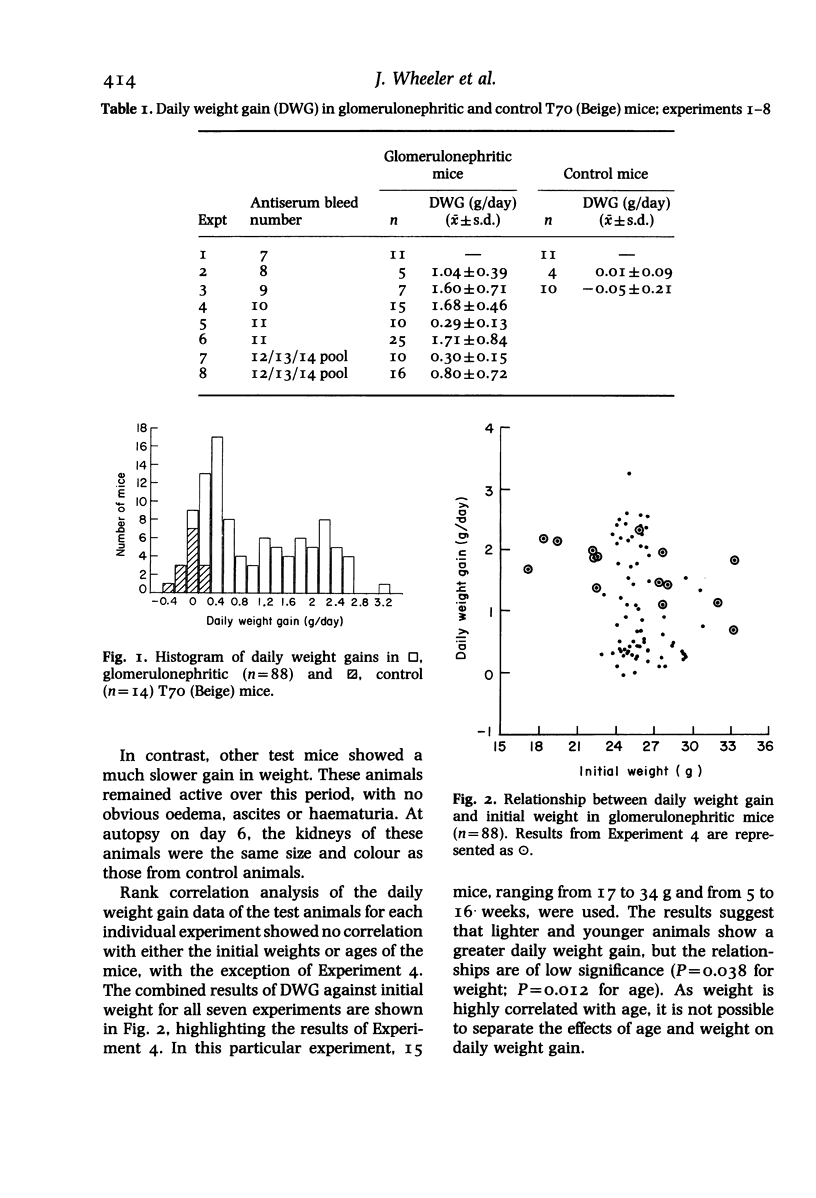
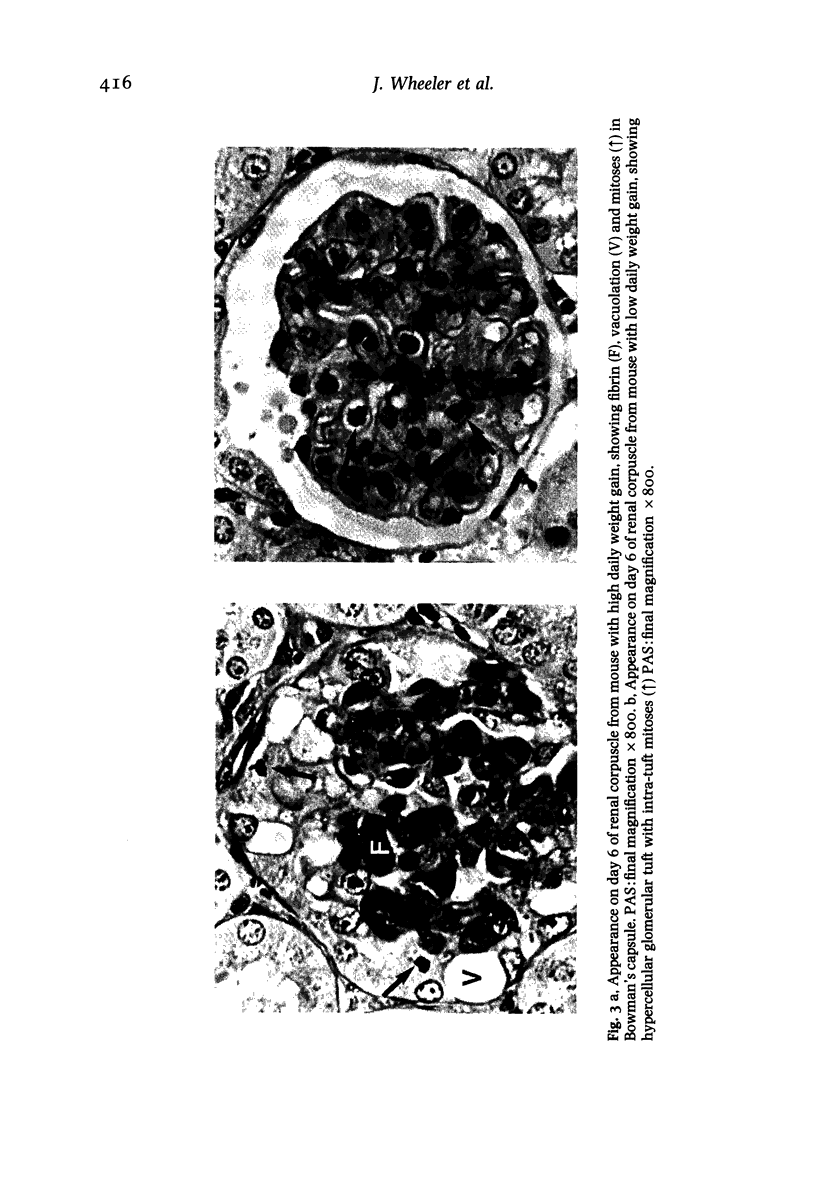
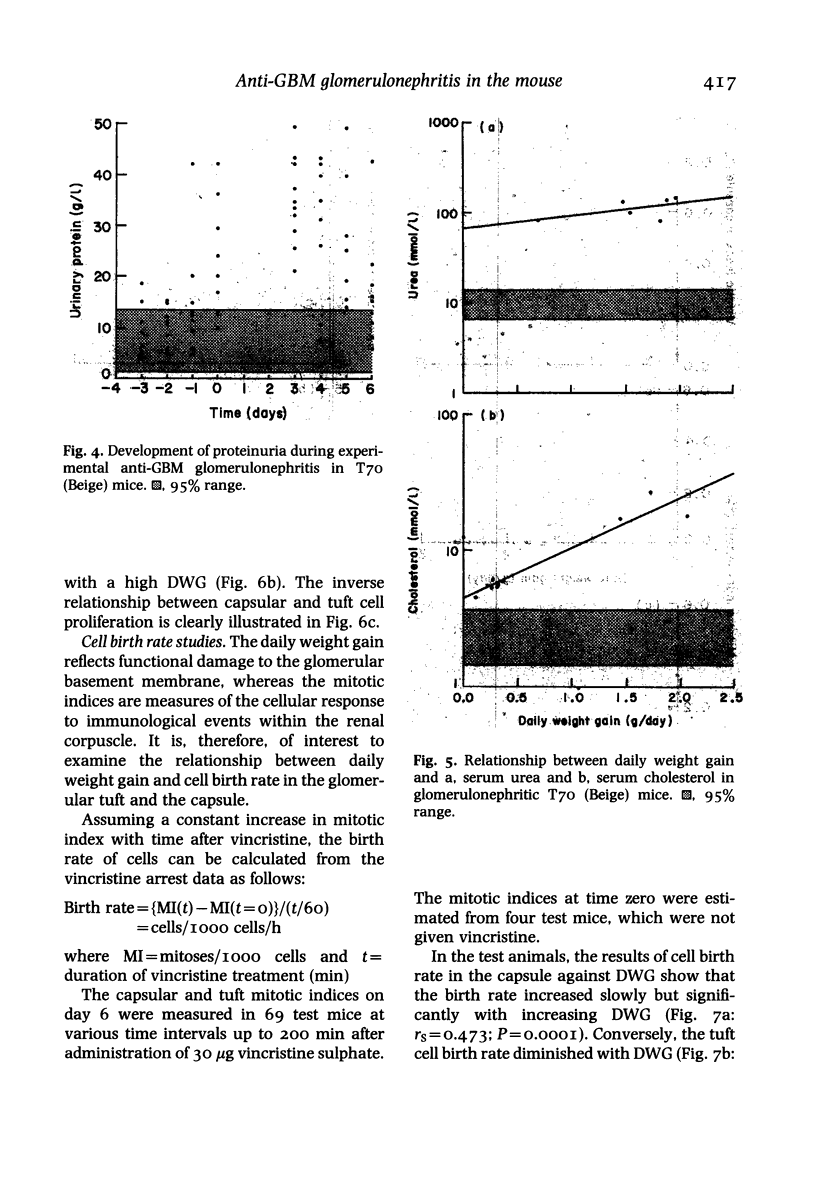
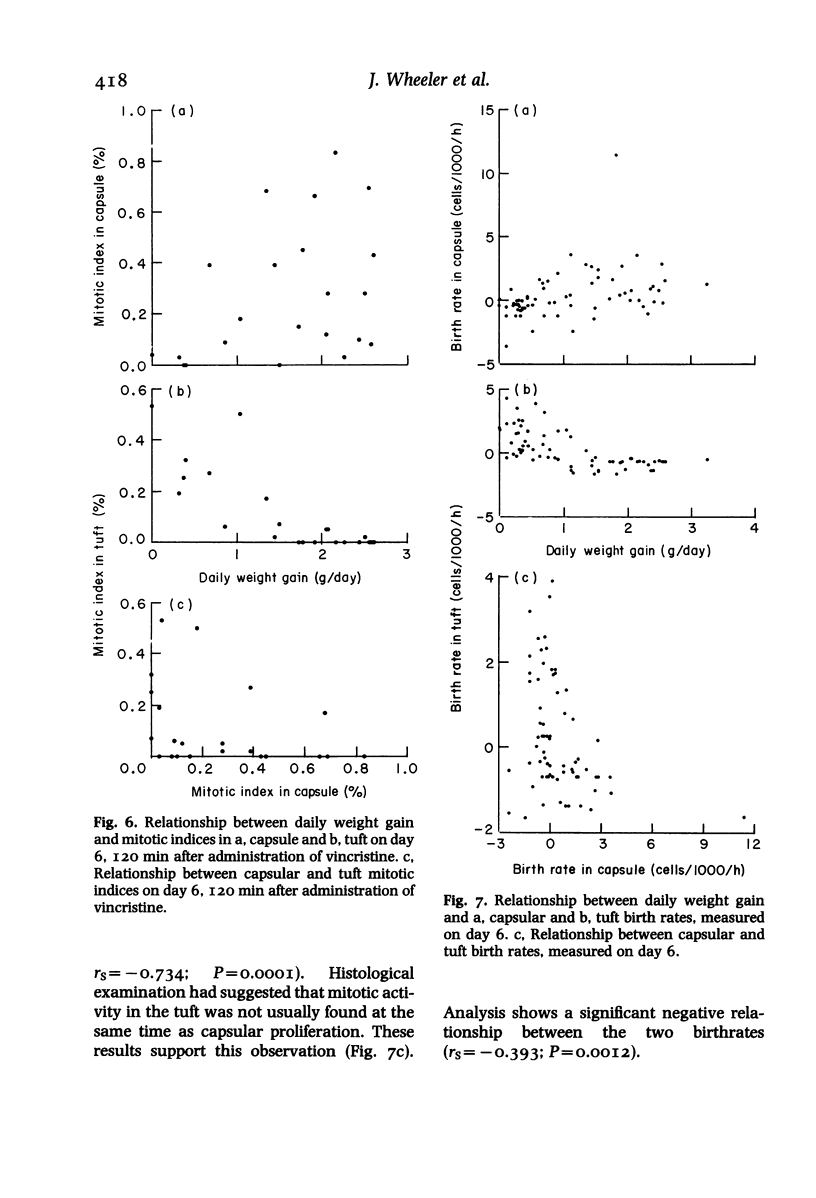
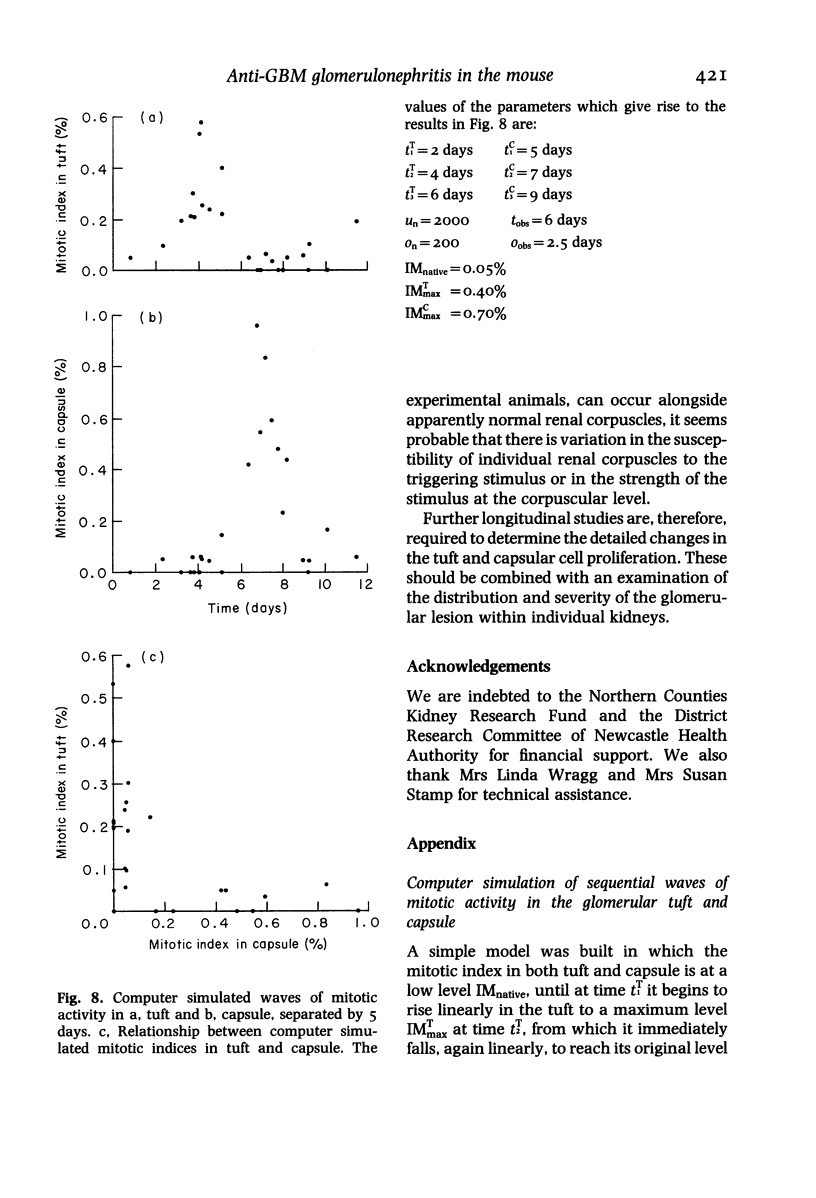
In a mouse model of anti-glomerular basement membrane (GBM) glomerulonephritis, associated with the nephrotic syndrome, a wide range of morphological and proliferative responses was seen in the renal corpuscle, at 6 days. The severity of the damage could be assessed by measuring the average daily weight gain between days 0 and 3 (DWG) of the animal. Those animals with a high DWG showed capsular proliferation, whereas animals with a low DWG showed predominantly tuft cell proliferation. Capsular cell birth rate increased with DWG whilst tuft cell birth rate was negatively related. A computer simulation suggests that the results are compatible with the induction of successive but overlapping waves of tuft and capsular cell proliferation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cattell V., Jamieson S. W. The origin of glomerular crescents in experimental nephrotoxic serum nephritis in the rabbit. Lab Invest. 1978 Dec;39(6):584–590. [PubMed] [Google Scholar]
- Foellmer H. G., Sterzel R. B., Kashgarian M. Progressive glomerular sclerosis in experimental antiglomerular basement membrane glomerulonephritis. Am J Kidney Dis. 1986 Jan;7(1):5–11. doi: 10.1016/s0272-6386(86)80050-6. [DOI] [PubMed] [Google Scholar]
- Hamada Y. [Sex difference and fine structure on epithelium cells of Bowman's capsule in mice (author's transl)]. Jikken Dobutsu. 1979 Oct;28(4):485–490. doi: 10.1538/expanim1978.28.4_485. [DOI] [PubMed] [Google Scholar]
- LITVAK R. M., BASERGA R. AN AUTORADIOGRAPHIC STUDY OF THE UPTAKE OF 3H-THYMIDINE BY KIDNEY CELLS OF MICE AT DIFFERENT AGES. Exp Cell Res. 1964 Feb;33:540–552. doi: 10.1016/0014-4827(64)90019-9. [DOI] [PubMed] [Google Scholar]
- Morley A. R., Wheeler J. Cell proliferation within Bowman's capsule in mice. J Pathol. 1985 Apr;145(4):315–327. doi: 10.1002/path.1711450405. [DOI] [PubMed] [Google Scholar]
- Nagai H., Yamada H., Nishigaki T., Nakazawa M., Koda A. The susceptibility of experimental glomerulonephritis in six different strains of mice. J Pharmacobiodyn. 1985 Jul;8(7):586–589. doi: 10.1248/bpb1978.8.586. [DOI] [PubMed] [Google Scholar]
- Pabst R., Sterzel R. B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983 Nov;24(5):626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- Sawtell N. M., Weiss M. A., Pesce A. J., Michael J. G. An immune complex glomerulopathy associated with glomerular capillary thrombosis in the laboratory mouse. A highly reproducible accelerated model utilizing cationized antigen. Lab Invest. 1987 Mar;56(3):256–263. [PubMed] [Google Scholar]
- Silva F. G., Hoyer J. R., Pirani C. L. Sequential studies of glomerular crescent formation in rats with antiglomerular basement membrane-induced glomerulonephritis and the role of coagulation factors. Lab Invest. 1984 Oct;51(4):404–415. [PubMed] [Google Scholar]
- Sterzel R. B., Pabst R. The temporal relationship between glomerular cell proliferation and monocyte infiltration in experimental glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):337–350. doi: 10.1007/BF02892829. [DOI] [PubMed] [Google Scholar]