Abstract
Production of mature erythrocytes requires multiple growth factors, but we do not know how their actions are coordinated. Here we show that erythroid progenitors from erythropoietin receptor (Epo-R)−/− fetal livers, infected in vitro with a retrovirus expressing the wild-type Epo-R, require addition of both Epo and stem cell factor (SCF) to form colony-forming unit erythroid (CFU-E) colonies. Thus, a functional interaction between KIT and the Epo-R, similar to what we reported in cultured cells, is essential for the function of CFU-E progenitors. In contrast, CFU-E colony formation in vitro by normal fetal liver progenitors requires only Epo; the essential interaction between activated KIT and the Epo-R must have occurred in vivo before or at the CFU-E progenitor stage. Using truncated dominant-negative mutant Epo-Rs, we show that KIT does not activate the Epo-R by inducing its dimerization, but presumably does so by phosphorylating tyrosine residue(s) in its cytosolic domain. By expressing mutant Epo-Rs containing only one of eight cytosolic tyrosines, we show that either tyrosine residue Y464 or Y479 suffices for Epo-dependent cell proliferation. However, only Epo-R F7Y479 is capable of supporting erythroid colony formation when expressed in Epo-R−/− fetal liver cells, indicating that Y464 either cannot send a differentiation signal or fails to respond to SCF/KIT activation. This work employs a novel experimental system to study the function of growth factors and their receptors in normal hematopoiesis.
Keywords: erythropoietin receptor/KIT, signal transduction/erythropoiesis
Hematopoietic progenitors require multiple growth factors for their optimal development and terminal differentiation. Previous studies suggested that stem cell factor (SCF), interleukin 3 (IL-3), and granulocyte-macrophage colony-stimulating factor (GM-CSF) are important for the generation and proliferation of erythroid progenitors. However, analysis of mice with null mutations in either GM-CSF (1, 2) or IL-3 receptor (3) genes indicated that GM-CSF and IL-3 are not crucial for erythropoiesis, or that other factors can compensate for their function. Mice deficient in either SCF or its receptor (KIT), however, suffer from severe anemia (4). Even though normal numbers of early erythroid progenitors [burst-forming-unit erythroids (BFU-Es)] are present in the fetal liver, the numbers of late progenitors [colony-forming-unit erythroids (CFU-Es)] are significantly reduced (5). Mice deficient in erythropoietin (Epo; ref. 6) or its receptor (Epo-R) (6–8) accumulate fetal liver CFU-Es that cannot differentiate and eventually undergo apoptosis. Thus SCF/KIT and Epo/Epo-R function as key switches for erythropoiesis: SCF and KIT are essential for the proliferation and differentiation of BFU-Es to CFU-Es, and Epo and the Epo-R are crucial for survival of CFU-Es and for their proliferation and irreversible terminal differentiation.
Using cultured erythroleukemia cells, we showed previously that activation of the KIT protein tyrosine kinase induces tyrosine phosphorylation of the Epo-R, and that KIT interacts with the Epo-R by physically associating with its cytoplasmic domain. Further, the ability of SCF to support proliferation of 32D cells expressing KIT requires coexpression of the Epo-R, demonstrating that at least one proliferative signal generated by KIT involves the Epo-R as a downstream signal-transduction protein (9).
Here we show that a functional interaction of activated KIT with the Epo-R at or around the CFU-E stage is crucial for normal erythroid differentiation. Epo activates the Epo-R by inducing its dimerization. However, we show that KIT activates the Epo-R probably through phosphorylation of tyrosine residue(s) in its cytosolic domain, rather than induction of Epo-R dimerization.
MATERIALS AND METHODS
Erythroid Colony Analysis.
Fetal livers were harvested from day-12.5 wild-type (wt) or Epo-R−/− embryos. Single cell suspensions were prepared and incubated with or without retrovirus expressing the wt or mutant Epo-R cDNAs as detailed in ref. 6. After infection, cells were plated in triplicate in α-methylcellulose (Stem Cell Technologies) without growth factor (−), or supplemented with Epo (3 μ/ml), or SCF (100 ng/ml), or Epo plus SCF (3 μ/ml Epo and 100 ng/ml SCF). Benzidine-positive colonies were counted 2–3 days after plating.
Cell Proliferation Assay.
32D cells coexpressing the wt EpoR and c-KIT (32D-EpoR/KIT cells), or coexpressing the wt EpoR and c-KIT and also a truncated mutant EpoR(1-257) (32D-EpoR/EpoR(1-257)/KIT cells) were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10% WEHI conditioned medium as a source of IL-3. Prior to proliferation assays, the cells were washed three times in RPMI 1640 medium supplemented with 2% FCS and plated at 1 × 104 cells/ml in RPMI 1640 medium containing 10% FCS and 10 μ/ml Epo or the concentrations of cytokines indicated in Fig. 2. After 3 days in culture, viable cells were counted using a Coulter counter and expressed as a percentage of the number of cells in a parallel culture in medium supplemented with 10% WEHI conditioned medium.
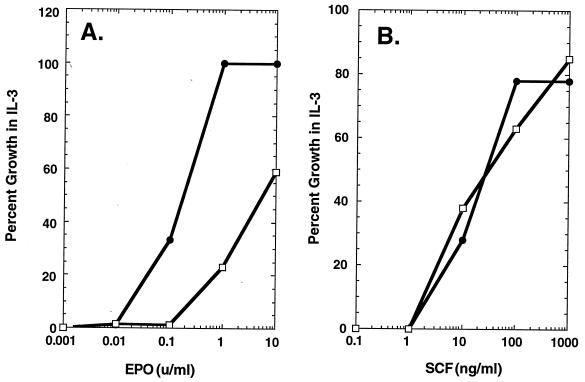
Figure 2.
Epo- and SCF-dependent cell proliferation. Epo- (A) and SCF-dependent (B) proliferation of 32D cells coexpressing the wt Epo-R and c-kit (32D-Epo-R/KIT cells, •), or coexpressing the wt Epo-R and c-kit as well as a truncated mutant Epo-R(1-257) (32D-Epo-R/Epo-R(1-257)/KIT cells, □).
RESULTS
Functional Interaction Between KIT and the Epo-R Is Essential for the Formation of CFU-Es in Vitro.
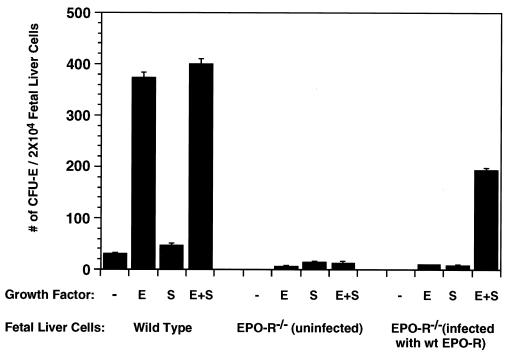
Fetal livers were harvested from day-12.5 Epo-R+/+, Epo-R+/−, or Epo-R−/− embryos. As judged by Northern blot analysis, Epo-R−/− fetal liver cells express approximately the same amount of KIT as do those from their Epo-R+/+ and Epo-R+/− littermates and, as expected, no Epo-R transcript could be detected in the Epo-R−/− fetal livers (data not shown). Fig. 1 shows that formation of CFU-E colonies from Epo-R+/+ fetal liver cells requires only Epo (Fig. 1 Left). No increase in the size or number of CFU-Es occurred when SCF was added to the culture, suggesting that Epo is the only growth factor needed for the proliferation and terminal differentiation of wt CFU-E progenitors. CFU-E colonies form from cultured Epo-R−/− fetal liver cells only if they were infected in vitro with a retrovirus expressing the wt Epo-R. Importantly, Fig. 1 (Right) shows that formation of CFU-Es from these cells requires addition of both Epo and SCF to the culture medium. The morphology of these colonies is normal, as is the extent of hemoglobinization, as monitored by benzidine staining (data not shown). We conclude that a functional interaction between the KIT and Epo-Rs at or just before the CFU-E stage is essential for these cells to respond to Epo and undergo subsequent cell proliferation followed by terminal differentiation. Thus, the physical and functional interaction of KIT and the Epo-R originally detected in cultured hematopoietic cells is required for normal erythroid differentiation. Because CFU-E colony formation in vitro by normal fetal liver progenitors requires only Epo, the essential interaction between activated KIT and the Epo-R must have occurred in vivo before the cells were placed into culture.
Figure 1.
Formation of erythroid colonies by wt and Epo-R−/− fetal liver cells. (Left) CFU-E colonies formed by wt fetal liver cells. (Center) CFU-E colonies formed by uninfected Epo-R−/− fetal liver cells. (Right) CFU-E colonies formed by Epo-R−/− fetal liver cells following infection with a retrovirus expressing the wt Epo-R cDNA. The average numbers of CFU-E colonies from three independent assays were expressed per 2 × 104 nucleated fetal liver cells.
KIT Does Not Activate Epo-R by Inducing Its Dimerization.
How does KIT activate the Epo-R in CFU-E progenitor cells? While Epo activates the Epo-R by inducing its dimerization (10–12), the experiment in Fig. 2 indicates that KIT does not signal by inducing dimerization of the Epo-R extracellular domain, but presumably does so by inducing (directly or indirectly) tyrosine phosphorylation of the Epo-R. When coexpressed in 32D cells with the wt Epo-R, Epo-R mutant 1-257—truncated at the beginning of its cytosolic domain and lacking the necessary sequences for KIT association (9)—forms inactive heterodimers with the wt Epo-R, resulting in inhibition of Epo- but not IL-3-mediated cell proliferation (11). Fig. 2A shows that, in 32D-Epo-R/KIT cells, co-expression of Epo-R(1-257) also has a dominant-negative effect on Epo signaling through the wt Epo-R; cell proliferation requires a concentration of Epo that is 10-fold higher than for parental 32D-Epo-R/KIT cells. In contrast, coexpression of Epo-R(1-257) in 32D-Epo-R/KIT cells has no effect on the ability of SCF to signal proliferation through the wt Epo-R. Were KIT to activate the Epo-R by inducing its dimerization, inhibition of SCF/KIT signaling by Epo-R(1-257) would be expected. These results suggest that SCF/KIT activates the Epo-R by phosphorylating its cytosolic domain rather than by inducing its dimerization, and that the Epo-R is a downstream signal-transduction protein for KIT.
Tyrosine Residues in the Epo-R Cytoplasmic Domain Are Essential for Epo-Dependent Cell Proliferation.
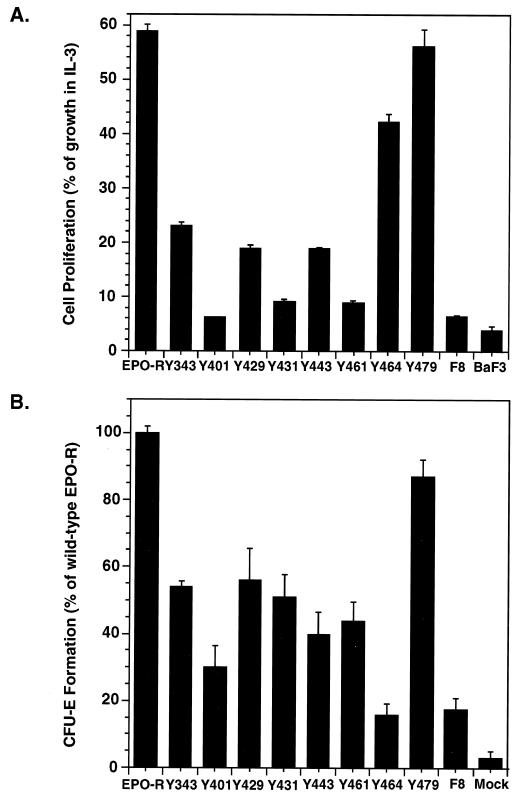
Homodimerization of the Epo-R in response to Epo binding transiently activates the receptor-associated protein tyrosine kinase JAK2 (13, 14). Subsequent tyrosine phosphorylation of the Epo-R creates “docking sites” for SH2 domain(s) in signaling molecules, such as the protein tyrosine phosphatases SH-PTP-1(15, 16) and SH-PTP-2 (17), PI3 kinase (18, 19), and STAT5 (20–22). To test the role of individual tyrosine residues in Epo-mediated cell proliferation, we generated a panel of Epo-R mutants that have none (F8) or only one of eight possible cytosolic tyrosine residues (F7Yxxx; ref. 21). Pools of transfected BaF3 cells expressing comparable numbers of cell surface receptors (data not shown) were utilized for cell proliferation assays. An Epo-R (F8) lacking all eight tyrosines in its cytosolic domain cannot support Epo-mediated proliferation of cultured BaF3 cells (compare F8 and BaF3 in Fig. 3A), suggesting that tyrosines in the Epo-R cytoplasmic domain are important for sending proliferative signals. In contrast, two of the Epo-R mutants containing only one cytosolic tyrosine residue, F7Y464 and F7Y479, support an almost normal level of Epo-mediated cell proliferation of BaF3 cells. Thus, signals generated by either Y464 and Y479 are sufficient for Epo-dependent cell proliferation. Epo-R mutants F7Y343, F7Y429, and F7443 also support significant Epo-mediated proliferation of BaF3 cells.
Figure 3.
Tyrosines in the Epo-R cytoplasmic domain are important for both proliferation and differentiation of erythroid progenitors. (A) Epo-dependent proliferation of parental BaF3 cells and BaF3 cells expressing the wt Epo-R (Epo-R) or mutant Epo-Rs containing no cytosolic tyrosines (F8) or a single cytosolic tyrosine (Yxxx) (21). The average cell numbers from four independent assays (± SD) were expressed as a percentage of the number of cells in a parallel culture in medium supplemented with 10% WEHI conditioned medium. (B) Epo-dependent proliferation and differentiation of CFU-E progenitors. Fetal liver cells were harvested from day-12.5 Epo-R−/− embryos and infected with retroviruses designed to express either the wt or the mutant Epo-Rs as indicated in A. The average numbers of CFU-E colonies from five independent analysis (± SE) were expressed as a percentage of the number of erythroid colonies formed by Epo-R−/− fetal liver cells infected with a retrovirus expressing the wt Epo-R.
Tyrosine Residues in the Epo-R Cytoplasmic Domain Are Essential for Epo-Dependent Erythroid Colony Formation.
To investigate the roles of individual tyrosines in the Epo-R in supporting erythroid colony formation—a test for both proliferation and differentiation of erythroid progenitors—the same panel of mutant Epo-Rs was introduced into Epo-R−/− fetal liver cells via retroviral infection. Infected fetal liver cells were cultured in vitro in the presence of both SCF and Epo, and CFU-E colonies were counted after 2 days. As shown in Fig. 3B, expression of Epo-R F8 in Epo-R−/− fetal liver cells supports formation of a very low number of CFU-Es, suggesting that tyrosines in the Epo-R cytoplasmic domain are important not only for cell proliferation but also for erythroid differentiation. Strikingly, an Epo-R containing only tyrosine Y479 is capable of supporting an almost normal level of erythroid colony formation, generating >85% of the colonies formed by the wt Epo-R. The size and morphology of these colonies were normal, as was the extent of hemoglobinization monitored by benzidine staining (data not shown). Tyrosine 464, though capable of supporting proliferation of BaF3 cells, is unable to support Epo- and SCF-dependent erythroid colony formation above the level mediated by Epo-R F8. An Epo-R containing any one of five cytosolic tyrosine residues—either Y343, Y429, Y431, Y443, or Y461—is capable of supporting the formation of significant numbers of Epo- and SCF-dependent CFU-Es from cultured Epo-R−/− fetal liver cells. Epo-R F7Y401 supported only a few CFU-Es, and the size of the colonies was smaller than that generated by fetal liver cells expressing wt or other mutant Epo-Rs (data not shown).
DISCUSSION
Our most important conclusion is that during erythroid differentiation, activation of the Epo-R occurs by two different mechanisms; these generate different intracellular signals essential for proliferation and terminal differentiation of committed erythroid progenitors. Erythroid progenitors from Epo-R−/− fetal livers, infected in vitro with a retrovirus expressing the wt Epo-R, require the addition of both Epo and SCF to form CFU-E colonies. Thus, signals from both Epo and SCF receptors are essential. At least one signal emanating from KIT must use the Epo-R as a downstream signal-transduction protein, because the Epo-R−/− fetal liver cells were exposed to SCF in vivo, yet required SCF in vitro after expression of the exogenous Epo-R. Indeed, CFU-E colony formation in vitro by normal fetal liver progenitors requires only Epo; the essential interaction between activated KIT and the Epo-R must have occurred in vivo before or at the CFU-E stage. This is consistent with our previous report that the ability of SCF to support proliferation of 32D cells expressing KIT requires coexpression of the Epo-R, demonstrating that at least one proliferative signal generated by KIT involves the Epo-R as a downstream signal-transduction protein (9).
Thus, proliferation and differentiation of Epo-R−/− fetal liver progenitors requires SCF-KIT-mediated activation—presumably phosphorylation—of the Epo-R followed by Epo-mediated activation of the Epo-R. If activated KIT directly phosphorylates the Epo-R, it must do so on any of several tyrosine residues, because Epo-Rs bearing any one of six tyrosines are capable of supporting the formation of significant numbers of CFU-Es. Tyrosine 479, which uniquely supports almost normal cell proliferation and erythroid colony formation, may be a preferred substrate of KIT. In contrast, tyrosine 464 supports the proliferation of hematopoietic cells yet does not support erythroid differentiation. Y464 presumably becomes phosphorylated after Epo-induced receptor dimerization and thus generates a proliferative signal, but it may not be a substrate for the KIT kinase and thus would be unable to support erythroid differentiation of Epo-R−/− progenitors. Alternatively, the signal emanating from Epo-activated Epo-R F7Y464 might be sufficient to support cell proliferation but not erythroid differentiation. Recently we showed that phosphotyrosine 479 is essential for sequential Epo-induced recruitment of PI3-kinase to the Epo-R and activation of mitogen-activating protein kinase (MAPK; ref. 19). Y464, on the other hand, is located within a consensus sequence for Grb2 binding. Neither Y464 nor Y479 in the Epo-R are involved in the maximum activation of JAK2 and STAT5 (21), two proteins also involved in the Epo-R signal-transduction pathway. The signal-transduction molecules downstream of PI3K-MAPK and Grb2 are not clear, and we do not know whether these pathways generate different intracellular signals.
Almost all hematopoietic cells bear more than one type of receptor on their surface and can respond to multiple ligands at the same time. How these receptors interact to generate an appropriate response—be it survival, proliferation, or differentiation—is an interesting and important question. Here we have shown that the Epo-R cannot trigger erythroid colony formation unless it also receives a signal, presumably phosphorylation, from the SCF/KIT signal-transduction pathway. This phenomenon may extend to other cytokine receptors, such as c-mpl (the thrombopoietin receptor), which has a structure similar to that of Epo-R. Because thrombopoietin together with SCF can stimulate the formation of a small number of Epo-R-independent erythroid colonies (8), an interaction between KIT and c-mpl may facilitate the differentiation of erythroid or megakaryocyte progenitors.
In this study, we have developed a unique biological system to study Epo-R signaling. Instead of using cell lines of nonerythroid origin with limited or no erythroid differentiation potential, we expressed mutant Epo-Rs in primary fetal liver cells from Epo-R−/− mice. This system allows us to study the function of KIT and the Epo-R in normal erythropoiesis.
Acknowledgments
We thank Dr. S. Watowich for the 32D-Epo-R(1-257) cell line. We also thank Drs. K. X. Luo, R. Lin, S. Watowich, X. D. Liu, and A. Sirotkin for many helpful discussions and for critically reading the manuscript. This work is supported by Grant HL32262 from the National Institutes of Health and by a grant from the Arris Pharmaceutical Corporation to H.F.L. H.W. was supported by a postdoctoral fellowship from the Damon Runyan–Walter Winchell Cancer Research Fund and U.K. was supported by a fellowship from the Deutsche Forschungsgemeinschaft.
ABBREVIATIONS
- Epo
erythropoietin
- Epo-R
Epo receptor
- CFU-E
colony-forming-unit erythroid
- BFU-E
burst-forming-unit erythroid
- SCF
stem cell factor
- IL-3
interleukin 3
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- wt
wild type
References
- 1.Dranoff G, Crawford A D, Sadelain M, Ream B, Rashid A, Bronson R T, Dickersin G R, Bachurski C J, Mark E L, Whitsett J A, Mulligan R C. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 2.Stanley E, Lieschke G L, Grail D, Metcalf D, Hodgson G, Gall J A, Maher D W, Cebon J, Sinickas V, Dunn A R. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishinakamura R, Nakayama N, Hirabayashi Y, Inoue T, Aud D, McNeil T, Azuma S, Yoshida S, Toyoda Y, Arai K, Miyajima A, Murray R. Immunity. 1995;2:211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein A, Forrester L, Reith A D, Dubreuil P, Rottapel R. Seminars in Hematology. 1991;28:138–142. [PubMed] [Google Scholar]
- 5.Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P. Genes Dev. 1989;3:816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Liu X, Jaenisch R, Lodish H F. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 7.Lin C S, Lim S K, D’Agati V, Costantini F. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 8.Kieran M, Perkins A, Orkin S, Zon L. Proc Natl Acad Sci USA. 1996;93:9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Klingmuller U, Besmer P, Lodish H F. Nature (London) 1995;377:242–246. doi: 10.1038/377242a0. [DOI] [PubMed] [Google Scholar]
- 10.Watowich S, Yoshimura A, Longmore G, Hilton D, Yoshimura Y, Lodish H F. Proc Natl Acad Sci USA. 1992;14:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watowich S, Hilton D, Lodish H F. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrightom N C, Farrell F X, Chang R, Kashysp A K, Barbone F P, Mulcahy L S, Johnson D L, Barrett R W, Jolliffe L K, Dower W J. Science. 1996;273:458–463. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura A, Lodish H F. Mol Cell Biol. 1992;12:706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witthuhn B A, Quelle F W, Silvennoinen T, Yi T, Tang B, Miura O, Ihle J N. Cell. 1992;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 15.Klingmuller U, Lorenz U, Cantley L C, Neel B G, Lodish H F. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 16.Yi T, Zhang J, Miura O, Ihle J N. Blood. 1995;85:87–95. [PubMed] [Google Scholar]
- 17.Tauchi T, Feng G S, Shen R, Hoatlin M, Bagby G, Kabat D, Lu L, Broxmeyer H E. J Biol Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 18.Damen J E, Cutler R L, Jiao H, Yi T, Krystal G. J Biol Chem. 1995;270:23402–23408. doi: 10.1074/jbc.270.40.23402. [DOI] [PubMed] [Google Scholar]
- 19.Klingmuller, U., Wu, H., Hsiao, J. G., Toker, A., Duckworth, B. C., Cantley, L. C. & Lodish, H. F. (1997) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 20.Damen J E, Wakao H, Miyajima A, Krosl J, Humphries R K, Cutler R, Krystal G. EMBO J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klingmuller U, Bergelson S, Hsiao J G, Lodish H F. Proc Natl Acad Sci USA. 1996;93:8324–8328. doi: 10.1073/pnas.93.16.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobert S, Chretien S, Gouileux F, Muller O, Pallard C, Dusanter-Fourt I, Groner B, Lacombe C, Gisselbrecht S, Mayeux P. EMBO J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]