Abstract
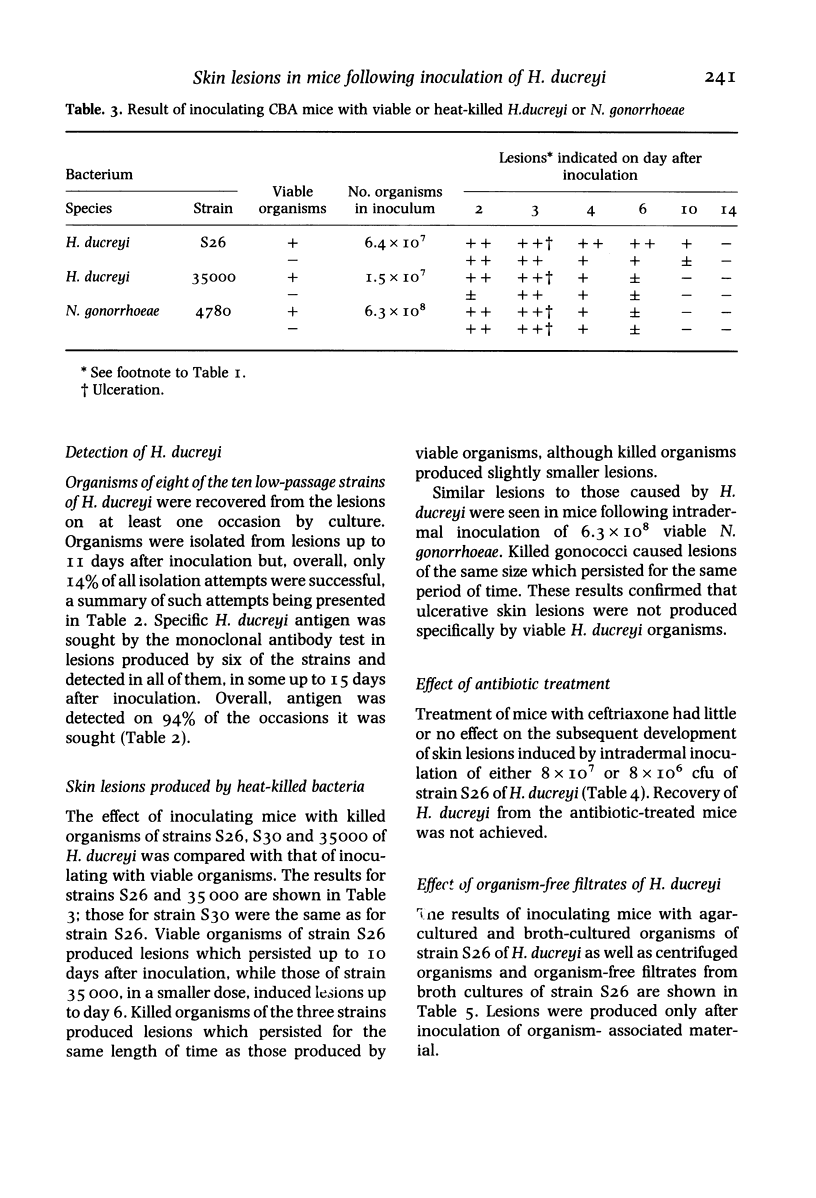
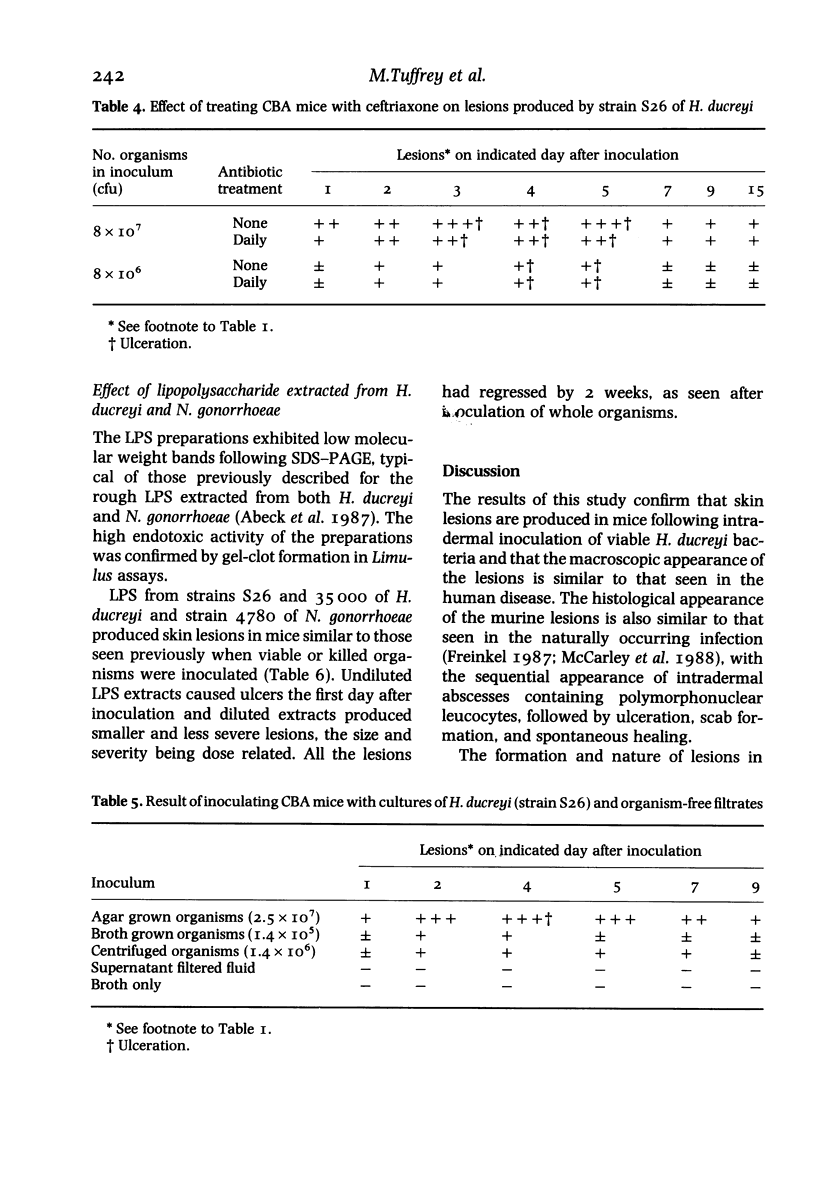
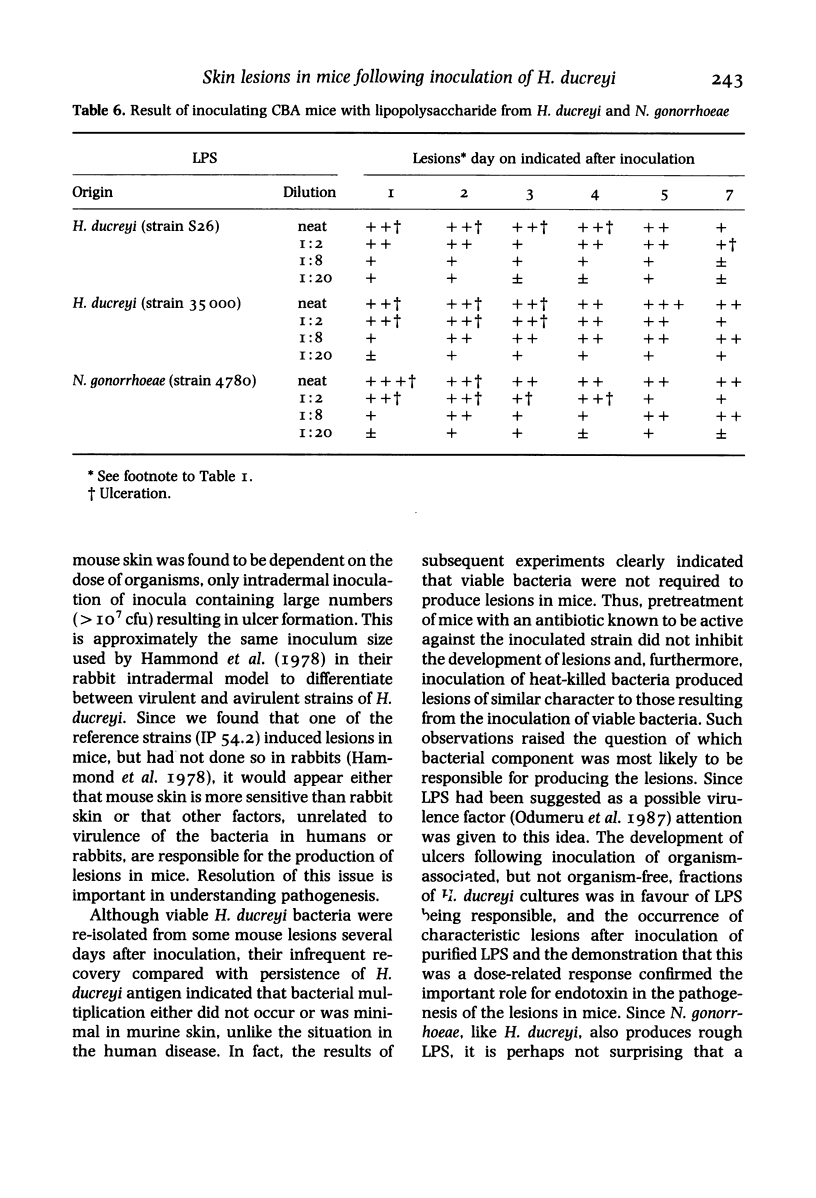
Twelve strains of H. ducreyi, which included two reference strains, were each inoculated intradermally into the flanks of CBA mice. All strains produced self-limited lesions which were macroscopically and microscopically typical of those seen in chancroid. Pustular nodules, about 5mm in diameter, developed at all inoculation sites by the second day when 10(7) organisms were inoculated. Approximately half of these lesions ulcerated and all had regressed by 2 weeks. Smaller nodules developed at about half the sites from the second to the fifth day when 10(6) or 10(5) organisms were given, but these did not ulcerate. No lesions were seen when 10(3) organisms were inoculated. Organisms were recovered from the lesions up to 11 days after inoculation. Specific H. ducreyi antigen, sought by a monoclonal antibody test, was detected in lesions up to 15 days following inoculation. Heat-killed organisms of H. ducreyi also produced nodules and ulcers although these were slightly smaller than those which developed after inoculation of viable bacteria. Similar lesions to those caused by H. ducreyi were produced after intradermal inoculation of about 10(8) viable or killed Neisseria gonorrhoeae organisms. Treatment of mice with ceftriaxone had little or no effect on the subsequent development of H. ducreyi-induced lesions. These findings indicated that the lesions were not produced specifically by viable H. ducreyi organisms. Ulcers were also produced following intradermal inoculation of purified lipopolysaccharide (LPS) from H. ducreyi or N. gonorrhoeae, but not by cell-free filtrates prepared from H. ducreyi cultures indicating a possible role for LPS in the pathogenesis of ulcerative skin lesions.
Full text
PDF

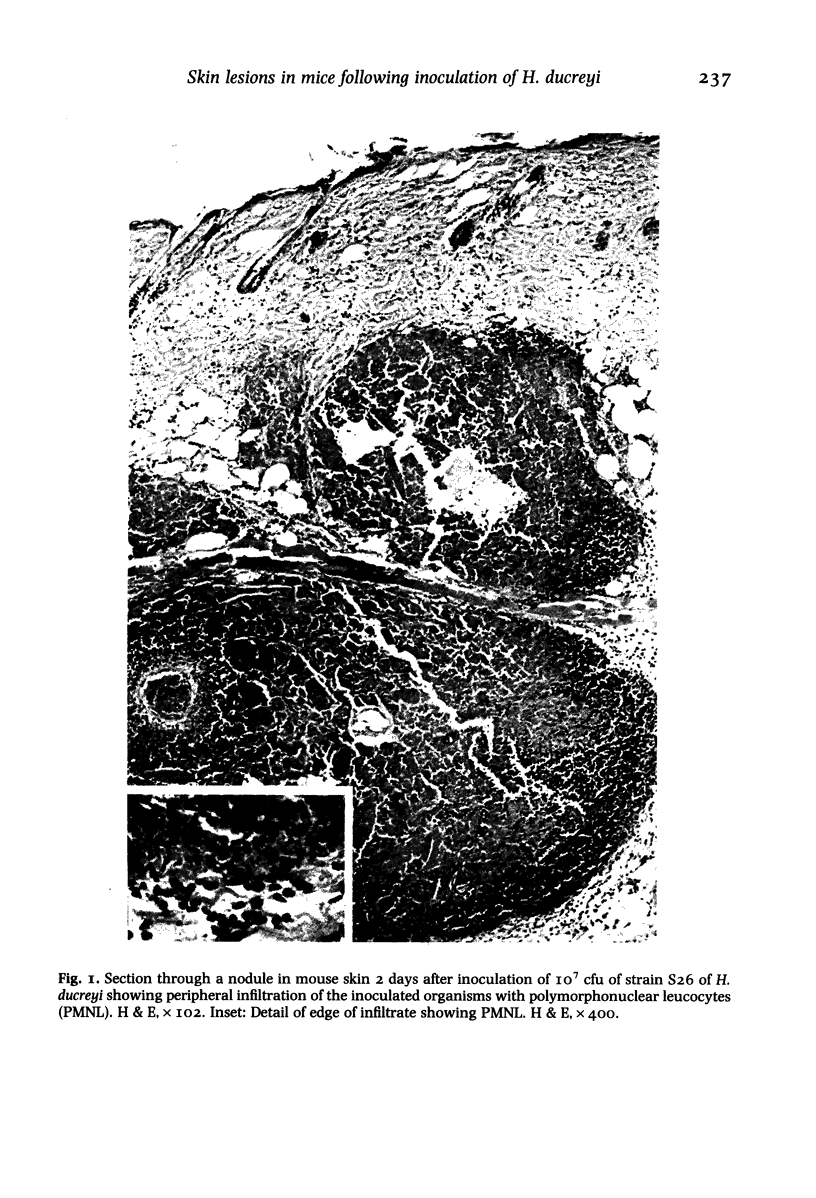
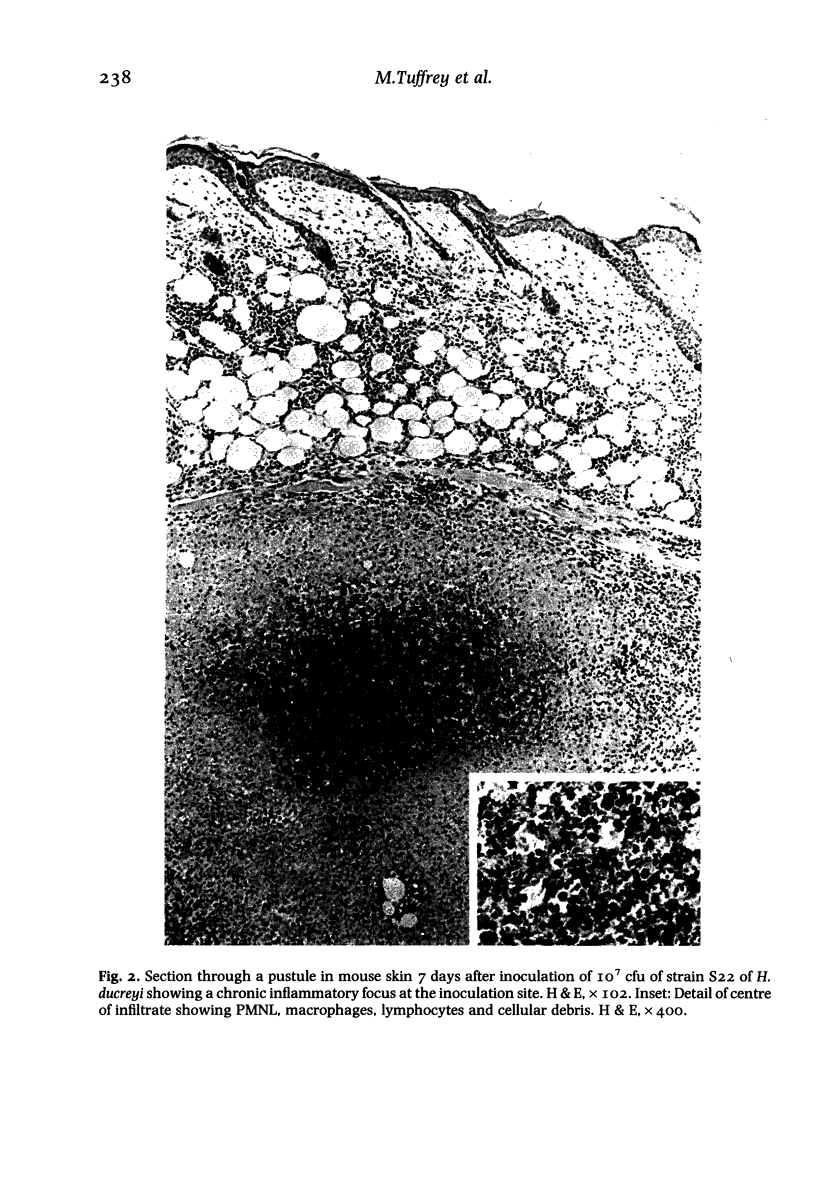

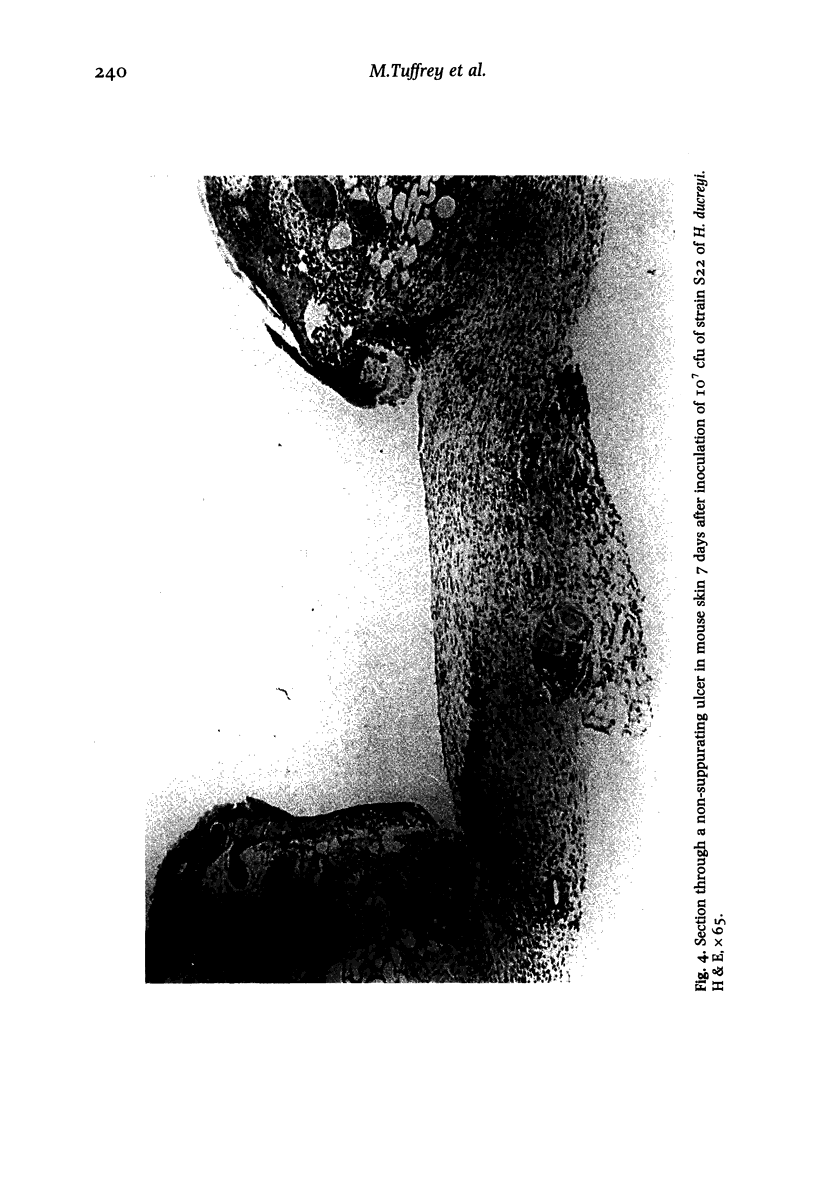

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Dangor Y., Ballard R. C. Antibiotic susceptibilities and plasmid profiles of Haemophilus ducreyi isolates from southern Africa. J Antimicrob Chemother. 1988 Oct;22(4):437–444. doi: 10.1093/jac/22.4.437. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- McCarley M. E., Cruz P. D., Jr, Sontheimer R. D. Chancroid: clinical variants and other findings from an epidemic in Dallas County, 1986-1987. J Am Acad Dermatol. 1988 Aug;19(2 Pt 1):330–337. doi: 10.1016/s0190-9622(88)70180-2. [DOI] [PubMed] [Google Scholar]
- Neubert U., Korting H. C., Luderschmidt C., Braun-Falco O. Preputial abscesses caused by beta-lactamase-producing gonococci. Cutis. 1985 Aug;36(2):161–163. [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Relationship between lipopolysaccharide composition and virulence of Haemophilus ducreyi. J Med Microbiol. 1987 Mar;23(2):155–162. doi: 10.1099/00222615-23-2-155. [DOI] [PubMed] [Google Scholar]
- Rajan V. S., Sng E. H., Lim A. L. The isolation of H. ducreyi in Singapore. Ann Acad Med Singapore. 1983 Jan;12(1):57–60. [PubMed] [Google Scholar]