Abstract
Background
The relationship between appendectomy and Crohn's disease is controversial. A Swedish–Danish cohort study was conducted to assess the risk of developing Crohn's disease after an appendectomy.
Methods
709 353 appendectomy patients in Sweden (since 1964) and Denmark (since 1977) were followed for first hospitalisations for Crohn's disease to 2004. Standardised incidence ratios (SIR) served as relative risks.
Results
Overall, 1655 Crohn's disease cases were observed during 11.1 million person‐years of follow‐up. Whereas appendectomy before the age of 10 years was not associated with the risk of Crohn's disease (SIR 1.00; 95% CI 0.80–1.25), the overall SIR of developing Crohn's disease was 1.52 (95% CI 1.45–1.59), being highest in the first 6 months (SIR 8.69; 95% CI 7.68–9.84). SIR diminished rapidly thereafter, with the risk of Crohn's disease reaching background levels after 5–10 years for Crohn's disease overall, as well as for Crohn's ileitis, ileocolonic Crohn's disease, Crohn's colitis and other/unspecified Crohn's disease. A long‐term increased risk of Crohn's disease up to 20 years after the appendectomy was seen only in appendectomy patients without appendicitis or mesenteric lymphadenitis.
Conclusion
The transient increased risk of Crohn's disease after an appendectomy is probably explained by diagnostic bias.
Meta‐analyses1,2 of case–control studies have concluded that individuals who have undergone an appendectomy were at a reduced risk of developing ulcerative colitis. The relationship between appendectomy and Crohn's disease is, however, less clear. Certain studies have shown that an appendectomy was a risk factor for developing Crohn's disease,3,4,5,6,7,8 whereas some concluded that it was protective9,10 and other studies drew no conclusions.11,12,13,14,15,16,17,18 A possible explanation for this discrepancy is that appendectomy may not be a risk factor, but may reflect a diagnostic uncertainty before the diagnosis of Crohn's disease; for example, patients presenting with right lower quadrant abdominal pain undergo an appendectomy for presumed appendicitis and then months later are diagnosed with Crohn's disease, unrecognised at the time of the appendectomy.
Several studies have stratified the risk of developing Crohn's disease after appendectomy on the basis of the duration between the appendectomy and the diagnosis of Crohn's disease.3,4,5,6,14 Such studies have consistently demonstrated a high rate of developing Crohn's disease within a year of an appendectomy. In all studies the risk dropped to background risk levels as the time interval between appendectomy and Crohn's disease widened, but in one study the risk of Crohn's disease remained significant even 10 or more years after the appendectomy.6
In an attempt to clarify the possible role of appendectomy in the etiology of Crohn's disease we have combined and updated population‐based cohort studies in Sweden and Denmark.5,6 This larger dataset has allowed us to follow appendectomy patients considerably longer (now up to 41 years after the appendectomy), and thus to capture more cases of Crohn's disease. Accordingly, we aim to determine the risk of developing Crohn's disease after an appendectomy and whether this risk was altered by the underlying cause of the appendectomy, the time between the appendectomy and the diagnosis of Crohn's disease, the extent of Crohn's disease, and the age at which the appendectomy took place.
Methods
Appendectomy cohort
A population‐based cohort of patients undergoing appendectomy in Swedish hospitals was created using anonymised data provided from the National Board of Health and Welfare in Sweden. In Sweden, 446 968 patients who underwent appendectomy during 1964–2004 were identified using national procedure codes for appendectomy that included: 4510, 4511, 4517, and 0058 during the period 1964–1996; and JEA00, JEA01, and JEA10 during the period 1997–2004. We excluded 304 appendectomy patients (0.07%) recorded as having both ulcerative colitis and Crohn's disease, as well as prevalent cases of ulcerative colitis (715, 0.16%) and Crohn's disease (1839, 0.41%). Finally, we excluded 349 patients (0.08%) who died in the interval between hospital discharge after the appendectomy and the start of follow‐up on the first day in the month after the appendectomy. The resulting Swedish cohort consisted of 443 761 patients, corresponding to 99.2% of all recorded appendectomy patients in Sweden between 1964 and 2004.
A population‐based cohort of patients undergoing appendectomy in Danish hospitals was created using data provided from the National Board of Health in Denmark. Since 1977, virtually all non‐psychiatric hospitalisations in Denmark were documented in the nationwide registration system that contains discharge abstracts consisting of a unique 10‐digit personal identifier, procedural codes and medical diagnoses codes.19 The cohort was identified using procedure codes for appendectomy that included: 43000 and 43001 during the period 1977–1995; and JEA00, JEA01, and JEA10 during the period 1996–2004. In total 273 099 patients underwent an appendectomy in Danish hospitals between 1977 and 2004. We excluded 2211 appendectomy patients (0.81%) who were not Danish citizens, 406 patients (0.15%) with invalid personal identifiers, and 1794 patients (0.66%) with permanent residence outside Denmark. In addition, we excluded 142 patients (0.05%) registered as having both ulcerative colitis and Crohn's disease, as well as prevalent cases of ulcerative colitis (394, 0.14%) and Crohn's disease (2069, 0.76%). Finally, we excluded 477 patients (0.17%) who died and 14 patients (0.005%) who emigrated from Denmark before the start of follow‐up. The resulting Danish cohort consisted of 265 592 patients, corresponding to 97.3% of all appendectomy patients in Denmark between 1977 and 2004.
For each patient, the operation code for appendectomy was accompanied by codes for relevant discharge diagnoses according to national modifications of the International Classification of Diseases (ICD) versions 7 to 10 in Sweden and versions 8 and 10 in Denmark. Cohort members with appendicitis were categorised as: (1) appendicitis with perforation, including appendicitis with diffuse or localised peritonitis and appendicitis with periappendicular abscess; or (2) appendicitis without perforation, including all other and unspecified cases of appendicitis. For cohort members without appendicitis, categories comprised: (3) mesenteric lymphadenitis; (4) appendiceal disease except appendicitis, including neoplasm, mucocele, lymphoid hyperplasia, invagination, fecalith and fistula; (5) non‐specific abdominal pain; and (6) other and unspecified disease. In the following, we refer to appendectomies in the three latter categories without appendiceal or mesenteric inflammation as appendectomies with non‐inflamed appendix.
Crohn's disease outcomes
The cohort of appendectomy patients was followed for first hospitalisations with a Crohn's disease diagnosis between 1964 and 2004 in Sweden and between 1977 and 2004 in Denmark. The diagnosis of Crohn's disease was based on hospital discharge registries and were identified using ICD codes: in Sweden ICD‐7 codes 572.00 or 572.09 (1964–1968), ICD‐8 codes 563.0 or 563.00 (1969–1986), ICD‐9 group 555 (1987–1996), and ICD‐10 group K50 (1997–2004); in Denmark ICD‐8 codes 563.00–563.09 (1977–1993), and ICD‐10 group K50 (1994–2004). Based on the sex, age, and calendar year distribution of Crohn's disease cases and person‐time at risk in the underlying general population, we generated a set of incidence rates of first Crohn's disease hospitalisation for each country in strata of sex, age (5‐year intervals) and calendar year.
ICD codes were used to stratify Crohn's disease by disease extent into the following categories: (a) Crohn's disease of the small intestine (Crohn's ileitis); (b) Crohn's disease of the large intestine (Crohn's colitis); (c) Crohn's disease of both the small and large intestine (ileocolonic Crohn's disease); and (d) Crohn's disease of other or unspecified location. ICD codes specific to disease location were only available when ICD‐9 and ICD‐10 codes were used in Sweden and when ICD‐10 codes were used in Denmark. Crohn's disease outcomes for this supplementary analysis were thus restricted to those Crohn's disease outcomes that occurred between 1987 and 2004 in Sweden and between 1994 and 2004 in Denmark.
Ethics approval was obtained from the Danish Data Inspection Board before commencement.
Statistical analyses
Standardised incidence ratios (SIR) of Crohn's disease were calculated as ratios of observed to expected first hospitalisations with a Crohn's disease diagnosis in the appendectomy cohort, with expected numbers based on background rates in the general population. The calculation of expected rates has been described previously5 and validated.20 Each cohort member contributed person‐time at risk of Crohn's disease from the first day of the month after the appendectomy and until death or 31 December 2004, whichever came first. Person‐years at risk were determined by linking appendectomy patients, by their unique national identification number, to the respective National Causes of Death Registry in Sweden and Denmark to obtain dates of death. Person‐years at risk were summed for cohort members and tabulated in strata of sex, age (5‐year intervals), and calendar year. Stratum‐specific person‐years were then multiplied by the corresponding stratum‐specific Crohn's disease rates in the general population and summed over strata to yield the expected numbers of Crohn's disease cases in the cohort.
Initially, SIR were calculated separately in Sweden and Denmark using country‐specific background rates. Combined Swedish‐Danish SIR estimates were obtained by dividing the sum of observed cases of Crohn's disease with the sum of expected cases of Crohn's disease in the two countries. SIR are presented stratified by country, sex, age at appendectomy (in 10‐year age intervals), age at risk of Crohn's disease (in 10‐year age intervals), time since appendectomy (< 0.5, 0.5–< 1, 1–4, 5–9, 10–14, 15–19, and ⩾ 20 years), Crohn's disease extent (Crohn's ileitis, Crohn's colitis, ileocolonic Crohn's disease, and unspecified Crohn's disease), and disease underlying the appendectomy (appendicitis with perforation, appendicitis without perforation, mesenteric lymphadenitis, appendiceal disease except appendicitis, non‐specific abdominal pain, other and unspecified disease). For all SIR estimates, we calculated 95% CI assuming a Poisson distribution of the observed Crohn's disease cases in the cohort. Formal comparisons of SIR across strata of explanatory variables and accompanying Wald tests for homogeneity were carried out as simple one‐factor log‐linear Poisson regressions on the observed Crohn's disease cases, with means proportional to the stratum‐specific expected numbers of Crohn's disease cases in the cohort. This is equivalent to using the log of stratum‐specific numbers of expected Crohn's disease cases as offset.
All analyses were carried out using SAS, version 8.2 (SAS Institute, Cary, North Carolina, USA). Throughout, 95% CI that excluded unity and two‐sided p values less than 0.05 were considered statistically significant.
Results
The cohort included 709 353 individuals who underwent an appendectomy in Swedish and Danish hospitals. The average follow‐up period was 15.6 years (SD 9.3) with a total of 11 078 855 person‐years accrued. The median age of appendectomy was 26 years (interquartile range 15–44) and more women (56%) underwent the operation. Two‐thirds of appendectomies occurred because of appendicitis, mesenteric lymphadenitis, or rarely because of appendiceal disease other than appendicitis. The remaining appendectomies were performed on normal‐appearing appendices during a laparotomy of a presumed appendicitis (table 1). The distribution of study characteristics of Swedish and Danish participants was equivalent.
Table 1 Characteristics for the study cohort of 709 353 patients followed for the development of Crohn's disease after an appendectomy in Swedish (1964–2004) and Danish (1977–2004) hospitals.
| Sweden | Denmark | Both countries | |
|---|---|---|---|
| No. patients (%) | No. patients (%) | No. patients (%) | |
| Sex | |||
| Male | 198 138 (44.6) | 113 336 (42.7) | 311 474 (43.9) |
| Female | 245 623 (55.4) | 152 256 (57.3) | 397 879 (56.1) |
| Year of appendectomy | |||
| 1964–1976 | 77 216 (17.4) | – | 77 216 (10.9) |
| 1977–1985 | 124 726 (28.1) | 110 879 (41.7) | 235 605 (33.2) |
| 1986–1994 | 125 263 (28.2) | 85 172 (32.1) | 210 435 (29.7) |
| 1995–2004 | 116 556 (26.3) | 69 541 (26.2) | 186 097 (26.2) |
| Age at appendectomy (years) | |||
| 0–9 | 34 092 (7.7) | 24 311 (9.2) | 58 403 (8.2) |
| 10–19 | 121 179 (27.3) | 76 966 (29.0) | 198 145 (27.9) |
| 20–29 | 91 317 (20.6) | 47 752 (18.0) | 139 069 (19.6) |
| 30–39 | 64 324 (14.5) | 36 493 (13.7) | 100 817 (14.2) |
| 40–49 | 51 027 (11.5) | 27 444 (10.3) | 78 471 (11.1) |
| ⩾50 | 81 822 (18.4) | 52 626 (19.8) | 134 448 (19.0) |
| Cause of appendectomy | |||
| Appendicitis | 296 367 (66.8) | 183 168 (69.0) | 479 535 (67.6) |
| With perforation | 54 934 (12.4) | 38 300 (14.4) | 93 234 (13.1) |
| Without perforation | 241 433 (54.4) | 144 868 (54.5) | 386 301 (54.5) |
| Mesenteric lymphadenitis | 24 064 (5.4) | 8119 (3.1) | 32 183 (4.5) |
| Other disease (non‐inflamed appendix) | 123 330 (27.8) | 74 305 (28.0) | 197 635 (7.9) |
| Appendiceal disease except appendicitis* | 1868 (0.4) | 1222 (0.5) | 3090 (0.4) |
| Non‐specific abdominal pain | 28 212 (6.4) | 13 433 (5.1) | 41 645 (5.9) |
| Other and unspecified disease | 93 250 (21.0) | 59 650 (22.5) | 152 900 (21.6) |
| Total | 443 761 (100) | 265 592 (100) | 709 353 (100) |
*Appendiceal disease except appendicitis includes neoplasm, mucocele, lymphoid hyperplasia, invagination, fecalith and fistula.
During follow‐up, 1070 cases of Crohn's disease were observed in Swedish and 585 in Danish cohort members. The overall SIR of developing Crohn's disease after an appendectomy in Swedish and Danish hospitals was 1.52 (95% CI 1.45–1.59; table 2). The risk of Crohn's disease was similarly elevated in men and women, but depended significantly on the age at appendectomy. Children who underwent an appendectomy before the age of 10 years were not at an increased risk (SIR 1.00; 95% CI 0.80–1.25), whereas appendectomies at all age groups above the age of 10 years were associated with a significantly elevated risk of Crohn's disease (table 2). The SIR of developing Crohn's disease after an appendectomy was significantly elevated across all age groups at risk of Crohn's disease. The highest SIR were observed for Crohn's disease diagnoses established before the age of 10 years (SIR 7.95; 95% CI 3.79–16.68) or during adolescence (SIR 2.88; 95% CI 2.51–3.30).
Table 2 Standardised incidence ratios and 95% CI of Crohn's disease among 709 353 patients undergoing appendectomy in Swedish (1964 to 2004) and Danish (1977 to 2004) hospitals.
| Person‐years | Obs | Exp | SIR (95% CI) | p Value* | |
|---|---|---|---|---|---|
| Overall | 11 078 855 | 1655 | 1089.7 | 1.52 (1.45 to 1.59) | to |
| Country | 0.054 | ||||
| Sweden | 7 250 881 | 1070 | 729.0 | 1.47 (1.38 to 1.56) | |
| Denmark | 3 827 974 | 585 | 360.7 | 1.62 (1.50 to 1.76) | |
| Sex | 0.42 | ||||
| Women | 6 467 929 | 1052 | 682.3 | 1.54 (1.45 to 1.64) | |
| Men | 4 610 926 | 603 | 407.4 | 1.48 (1.37 to 1.60) | |
| Age at appendectomy (years) | |||||
| 0 to 9 | 952 028 | 77 | 77.1 | 1.00 (0.80 to 1.25) | <0.001 |
| 10 to 19 | 3 358 272 | 533 | 370.1 | 1.44 (1.32 to 1.57) | |
| 20 to 29 | 2 376 137 | 452 | 267.2 | 1.69 (1.54 to 1.85) | |
| 30 to 39 | 1 672 986 | 236 | 150.7 | 1.57 (1.38 to 1.78) | |
| 40 to 49 | 1 267 122 | 163 | 105.1 | 1.55 (1.33 to 1.81) | |
| ⩾50 | 1 452 309 | 194 | 119.5 | 1.62 (1.41 to 1.87) | |
| Age at risk of Crohn's disease (years) | |||||
| 0 to 9 | 144 077 | 7 | 0.9 | 7.95 (3.79 to 16.68) | <0.001 |
| 10 to 19 | 1 357 510 | 204 | 71.9 | 2.88 (2.51 to 3.30) | |
| 20 to 29 | 2 425 751 | 538 | 370.9 | 1.45 (1.33 to 1.58) | |
| 30 to 39 | 2 344 822 | 329 | 245.6 | 1.34 (1.20 to 1.49) | |
| 40 to 49 | 1 786 427 | 224 | 147.9 | 1.51 (1.33 to 1.73) | |
| ⩾50 | 3 020 268 | 353 | 253.5 | 1.39 (1.25 to 1.55) | |
Exp, Expected number of patients with Crohn's disease; Obs, observed number of patients with Crohn's disease; SIR, standardised incidence ratio.
*Test of homogeneity.
The risk of Crohn's disease was significantly elevated whether the underlying cause of the appendectomy was inflammatory (appendicitis or mesenteric lymphadenitis) or not. The risk was, however, considerably higher in patients with appendicitis complicated by perforation (SIR 1.89; 95% CI 1.66–2.16) and in patients whose resected appendices were non‐inflamed (SIR 1.97; 95% CI 1.82–2.14) compared with patients with non‐perforated appendicitis (SIR 1.19; 95% CI 1.11–1.28; p value for homogeneity < 0.001; table 3).
Table 3 Standardised incidence ratios and 95% CI of Crohn's disease among 709 353 patients undergoing appendectomy in Swedish (1964 to 2004) and Danish (1977 to 2004) hospitals stratified by cause of appendectomy.
| Cause of appendectomy | Person‐years | Obs | Exp | SIR (95% CI) | *p Value |
|---|---|---|---|---|---|
| Appendicitis | 7 188 320 | 924 | 707.6 | 1.31 (1.22 to 1.39) | <0.001 |
| With perforation | 1 234 469 | 216 | 114.2 | 1.89 (1.66 to 2.16) | |
| Without perforation | 5 953 851 | 708 | 593.5 | 1.19 (1.11 to 1.28) | |
| Mesenteric lymphadenitis | 645 698 | 111 | 68.0 | 1.63 (1.36 to 1.97) | |
| Other disease (non‐inflamed appendix) | 3 244 837 | 620 | 314.1 | 1.97 (1.82 to 2.14) | |
| Appendiceal disease except appendicitis | 43 437 | 8 | 4.2 | 1.92 (0.96 to 3.83) | |
| Non‐specific abdominal pain | 654 377 | 138 | 69.6 | 1.98 (1.68 to 2.34) | |
| Other and unspecified disease | 2 547 023 | 474 | 240.4 | 1.97 (1.80 to 2.16) |
Exp, Expected number of patients with Crohn's disease; Obs, observed number of patients with Crohn's disease; SIR, standardised incidence ratio.
*Test of homogeneity. P value < 0.001 whether using a three‐way (appendicitis, mesenteric lymphadenitis, other disease) or a six‐way (appendicitis with perforation, appendicitis without perforation, mesenteric lymphadenitis, appendiceal disease except appendicitis, non‐specific abdominal pain, other and unspecified disease) categorisation of the cause of appendectomy.
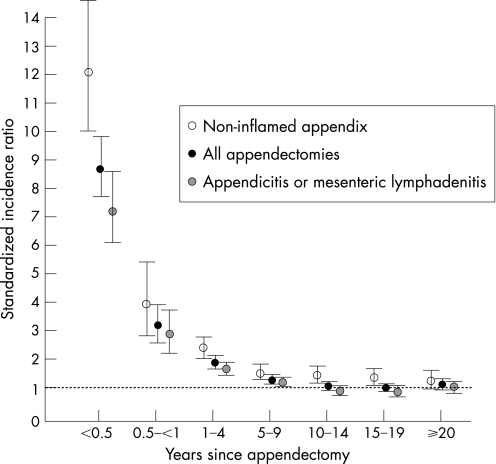
The highest risk of Crohn's disease occurred within the first 6 months of undergoing an appendectomy (SIR 8.69; 95% CI 7.68–9.84); the subsequent 6‐month period was associated with over a threefold increased risk (fig 1). Stratification of the cohort by the underlying cause of the appendectomy revealed that patients with perforated appendicitis, non‐perforated appendicitis, and mesenteric lymphadenitis were at an elevated risk of Crohn's disease only for the first 5 years after appendectomy (table 4); however, when patients with an inflammatory etiology (i.e. appendicitis or mesenteric lymphadenitis) were combined, the risk of developing Crohn's disease remained significant up to 10 years after the appendectomy. Patients who underwent an appendectomy with a non‐inflamed appendix remained at a significantly elevated risk for up to 20 years (fig 1, table 4).
Figure 1 Standardised incidence ratios of developing Crohn's disease by the time since appendectomy in Swedish (1964–2004) and Danish (1977–2004) hospitals, stratified by cause of appendectomy. Bars indicate 95% CI.
Table 4 Standardised incidence ratios and 95% CI of developing Crohn's disease by the time since appendectomy, stratified by cause of appendectomy.
| Time since appendectomy (years) | Overall SIR (95% CI) | Appendicitis or mesenteric lymphadenitis SIR (95% CI) | Perforated appendicitis SIR (95% CI) | Non‐perforated appendicitis SIR (95% CI) | Mesenteric lymphadenitis SIR (95% CI) | Non‐inflamed appendix* SIR (95% CI) |
|---|---|---|---|---|---|---|
| <0.5 | 8.69 (7.68 to 9.84) | 7.16 (6.08 to 8.44) | 12.12 (8.92 to 16.46) | 6.10 (4.99 to 7.46) | 6.93 (3.30 to 14.53) | 12.12 (10.03 to 14.63) |
| 0.5–<1 | 3.16 (2.58 to 3.89) | 2.85 (2.20 to 3.69) | 5.99 (3.86 to 9.28) | 2.11 (1.50 to 2.97) | 3.78 (1.42 to 10.06) | 3.89 (2.78 to 5.44) |
| 1–4 | 1.86 (1.69 to 2.05) | 1.66 (1.47 to 1.87) | 2.38 (1.85 to 3.05) | 1.42 (1.22 to 1.64) | 2.86 (1.99 to 4.12) | 2.36 (2.02 to 2.76) |
| 5–9 | 1.25 (1.12 to 1.39) | 1.15 (1.01 to 1.31) | 1.23 (0.88 to 1.70) | 1.12 (0.96 to 1.30) | 1.31 (0.86 to 2.01) | 1.51 (1.25 to 1.81) |
| 10–14 | 1.03 (0.91 to 1.17) | 0.88 (0.75 to 1.03) | 1.28 (0.90 to 1.82) | 0.73 (0.59 to 0.89) | 1.45 (0.97 to 2.17) | 1.44 (1.17 to 1.77) |
| 15–19 | 0.98 (0.84 to 1.15) | 0.85 (0.69 to 1.03) | 1.05 (0.65 to 1.68) | 0.81 (0.64 to 1.03) | 0.80 (0.43 to 1.50) | 1.31 (1.02 to 1.69) |
| ⩾20 | 1.09 (0.93 to 1.27) | 1.01 (0.82 to 1.23) | 0.84 (0.45 to 1.55) | 0.96 (0.76 to 1.22) | 1.48 (0.91 to 2.41) | 1.25 (0.97 to 1.61) |
SIR, Standardised incidence ratio.
*Non‐inflamed appendix includes appendectomy patients without appendicitis or mesenteric lymphadenitis.
Stratified by Crohn's disease extent and time since the appendectomy (table 5), SIR were highest for Crohn's ileitis diagnosed within the first 6 months after an appendectomy (SIR 14.14; 95% CI 11.00–18.18). A rather similar pattern of rapidly declining SIR was seen for each of the four studied Crohn's disease locations, however, with no statistically significant increase in risk remaining after 10 years for any specific Crohn's disease extent. Indeed, when combining Crohn's ileitis, ileocolonic Crohn's disease and Crohn's colitis, the risk in the combined period 5 or more years after the appendectomy was inconspicuous (SIR 1.05; 95% CI 0.95–1.16), whereas the risk of Crohn's disease of other or unspecified extent reached background levels 10 years after the appendectomy (table 5).
Table 5 Standardised incidence ratios and 95% CI of developing Crohn's disease by time since appendectomy stratified by Crohn's disease extent.
| Time since appendectomy (years) | Crohn's ileitis SIR (95% CI) | Ileocolonic Crohn's disease SIR (95% CI) | Crohn's colitis SIR (95% CI) | Other and unspecified Crohn's disease SIR (95% CI) |
|---|---|---|---|---|
| <0.5 | 14.14 (11.00 to 18.18) | 8.43 (4.99 to 14.23) | 9.46 (6.10 to 14.66) | 6.87 (5.04 to 9.36) |
| 0.5–<1 | 4.09 (2.57 to 6.48) | 1.77 (0.57 to 5.49) | 2.79 (1.25 to 6.21) | 4.05 (2.71 to 6.04) |
| 1–4 | 1.84 (1.46 to 2.32) | 1.57 (1.04 to 2.36) | 1.61 (1.13 to 2.30) | 2.28 (1.90 to 2.73) |
| 5–9 | 1.16 (0.90 to 1.48) | 1.61 (1.15 to 2.27) | 1.29 (0.92 to 1.81) | 1.47 (1.22 to 1.78) |
| 10–14 | 1.25 (0.99 to 1.58) | 0.81 (0.50 to 1.30) | 0.56 (0.36 to 0.92) | 1.05 (0.85 to 1.31) |
| 15–19 | 0.76 (0.54 to 1.06) | 1.03 (0.64 to 1.66) | 1.02 (0.69 to 1.50) | 1.15 (0.92 to 1.44) |
| ⩾20 | 1.01 (0.74 to 1.38) | 1.35 (0.86 to 2.11) | 0.83 (0.54 to 1.29) | 1.17 (0.94 to 1.47) |
SIR, standardised incidence ratio.
Discussion
Appendectomy patients were generally at an elevated risk of being hospitalised with Crohn's disease for 5–10 years after the appendectomy. For patients undergoing appendectomy for a suspicion of appendicitis, but whose appendix and mesenteric lymph nodes showed no signs of inflammation, the excess risk lasted up to 20 years after the operation. The observed patterns of rather extreme SIR in the first year after appendectomy followed by gradually diminishing SIR in subsequent periods of follow‐up were found consistently in Sweden and Denmark and in both sexes. These findings can be explained by well‐known differential diagnostic problems that may result in unnecessary appendectomies in some patients who present with symptoms of incipient Crohn's disease. Such cases of Crohn's disease will probably come to the clinician's attention after the appendectomy, a bias that is likely to be most pronounced within the first few years after an appendectomy. The markedly elevated early risk of developing Crohn's ileitis and the lack of an increased risk of Crohn's colitis beyond 5 years after the appendectomy further supported the presence of a diagnostic bias.
The present study extended the follow‐up period of two previously published cohort studies in Sweden and Denmark;5,6 thus allowing for a threefold increase in observed cases of Crohn's disease among patients undergoing an appendectomy, and increasing the power to detect differences across strata of appendectomy patients. The study was population‐based and we used validated electronic hospitalisation databases to identify Crohn's disease outcomes.
Previous studies have reported widely variable results including positive,4,5,7,8 inverse9,10 and null11,12,13,14,15,16,17,18 associations between appendectomy and the risk of Crohn's disease. The observed heterogeneity between studies may be explained by methodological issues. In two studies17,18 investigators included appendectomies conducted after the diagnosis of Crohn's disease. Although the majority of case–control studies appropriately excluded appendectomies that occurred after the diagnosis of Crohn's disease among case subjects, only two studies14,15 applied a similar restriction in controls. Furthermore, some studies were likely to be influenced by ascertainment bias, as appendectomy histories in cases were identified by a different mechanism than in controls.9,10,11,14 Finally, in several studies7,9,10,11,12 cases were individually matched to controls; however, matching was not accounted for in the statistical analysis.
Although the current study aimed to overcome the limitations of previous studies, the hospitalisation databases were not designed to capture patient‐specific confounder information such as smoking. Smoking was a possible confounder because it is a risk factor for developing Crohn's disease,21 and it may be related to developing appendicitis.11,22 Controlling for smoking, however, did not materially change relative risk estimates in those studies with smoking information available.7,9,10,13,14,16 In addition, recorded data in hospital discharge registries are subject to varying degrees of misclassification, and would not include patients with mild Crohn's disease who were not hospitalised. Previous validation studies have, however, shown that misclassification was low, as the accuracy of a Crohn's disease diagnosis from hospital discharge abstracts was 97%,20 and appendicitis was accurately coded in 94% of cases.23 Such minor degrees of misclassification are unlikely to have produced any major distortion of the SIR.
Several studies have argued that the risk associated with appendectomy may not be biological, but an effect of a diagnostic bias.3,4,5,14 Crohn's disease can present with abdominal pain that mimics appendicitis. In addition, Crohn's disease can rarely involve the appendix, leading to appendicitis.24 The delay from symptom onset to the diagnosis of Crohn's disease can further confound this relationship, as 40% experience gastrointestinal symptoms for 3 or more years before diagnosis,25 and the mean latency period has been reported to be as high as 7 years.26 Although most gastrointestinal symptoms retrospectively reported by patients with a prolonged prodromal period were not consistent with appendicitis, the findings highlight the potential influence of a diagnostic bias. Therefore, for an appendectomy to be a true risk factor its effect must persist for an extended period of time. The current study demonstrated that the greatest risk occurred within the first year of an appendectomy, particularly in the first 6 months; a finding consistent with a diagnostic bias. The risk associated with appendectomy for verified appendicitis or mesenteric lymphadenitis remained significantly elevated 5–10 years after the appendectomy; among individuals who underwent an appendectomy without such inflammation the risk persisted for up to 20 years. These time‐dependent results need to be interpreted with caution, however, because the diagnosis of Crohn's disease was based on hospital registries and did not include ambulatory clinic diagnoses that may have occurred earlier.
Among the studied Crohn's disease locations, the highest SIR observed was for Crohn's ileitis diagnosed shortly after the appendectomy. These findings were probably explained by a diagnostic bias because patients with small bowel disease more commonly present with right lower quadrant pain that may mimic appendicitis. Previous studies have also shown higher relative risk estimates for Crohn's ileitis than for Crohn's colitis.14,27 In our study the risk of Crohn's colitis was only significantly elevated in the first 5 years after the appendectomy.
An inverse association between appendectomy and the development of ulcerative colitis has been consistently reported.1,28,29 The inverse relationship was found after appendicitis and not after appendectomy with a non‐inflamed appendix. On the contrary, the increased risk of developing Crohn's disease was observed after both appendectomy for appendicitis and with a non‐inflamed appendix. Furthermore, the decreased risk of developing ulcerative colitis only occurred in patients who underwent appendectomy in childhood;28 in contrast, the increased risk of Crohn's disease occurred in adults, but not in children. These paradoxical findings might be explained by a diagnostic bias because Crohn's disease patients are more likely to mimic the presentation of appendicitis compared with ulcerative colitis patients.
Children who underwent appendectomy before the age of 10 years were not at greater risk of developing Crohn's disease, except for the rare cases of Crohn's disease diagnosed before the age of 10 years. Presumably, the effect of appendectomy should be present across all age groups unless the underlying mechanism depends on other etiological factors that differ between juvenile‐onset and adult‐onset Crohn's disease. Juvenile‐onset Crohn's disease patients may have a higher frequency of Crohn's disease susceptibility genes compared with adult‐onset Crohn's disease patients.30,31,32 The risk of developing Crohn's disease in children may thus have a stronger genetic influence, whereas in adults, other environmental factors such as removal of the appendix or the occurrence of appendicitis may be more important. Of the 77 Crohn's disease patients who had undergone appendectomy before the age of 10 years, only seven had also been diagnosed with Crohn's disease under the age of 10 years (SIR 7.95). Presumably, differential diagnostic difficulties explained the high SIR because six of these seven childhood Crohn's disease cases were diagnosed within 2 years of the appendectomy.
Perforated appendicitis was associated with a greater risk of developing Crohn's disease compared with non‐perforated appendicitis. For neither of the two, however, did the excess risk persist beyond 5 years. In addition to the influence of a diagnostic bias, the elevated risk observed after perforated appendicitis might be explained by the rare occurrence of Crohn's disease involving the appendix that can lead to perforation.24 Alternatively, this finding may result from a biological association between perforated appendicitis and Crohn's disease. For example, a previous study demonstrated that perforated appendicitis, compared with non‐perforated appendicitis and appendectomy for the suspicion of appendicitis with a non‐inflamed appendix, was associated with a preference for a T‐cell helper type 1‐dominated immune response, which is characteristic of Crohn's disease.33
The association between appendectomy and Crohn's disease remains difficult to clarify. The heterogeneity of results in the literature and in the present study is most likely caused by the time‐dependent complexity of the relationship and the influence of non‐biological distorting factors originating in differential diagnostic difficulties. The markedly elevated risk shortly after the appendectomy, the disappearance of an excess risk in most subgroups after 5 years, and the extent‐specific findings observed for Crohn's ileitis and Crohn's colitis suggest that a diagnostic bias explains the increased risk of Crohn's disease observed after an appendectomy.
Abbreviations
ICD - International Classification of Diseases
SIR - standardized incidence ratio
Footnotes
Funding: This study was supported by unrestricted research grants from Aase and Ejnar Danielsen's Foundation, Civil engineer Frode V Nyegaard and Wife's Foundation, and the Gangsted Foundation.
Conflict of interest: None declared.
References
- 1.Koutroubakis I E, Vlachonikolis I G. Appendectomy and the development of ulcerative colitis: results of a metaanalysis of published case‐control studies. Am J Gastroenterol 200095171–176. [DOI] [PubMed] [Google Scholar]
- 2.Koutroubakis I E, Vlachonikolis I G, Kouroumalis E A. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: a critical review. Inflamm Bowel Dis 20028277–286. [DOI] [PubMed] [Google Scholar]
- 3.Frisch M, Gridley G. Appendectomy in adulthood and the risk of inflammatory bowel diseases. Scand J Gastroenterol 2002371175–1177. [DOI] [PubMed] [Google Scholar]
- 4.Kurina L M, Goldacre M J, Yeates D.et al Appendicectomy, tonsillectomy, and inflammatory bowel disease: a case–control record linkage study. J Epidemiol Commun Health 200256551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisch M, Johansen C, Mellemkjaer L.et al Appendectomy and subsequent risk of inflammatory bowel diseases. Surgery 200113036–43. [DOI] [PubMed] [Google Scholar]
- 6.Andersson R E, Olaison G, Tysk C.et al Appendectomy is followed by increased risk of Crohn's disease. Gastroenterology 200312440–46. [DOI] [PubMed] [Google Scholar]
- 7.Koutroubakis I E, Vlachonikolis I G, Kapsoritakis A.et al Appendectomy, tonsillectomy, and risk of inflammatory bowel disease: case–controlled study in Crete. Dis Colon Rectum 199942225–230. [DOI] [PubMed] [Google Scholar]
- 8.Caserta L, de Filippo F R, Riegler G. Relationship between anamnestic evidence of appendectomy and onset and clinical course of Crohn's disease. Am J Gastroenterol 200297207–208. [DOI] [PubMed] [Google Scholar]
- 9.Lopez Ramos D, Gabriel R, Cantero Perona J.et al Association of MALTectomy (appendectomy and tonsillectomy) and inflammatory bowel disease: a familial case–control study. Rev Esp Enferm Dig 200193303–314. [PubMed] [Google Scholar]
- 10.Radford‐Smith G L, Edwards J E, Purdie D M.et al Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut 200251808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duggan A E, Usmani I, Neal K R.et al Appendicectomy, childhood hygiene, Helicobacter pylori status, and risk of inflammatory bowel disease: a case control study. Gut 199843494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reif S, Lavy A, Keter D.et al Appendectomy is more frequent but not a risk factor in Crohn's disease while being protective in ulcerative colitis: a comparison of surgical procedures in inflammatory bowel disease. Am J Gastroenterol 200196829–832. [DOI] [PubMed] [Google Scholar]
- 13.Garcia Rodriguez L A, Gonzalez‐Perez A, Johansson S.et al Risk factors for inflammatory bowel disease in the general population. Aliment Pharmacol Ther 200522309–315. [DOI] [PubMed] [Google Scholar]
- 14.Russel M G, Dorant E, Brummer R J.et al Appendectomy and the risk of developing ulcerative colitis or Crohn's disease: results of a large case–control study. South Limburg Inflammatory Bowel Disease Study Group. Gastroenterology 1997113377–382. [DOI] [PubMed] [Google Scholar]
- 15.Gent A E, Hellier M D, Grace R H.et al Inflammatory bowel disease and domestic hygiene in infancy. Lancet 1994343766–767. [DOI] [PubMed] [Google Scholar]
- 16.Sicilia B, Lopez Miguel C, Arribas F.et al Environmental risk factors and Crohn's disease: a population‐based, case–control study in Spain. Dig Liver Dis 200133762–767. [DOI] [PubMed] [Google Scholar]
- 17.Breslin N P, McDonnell C, O'Morain C. Surgical and smoking history in inflammatory bowel disease: a case–control study. Inflamm Bowel Dis 199731–5. [PubMed] [Google Scholar]
- 18.Smithson J E, Radford‐Smith G, Jewell G P. Appendectomy and tonsillectomy in patients with inflammatory bowel disease. J Clin Gastroenterol 199521283–286. [DOI] [PubMed] [Google Scholar]
- 19.Andersen T F, Madsen M, Jorgensen J.et al The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 199946263–268. [PubMed] [Google Scholar]
- 20.Fonager K, Sorensen H T, Rasmussen S N.et al Assessment of the diagnoses of Crohn's disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol 199631154–159. [DOI] [PubMed] [Google Scholar]
- 21.Calkins B M. A meta‐analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci 1989341841–1854. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery S M, Pounder R E, Wakefield A J. Smoking in adults and passive smoking in children are associated with acute appendicitis. Lancet 1999353379. [DOI] [PubMed] [Google Scholar]
- 23.Andersson R, Hugander A, Thulin A.et al Indications for operation in suspected appendicitis and incidence of perforation. BMJ 1994308107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oren R, Rachmilewitz D. Preoperative clues to Crohn's disease in suspected, acute appendicitis. Report of 12 cases and review of the literature. J Clin Gastroenterol 199215306–310. [DOI] [PubMed] [Google Scholar]
- 25.Burgmann T, Clara I, Graff L.et al The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis – how much is irritable bowel syndrome? Clin Gastroenterol Hepatol 20064614–620. [DOI] [PubMed] [Google Scholar]
- 26.Pimentel M, Chang M, Chow E J.et al Identification of a prodromal period in Crohn's disease but not ulcerative colitis. Am J Gastroenterol 2000953458–3462. [DOI] [PubMed] [Google Scholar]
- 27.Cosnes J, Seksik P, Nion‐Larmurier I.et al Prior appendectomy and the phenotype and course of Crohn's disease. World J Gastroenterol 2006121235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson R, Olaison G, Tysk C.et al Appendectomy and protection against ulcerative colitis. N Engl J Med 2001344808–814. [DOI] [PubMed] [Google Scholar]
- 29.Frisch M. Inverse association between appendicectomy and ulcerative colitis. BMJ 2006332561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss B, Shamir R, Bujanover Y.et al NOD2/CARD15 mutation analysis and genotype–phenotype correlation in Jewish pediatric patients compared with adults with Crohn's disease. J Pediatr 2004145208–212. [DOI] [PubMed] [Google Scholar]
- 31.Bene J, Magyari L, Talian G.et al Prevalence of SLC22A4, SLC22A5 and CARD15 gene mutations in Hungarian pediatric patients with Crohn's disease. World J Gastroenterol 2006125550–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leshinsky‐Silver E, Karban A, Buzhakor E.et al Is age of onset of Crohn's disease governed by mutations in NOD2/caspase recruitment domains 15 and Toll‐like receptor 4? Evaluation of a pediatric cohort. Pediatr Res 200558499–504. [DOI] [PubMed] [Google Scholar]
- 33.Ruber M, Berg A, Ekerfelt C.et al Different cytokine profiles in patients wiht a history of gangrenous or phlegmonous appendicitis. Clin Exp Immunol 2006143117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]